Abstract—
Recent years have seen increasingly growing attention to decarbonizing the energy sector and decreasing, owing to this policy, the consumption of fossil fuels. Since recycling of waste is among the urgent problems at present, then, seeing that biomass accounts for a significant fraction of waste, efforts taken to more efficiently use it as fuel are becoming of issue. Torrefaction is one of the most acceptable technologies for obtaining high-quality fuel from biomass, the application of which makes it possible to increase the heating value of biofuel, decrease its hydrophilicity, and improve its grindability, all with relatively moderate energy expenditures. The torrefaction process can be further improved by performing it in a gaseous medium with some content of oxygen. This will make it possible to decrease both the energy expenditures for conducting the process and the time taken to perform it. The article presents the results from studying the oxidative torrefaction of three kinds of finely dispersed biomass: ground sunflower husk, chicken litter, and wood sawdust. The process is performed in a fluidized bed with biomass fluidization by subjecting it to smoke gases at a temperature of 250°С and with the oxygen content equal to 2–3 vol %. The study results have shown that, given the polydispersed composition and complex shape of biomass particles, their stable fluidization is possible in a rather narrow range of gas velocities. The oxidative torrefaction process itself takes from 5 to 15 min for its completion, depending on the biomass kind. Such a wide interval of time is due to the presence or almost complete absence of exothermal reactions developing in the process depending on the kind of raw material used. The maximal and minimal exothermal effects take place in performing oxidative torrefaction of sunflower husk and chicken litter, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The amount of carbon dioxide (CO2) that is released in firing plant biomass is the same as that which the plant absorbed for its entire vegetation time. This carbon dioxide is a carbon-neutral readily accessible fuel [1, 2], because the time taken by the plant biomass to grow until its combustion is hardly more than a few decades in contrast to fossil fuel, which was formed over millions of years. However, biomass in its initial state has a significantly lower heating value and is characterized by a significantly higher moisture content and low bulk density in comparison with fossil fuel [3, 4], circumstances that impose limitations on its use for energy-generation purposes. Pelletized biomass is free from some of the above-mentioned drawbacks, but it still remains hydrophilic, a feature that adds difficulty to its transportation and storage. In addition, despite their being widely used in domestic heat supply, pellets cannot be used on a wide scale in large power installations in view of their high cost.
The content of moisture and volatiles in biomass can be decreased, its heating value can be increased, and its hydrophobicity and grindability can be improved by subjecting biomass to thermal treatment in oxygen-free medium at a temperature of 200–300°C. This process is called torrefaction. In the course of torrefaction, moisture is removed from the initial biomass, as a result of which the content of oxygen in it becomes significantly lower. As a result, the gaseous products obtained from the gasification of torrefied biomass have a higher heating value than those obtained from the gasification of initial biomass. Torrefied biomass has a combustion rate lower than that of the initial biomass, due to which it becomes possible to complete the combustion process in the boiler furnace and rule out the possibility of combustion in the furnace smoke conduits, thereby minimizing the danger of ash melting and dense ash deposits forming on the boiler’s convective heating surfaces. Owing to its improved hydrophobicity, torrefied biomass can be stockpiled not in indoors but outdoors under a shelter, which helps to achieve significantly lower costs for storing such biofuel and to simplify its handling.
Data are available according to which the energy resources consumed for biomass grinding are a factor of 3.5–4.0 higher than those for coal processing [5]. These expenditures can be decreased by improving the torrefied biomass' grindability. Thus, it can be concluded from what was said above that torrefied biomass is a product more suitable for its combustion jointly with coal in comparison with the initial biomass [6–10].
Torrefaction is a rather energy consuming process. The costs for operation of torrefaction facilities can be decreased by using air and gaseous torrefaction products instead of nitrogen. Such a process is called oxidative torrefaction. The accomplished works on studying the effect that the oxygen concentration in the gas supplied to the reactor and the torrefaction temperature have on the biomass properties [11] have shown that the change in the O2 content in the range from 2 to 21 vol % does not have a significant influence on the solid product composition at low temperatures (below 280°С). This helps prevent oxidation of the volatiles released in the course of torrefaction.
