Abstract
In this paper, the effects of temperature, organic solvents (benzene and toluene) and halogen salts on the electric conductivity of the four kinds of quaternary ammonium salt-type AlCl3 ionic liquids, including phenyltrimethylammonium chloride, benzyltrimethylammonium chloride, benzyltriethylammonium chloride, benzyltributylammonium chloride, and tetramethylammonium chloride, were studied. The experimental results showed that the conductivity of each ionic liquid system tested rises with increasing temperature. Among all the tested systems, the TMPAC-AlCl3 system shows the highest conductivity and lowest value of apparent activation energy calculated according to the Arrhenius equation of 19.29 kJ/mol. The addition of benzene (or toluene or ethylbenzene) to ionic liquids induces a considerable increase of the electric conductivity in mixed systems with a rising volume fraction of benzene (or toluene or ethylbenzene), and then when each kind of mixed system has about 50 vol % of benzene (or toluene or ethylbenzene), its electric conductivity generally reaches the highest value of 14.5 mS/cm. Among all of the alkaline halides tested, the solubility of LiCl in TMPAC-AlCl3 has the highest value, which has a maximum value of approximately 50 g L‒1. At temperatures above 60°C, the electrolyte containing 2 g LiCl has the highest conductivity of 7.45 mS/cm, when the concentration of LiCl in the electrolyte is close to saturation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Room-temperature ionic liquids or room-temperature molten salts are molten salt systems consisting of specific anions and cations that appear as liquids at or near room temperature [1, 2]. Compared to solid, traditional liquid materials, it exhibits unique physical and chemical properties and functions, making it a new media class [3, 4]. In recent years, due to their unique physical and chemical properties, such as high electrical conductivity [5], low saturation vapor pressure [6], good solubility [7], adjustable acid and base [8], good chemical thermal stability and other excellent properties [9], ionic liquids have received widespread attention, considered a new generation of green solvents, and widely used in electrodeposition and other fields [10, 12].
According to their different cationic structures, three types of ILs have been considered suitable baths for electroplating/electrodeposition of Al: alkyl imidazole, alkyl quaternary phosphonium and alkyl quaternary ammonium [13, 14]. Chloroaluminates are the simplest compounds consisting of. They are usually made by mixing AlCl3 (anhydrous) with lL containing halides (i.e., Cl–, F–, Br–, I–). Although AlCl3-BPC and AlCl3-[EMIm]Cl ionic liquids have been extensively studied as electrolytes for the deposition of aluminum, there are still many difficulties in their application [15, 16]. Alkylpyridine is easily reduced as a cation, shortening the electrochemical potential window of the ionic liquid. AlCl3-[EMIm]Cl ionic liquids are not as easily reduced as the pyridine ion, but the highly exothermic and costly reactions between the AlCl3 and [EMIm]Cl components greatly limit the application of this electrolyte in many fields, especially in engineering. Quaternary ammonium salt compounds have attracted a large number of researchers and engineers due to their ease of synthesis, low cost, good ionic conductivity, and high electrochemical stability [17], such as the compound trimethylphenylammonium chloride (TMPAC). The ILs formed from AlCl3 and TMPAC have electrochemical stability comparable to that of AlCl3-[EMIm]Cl, and the conductivity of AlCl3-TMPAC is comparable to that of AlCl3-BPC, but lower than that of AlCl3-[EMIm]Cl [15, 18]. It also has a low melting point, for example, for a 2 : 1 molar ratio of AlCl3-TMPAC, the melting point is as low as –75°C [19, 20].
As a representative of quaternary ammonium-based ILs, trimethyl-phenyl-ammonium chloride and anhydrous aluminum chloride (TMPAC-AlCl3) IL has been employed for electroplating of Al. However, the low conductivity of TMPAC-AlCl3 IL makes it fall short when used as the electrolyte for Al electroplating. In previous studies, it was found that benzene, toluene and ethylbenzene each exhibited good inter-solubility with ionic liquids, which reduced the viscosity of the original system, enhanced its fluidity and increased its conductivity [21, 22]. For example, the conductivity of TMPAC-AlCl3 IL can be improved by introducing benzene into TMPAC-AlCl3 IL [10]. As a consequence, the capability of TMPAC-AlCl3 for electroplating Al has been greatly promoted. Up to now, the impact of the addition of benzene is studied on the addition of benzene to the effect of TMPAC-AlCl3 ILs conductivity, and electrode-AlCl3 ILs containing 50 vol % are the electrodeposition of aluminum, and it yields relatively dense aluminum coating. Yin Xiaomei et al. reported the electrical conductivity of TMPAC-AlCl3 ILs is affected by the cost and temperature [23, 24]. The results show that when TMPAC and AlCl3 molar ratio is 1 : 2, the electrical conductivity of TMPAC-AlCl3 ionic liquid conductivity is the largest and good metallic crystalline Al coatings are observed by galvanostatic deposition of Al by using TMPAC-AlCl3 IL with 50 vol % of toluene. Wang et al. reported the relationship between the conductivity of AlCl3-BMIC ILs with temperature and composition [25]. When the mole fraction (x) of AlCl3 in the ionic liquid x(AlCl3) < 0.667, the conductivity of the ionic liquid increased with the increase of x(AlCl3) and temperature.
Although alkyl quaternary ammonium salt cations have the advantages of low cost, good ionic conductivity, a wide electrochemical window and have found application in metal electrodeposition [26, 27]. However, knowledge of the important physical and chemical properties of the system, such as electrical conductivity, is still limited, which is not conducive to research and applications. This paper takes quaternary ammonium salts ionic liquids as the object of study, by adding organic solvents, alkali metal halide salts, etc. to quaternary ammonium salts ionic liquids as conductive solvents or electrolytes, the effect of temperature, viscosity, additives benzene, etc. on the conductivity of ionic liquids was studied. The temperature-conductivity and alkali metal halide-conductivity relationship curves were plotted using the Arrhenius formula respectively, and the activation energy was calculated, laying the foundation for further related research and the application of quaternary ammonium salt-based ionic liquids.
EXPERIMENTAL
Experimental Reagents
All the as-used ILs with a purity higher than 98% were purchased from Jiangsu Jintan Huadong Chemical Research Institute, and they were used without further purification. AlCl3 was purchased from Sinopharm Shenyang Chemical Reagent Co.
To prepare the electrolyte with a characteristic chemical formulation. Mixed TMPAC and anhydrous aluminum chloride (AlCl3) were put into a dry three-mouth flask with a molar ratio of 1 : 2, and heated for 60 min, the TMPAC-AlCl3 ionic liquid with brown yellow color is obtained. The other three ILs-based electrolytes, TMBAC-AlCl3, TEBAC-AlCl3, and TBBAC-AlCl3, were prepared accordingly by using the same method.
Determination of the viscosity of ILs
In this paper, the viscosity value of the sample is measured by Shanghai Right One RVDV-1 digital viscometer (η), which has a measurement accuracy of ±5 × 105 mPa s and a temperature control accuracy of ±0.05 K. The measurement is carried out according to the principle that the rotor is subjected to the resistance of the liquid, so that the resistance generated by the filament resists the resistance, and the liquid to be measured needs to be submerged in the rotor to make the measured data accurate. In the measurement of ionic liquid viscosity, the measured time should be greater than 200 s, a total of three repetitions, calculate the average value of the three groups of viscosity