The authors of [12–14] have determined that the oxidative torrefaction characteristics (the solid product mass yield and properties) essentially depend on the nature of the initial raw material. In particular, they have found that oxidative torrefaction is more suitable for processing wood biomass than fibrous (nonwood) biomass. Apart from the shape of particles, the oxidative torrefaction process depends on the particle size, including the surface area of the processed particles that is contacting with the gas medium [12].
The aim of this work is to study the oxidative torrefaction of various biomass kinds: wood waste, sunflower husk, and chicken litter. For achieving the maximal possible surface area through which the biomass contacts with oxygen-containing gas, it is proposed to grind the biomass and to perform the torrefaction process in a fluidized bed [15, 16].
EXPERIMENTAL SETUP
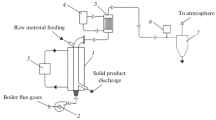
For studying the oxidative torrefaction of ground biomass, an experimental setup was constructed (Fig. 1). It consists of a fluidized bed-containing reactor covered with a “jacket” into which a liquid heat carrier (TLV-330 thermal oil) heated in an electrical boiler is supplied. Physically, the reactor is a steel pipe 108 mm in diameter (the wall thickness is 4 mm) and 1000 mm in height. The initial biomass is fed in portions into the reactor from the top through a connection pipe, and the torrefied biomass is taken out from the bottom.
According to the study results reported in [15, 16], it is impossible to achieve heterogeneity of biomass particles in the fluidized inert material bed during torrefaction. It is also difficult to separate biomass particles from the inert material. Therefore, it was decided to discard the use of inert material.
In this study, a periodically operating reactor was used, the fluidized bed in which was formed by ground particles of biomass itself. The biomass bed in the reactor was fluidized by flue gases from the boiler, in which biofuel (granulated sunflower husk) was combusted. The flue gases were supplied to the reactor’s lower part by means of an exhaust fan (not shown in Fig. 1). Flue gases were produced using a flame tube/flue tube hot water boiler with a capacity of 500 kW equipped with a furnace for burning granulated sunflower husk in a fluidized bed.
The gases flowing out from the reactor contained condensable and noncondensable gaseous biomass torrefaction products. These gases were cooled in a heat exchanger by water that was set to circulate, by means of a pump (not shown in Fig. 1), between the heat exchanger and a water storage tank. The obtained condensate was removed from the system. After that, noncondensable gaseous torrefaction products were mechanically purified in a cyclone and discharged into the atmosphere.
Prior to the beginning of experiments, the setup was purged with flue gases, which were cooled in the heat exchanger and, after the removal of moisture from them, were subjected to a chemical analysis by means of a Vario Plus Industrial flow-type gas analyzer (the error of readings is 5–10% of the measured value depending on the used electrochemical sensor).
The characteristics of the studied biomass kinds are given in Table 1.
The mass of ground sunflower husk, litter, and wood sawdust particles before and after torrefaction was measured using an Acom JW-1-200 RS 232 C laboratory scale (the maximal and minimal mass values are 200 and 0.2 g, and the measurement step is 0.01 g). The husk moisture content was determined using an Ohaus MB45 moisture analyzer (the measurement error is ±0.05%).
For determining the fraction of volatiles, a thermogravimetric analysis (TGA) was carried out using a NETSCH STA 409 PC/PG thermoanalyzer (with a sensitivity of 0.002 mg). The ash content was measured by burning a sample in a muffle furnace at a temperature of 600°С until a constant mass was achieved. Prior to determining the ash content, content of volatiles, and elemental composition, the samples were dried to the relative moisture content equal to 0.1%.
The elemental composition (C, H, N, S) that the studied biomass had before and after the thermal treatment was analyzed using an Elementar Vario Macro Cube analyzer (with the measurement error equal to 0.1%). The oxygen content CO, % per dry mass was calculated proceeding from the following material balance:
where CС, CH, CN, and CS are the contents of carbon, hydrogen, nitrogen, and sulfur, respectively, in the sample, and A is the ash content in raw material, % per dry mass. These data were used for determining the initial and torrefied biomass heating values.
After the first experiments on biomass oxidative torrefaction, it was found that the cap of the gas distribution grate located in the reactor’s lower part was prone to becoming clogged with dense deposits consisting of condensed sulfur containing substances released from the gaseous granulated sunflower husk combustion products. The problem was solved by modifying the cap design.
The biomass under study was a polydisperse mixture of particles with a complex geometrical shape. For more detailed studying of their fluidization process, a setup was constructed, the key component of which was a reactor 108 mm in diameter (with an inner diameter of 100 mm) and 2000 mm in height. The reactor walls were made of acryl glass. The bed in the reactor was fluidized by air at room temperature.
The setup enabled us to determine the range of air velocities at which the bed of biomass particles remained in a stable fluidized state. In studying the process, the replacement of flue gases with t = 200°С by air with t = 20°С is quite justified because, according to [17], the ratio between the fluidization numbers at 20 and 200°С can be estimated by the square root of the densities of gases at the corresponding temperatures. This ratio for the densities of air and flue gases at the above-mentioned temperature is equal to 1.23. Thus, it can be concluded that fluidization by air and flue gases takes place in the case considered with approximately the same fluidization numbers. The observation results are given in Tables 2–4.
An analysis of these data has shown that finely dispersed biomass particles having a small mass and complex shape are stably fluidized in rather narrow ranges of the ratio of bed initial height to reactor diameter and gas velocities. For this reason, in the torrefaction experiments, the fixed bed of biomass particles in the reactor had a height of 100–170 mm, and the gas velocity related to the empty torrefaction reactor section was from 0.67 to 0.85 m/s at room temperature.
According to the data presented in Tables 2–4, with the gas velocity maintained in the range 0.67–0.85 m/s, the fluidized bed for all three biomass kinds (sunflower husk, ground litter, and wood sawdust) is in a stable fluidized state. Therefore, during the torrefaction experiments, the gas velocity was maintained particularly in this range. At higher gas velocities, the fluidization of particles of one biomass kind or another became unstable. Since the gas velocity in the bed may have an effect on the torrefaction rate, it was decided to maintain the same gas velocity in the bed to exclude the influence of this parameter in fluidizing the particles of all three biomass kinds.
The temperature of the heat carrier supplied to the reactor “jacket” was 250°C, and the temperature of flue gases forwarded into the reactor was 200°С. The flue gases in the fluidized bed contained approximately 8.5% of water vapor and had the following chemical composition, vol %:
Oxygen | 2–3 |
Carbon dioxide | 16.0–17.3 |
Nitrogen | 70 |
Carbon monoxide | 0.75–1.25 |
A high content of carbon monoxide was because the amount of blast air supplied to the boiler was limited to decrease the oxygen concentration in flue gases and to prevent biofuel from igniting during its torrefaction. Nonetheless, in regard to carbon monoxide emissions, the boiler operation was in compliance with the requirements of the State Standard GOST 30735-2001 [18], which specifies the maximal level of CO emissions equal to 24 000 mg/m3 (in this study, taking into account the dilution factor equal to 1.17 calculated as the dissolved substance mass to the solvent mass ratio, the maximal CO concentration did not exceed 14 625 mg/m3).
In the considered temperature range, carbon dioxide does not interact with carbon; therefore, the presence of CO2 in the flue gases should not affect the torrefaction rate.
The biomass was held in the reactor for a specified time interval, after which the thermally treated samples were unloaded into a hermetically closed reservoir for a subsequent analysis. Biomass was held in the reactor for different the periods of time, which were equal to 3, 5, 10, and 15 min.
In the course of experiments, we continuously determined the fluidized bed temperature and pressure difference across it taking into account the pressure drop across the gas distribution grate (the measurements were carried out using a Testo 521 differential micromanometer with an error of ±0.4%).
As regards the gaseous products, the Vario Plus Industrial gas analyzer design options include the possibility of determining the composition of measured medium with a temperature of up to 1700°С. However, during torrefaction, its gaseous products may contain a large quantity of moisture; therefore, prior to supplying flue gases to the gas analyzer, they were cooled in a shell-and-tube heat exchanger for approximately 2 s to a temperature below 100°С, due to which moisture was removed from them, and stable operation of the gas analyzer was ensured. From the amount of information produced by the gas analyzer, only those data were used in the subsequent torrefaction process analysis that pointed to a change in the carbon monoxide concentration.
RESULTS AND DISCUSSION
Figure 2 shows the change with time τ of the litter, husk, and sawdust fluidized bed temperature in the course of torrefaction. The thermocouple measuring the fluidized bed temperature was installed a few millimeters above the bed of fixed biomass particles. During the transition into fluidized state, the bed of relatively dry chicken litter particles is rapidly heated, and the thermocouple records a monotonic growth of temperature to t = 235°С, which is a certain intermediate value between the fluidizing gas and hot liquid heat carrier temperatures (see Fig. 2a).
For wetter husk particles, the bed transition into a fluidized state and its heating are accompanied by drying the particles, and the bed temperature remains constant for τ = 3.5 min, after which it begins to rapidly grow to t = 350°С (see Fig. 2b).
For sawdust particles, which have a still higher wetness, their fluidized state entails a rich release of water vapor. As this takes place, the bed temperature decreases and begins to grow only in τ = 1.5 min after the process starting moment and reaches 220°С (see Fig. 2c).
As is known from [19–24], exothermal reactions occur during the biomass heating, as a result of which the hemicellulose, cellulose, and lignin that are present in the biomass composition are destructed. As applied to wood, these reactions run in the temperature range 200–500°С, but the maximum amount of heat releases at 370°С [19]; it is from 425 to 1113 kJ/kg at 230°С; from 22 to 1375 kJ/kg at 250°С, and from 1160 to 1516 kJ/kg at 280°С [25].
In this study, the torrefaction was performed at a temperature of up to 250°С; therefore, a noticeable exothermal effect was not observed, and the graph showing the temperature variation in the fluidized bed of wood particles confirms this (see Fig. 2c).
In the presented experiments, pine sawdust was used, which contained 42–43% of cellulose, 29–30% of lignin, and 18–25% of hemicellulose [26].
Sunflower husk contains 23% cellulose, 18% hemicellulose, and 29% lignin [24]; i.e., sunflower husk is close to pine sawdust by the content of the above-mentioned substances. However, as can be seen from Fig. 2b, a noticeable exothermal effect is observed in torrefying sunflower husk, due to which the bed of particles is heated to a temperature that exceeds the fluidizing agent and liquid heat carrier temperatures by 150°С and 100°С, respectively. It can be conjectured that this effect is due to the ether oils that release from husk particles in heating them.
Lignin, cellulose, and hemicellulose may enter in chicken litter with feedstuff or when it is mixed with bedding (wood sawdust or ground straw). In view of this, the content of these substances in chicken litter is much smaller than it is in wood sawdust or sunflower husk. Thus, chicken litter contains only 3.4% of lignin [27]; therefore, an exothermal effect is not observed during its torrefaction, and the data shown in Fig. 2a confirm this.
An analysis of the graphs characterizing the change with time τ of CO concentration in gaseous torrefaction products (Fig. 3) and the change of pressure difference (Fig. 4) across the bed of torrefied biomass particles shows that this process proceeds in two stages. During 2 min after the torrefaction process starting, a drastic drop of carbon monoxide concentration in gaseous products is observed for the bed of wood sawdust (see Fig. 3a), sunflower husk particles (see Fig. 3b), and chicken litter particles (see Fig. 3c). This may be connected with the final oxidation of СО to СО2 at temperatures below 200°С. After that, the CO concentration begins to grow and exceeds the carbon monoxide concentration in the flue gases suppled for fluidization. The growth in the CO content is connected with the biomass destruction processes resulting in breaking of chemical bonds in macromolecules and release of volatiles in heating the material in the course of its oxidative torrefaction.
Biomass torrefaction is accompanied by the release of moisture and a part of volatiles, which results in a loss of mass of the particle being treated. This loss of mass can be estimated from the change of pressure difference in the fluidized bed of biomass particles being subjected to torrefaction. The process is completed with a constant pressure difference.
It can be supposed proceeding from this reasoning that torrefaction is completed when the pressure difference across the bed does not change any longer. For wood sawdust this process completes in approximately 1.5–2 min (see Fig. 4a); it mainly completes in 7 min for husk particles (see Fig. 4b), and it completes in 9 min for chicken litter (see Fig. 4c) after the start of the experiment.
How complete the biomass torrefaction process is can be estimated from the change of pressure difference across the fluidized bed. This is confirmed by an analysis of the data given in Table 5, which reflects the results from measurements of moisture content, ash content, and heating value of the initial samples of the three biomass kinds and biocarbon extracted from them.
The characteristics of the studied samples of solid biomass products are close to the characteristics of similar samples obtained in a dense bed, moving dense bed, and agitated dense bed. Thus, the authors of [28] describe a solid product obtained from torrefaction of granulated sunflower husk that had the following characteristics:
Moisture content, % | 6.7 |
Ash content, % | 6.1 |
Lower heating value, MJ/kg | 20.7 |
This product was obtained in a dense bed of granules moving from the top down in the course of torrefaction for 45 min, but its characteristics are close to those of the solid torrefaction product we obtained from sunflower husk.
In [29], in which torrefaction of wood sawdust in nitrogen medium was studied (the initial product had ash and moisture contents equal to 1.3 and 7.72%, respectively), the higher heating values were determined for different periods of time of holding in a fluidized bed reactor and at different process temperatures (Table 6).
In torrefying chicken litter in oxygen-free medium, the higher heating value increases from 17.03 to 19.07 MJ/kg (with the process going for 60 min at 220°С [30]).
CONCLUSIONS
(1) Oxidative torrefaction of various biomass kinds in a fluidized bed composed of particles of ground biomass itself and blown with flue gases with a low oxygen content appears to be possible. However, in view of the complex shape and low bulk density of biomass particles, they can be fluidized in a stable manner within a rather narrow range of gas velocities.
(2) During oxidative torrefaction of sunflower husk, an exothermal effect is observed, which is connected with the release of ether oils and their incomplete combustion. During the torrefaction of wood sawdust and chicken litter, such an effect either does not exist or manifests itself very weakly.
(3) Oxidative torrefaction in a fluidized bed in flue gas medium allows the process to be completed within 2–10 min and obtain a solid product whose characteristics are commensurable with those of the torrefaction product obtained in a moving dense bed.
(4) The time of oxidative torrefaction in a fluidized bed can be monitored from the change of pressure difference across the bed: if it stops decreasing, this means that the process is complete. This statement is valid, however, for periodically operating reactors.
REFERENCES
P. McNamee, P. W. R. Adams, M. C. McManus, B. Dooley, L. I. Darvell, A. Williams, and J. M. Jones, “An assessment of the torrefaction of North American pine and life cycle greenhouse gas emissions,” Energy Convers. Manage. 113, 177–188 (2016). https://doi.org/10.1016/j.enconman.2016.01.006
L. Jiang, X. Yuan, Z. Xiao, J. Liang, H. Li, L. Cao, H. Wang, X. Chen, and G. Zeng, “A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties,” Energy Convers. Manage. 126, 509–515 (2016). https://doi.org/10.1016/j.enconman.2016.08.035
S. Wang, G. Dai, H. Yang, and Z. Luo, “Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review,” Prog. Energy Combust. Sci. 62, 33–86 (2017). https://doi.org/10.1016/j.pecs.2017.05.004
H. Li, X. Liu, R. Legros, X. T. Bi, C. J. Lim, and S. Sokhansanj, “Pelletization of torrefied sawdust and properties of torrefied pellets,” Appl. Energy 93, 680–385 (2012). https://doi.org/10.1016/j.apenergy.2012.01.002
J. S. Tumuluru, S. Sokhansanj, T. W. Christopher, and D. B. Richard, Biomass Torrefaction Process Review and Moving Bed Torrefaction System Model Development (Idaho National Laboratory, 2010). https://doi.org/10.2172/1042391
W.-H. Chen, J. Peng, and X. T. Bi, “A state-of-the-art review of biomass torrefaction, densification and applications,” Renewable Sustainable Energy Rev. 44, 847–866 (2015). https://doi.org/10.1016/j.rser.2014.12.039
M. A. Sukiran, F. Abnisa, W. M. A. Wan Daud, N. Abu Bakar, and S. K. Loh, “A review of torrefaction of oil palm solid wastes for biofuel production,” Energy Convers. Manage. 149, 101–120 (2017). https://doi.org/10.1016/j.enconman.2017.07.011
D. Chen, A. Gao, K. Cen, J. Zhang, X. Cao, and Z. Ma, “Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin,” Energy Convers. Manage. 169, 228–237 (2018). https://doi.org/10.1016/j.enconman.2018.05.063
D. Chen, Y. Li, M. Deng, J. Wang, M. Chen, B. Yan, and Q. Yuan, “Effect of torrefaction pretreatment and catalytic pyrolysis on the pyrolysis poly-generation of pine wood,” Bioresour. Technol. 214, 615–622 (2016). https://doi.org/10.1016/j.biortech.2016.04.058
D. Chen, J. Mei, H. Li, Y. Li, M. Lu, T. Ma, and Z. Ma, “Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products,” Bioresour. Technol. 228, 62–68 (2017). https://doi.org/10.1016/j.biortech.2016.12.088
P. Rousset, L. Macedo, J.-M. Commandré, and A. Moreira, “Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product,” J. Anal. Appl. Pyrolysis 96, 86–91 (2012). https://doi.org/10.1016/j.jaap.2012.03.009
W. H. Chen, K. M. Lu, W. J. Lee, S. H. Liu, and T. C. Lin, “Non-oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass,” Appl. Energy 114, 104–113 (2014). https://doi.org/10.1016/j.apenergy.2013.09.045
S. W. Park, C. H. Jang, K. R. Baek, and J. K. Yang, “Torrefaction and low-temperature carbonization of woody biomass: Evaluation of fuel characteristics of the products,” Energy 45, 676–685 (2012). https://doi.org/10.1016/j.energy.2012.07.024
K. M. Lu, W. J. Lee, W. H. Chen, S. H. Liu, and T. C. Lin, “Torrefaction and low temperature carbonization of oil palm fiber and eucalyptus in nitrogen and air atmospheres,” Bioresour. Technol. 123, 98–105 (2012). https://doi.org/10.1016/j.biortech.2012.07.096
Z. Wang, H. Li, C. J. Lim, and J. R. Grace, “Oxidative torrefaction of spruce-pine-fir sawdust in a slot-rectangular spouted bed reactor,” Energy Convers. Manage. 174, 276–287 (2018). https://doi.org/10.1016/j.enconman.2018.08.035
Z. Wang, Biomass Torrefaction in Slot-Rectangular Spouted Beds, Doctoral Dissertation in Chemical and Biological Engineering (Univ. of British Columbia, Vancouver, 2017). https://doi.org/10.14288/1.0362042
S. S. Zabrodskii, High-Temperature Plants with Pseudo-Fluidized Bed (Energiya, Moscow, 1971) [in Russian].
GOST 30735-2001. Heating Hot-Water Boilers with Capacity from 0,1 to 4,0 MW. General Specifications (Mezhgos. Sov. po Stand., Metrol. Sertifikatsii, Bishkek, 2001).
A. Yu. Krylova, E. G. Gorlov, and A. V. Shumovskii, “Production of biocoal by the pyrolysis of biomass,” Solid Fuel Chem. 53, 369–376 (2019). https://doi.org/10.3103/S0361521919060107
Yu. G. Sokolovskaya and P. L. Falyushin, “Pyrolysis of furniture production waste,” Prirodopol’zovanie, No. 20, 143–146 (2011).
H. Yang, R. Yan, H. Chen, D. Lee, and C. Zheng, “Characteristics of hemicellulose, cellulose and lignin pyrolysis,” Fuel 86, 1781–1788 (2007). https://doi.org/10.1016/j.fuel.2006.12.013
C. Gomez, E. Velo, F. Barontini, and V. Cozzani, “Influence of secondary reactions on the heat of pyrolysis of biomass,” Ind. Eng. Chem. Res. 48, 10222–10233 (2009). https://doi.org/10.1021/ie9007985
I. Milosavljevic, V. Oja, and E. M. Suuberg, “Thermal effects in cellulose pyrolysis: Relationship to char formation processes,” Ind. Eng. Chem. Res. 35, 653–662 (1996). https://doi.org/10.1021/ie950438l
A. Ohliger, M. Förster, and R. Kneer, “Torrefaction of beechwood: A parametric study including heat of reaction and grindability,” Fuel 104, 607–613 (2013). https://doi.org/10.1016/j.fuel.2012.06.112
A. L. Shevchenko, G. A. Sytchev, and V. M. Zaichenko, “Possibility of the use of exothermic-reactions heat from thermal destruction of biomass to increase the energy efficiency of the torrefaction process,” J. Phys.: Conf. Ser. 1147, 012093 (2019). https://doi.org/10.1088/1742-6596/1147/1/012093
A. P. Sinitsin and O. A. Sinitsina, “Bioconversion of renewable plant biomass using the example of second generation biofuels: Pretreatment, enzymes, processes, economics, generations,” Usp. Biol. Khim. 61, 347–414 (2021).
M. V. Korznikova, A. Yu. Blokhin, and Yu. P. Kozlov, “Assessment of the degree of conversion of organic matter of animal and poultry waste into biogas (using the example of the Russian Federation),” Vestn. VGU, Ser.: Khim. Biol. Farm., No. 2, 108–111 (2008).
N. Kienz, N. Margaritis, R. Isemin, V. Zaychenko, C. Strasser, D.-S. Kourkoumpas, P. Grammelis, D. Klimov, O. Larina, S. Sytchev, and A. Mikhalev, “Applicability of torrefied sunflower husk pellets in small and medium scale furnaces,” Waste Biomass Valorization 12, 2579–2596 (2021). https://doi.org/10.1007/s12649-020-01170-7
Y.-K. Chih, W.-H. Chen, H. C. Ong, and P. L. Show, “Product characteristics of torrefied wood sawdust in normal and vacuum environments,” Energies 12, 3844 (2019). https://doi.org/10.3390/en12203844
Ya. D. Pudova, O. M. Larina, and V. M. Zaichenko, “Effect of temperature process at chicken litter torrefaction on properties of products obtained,” in Proc. 34th Int. Conf. on Interaction of Intense Energy Fluxes with Matter, Elbrus, Kabardino-Balkaria, Russia, Mar. 1–6, 2019; J. Phys.: Conf. Ser. 1556, 012020 (2019). https://doi.org/10.1088/1742-6596/1556/1/012020
Funding
This study was financially supported by the Russian Foundation for Basic Research (scientific project no. 20-38-90013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Filatov
Rights and permissions
About this article
Cite this article
Kokh-Tatarenko, V.S., Kuz’min, S.N., Nebyvaev, A.V. et al. Oxidative Torrefaction of Some Biomass Kinds in a Fluidized Bed. Therm. Eng. 69, 93–100 (2022). https://doi.org/10.1134/S0040601522020021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601522020021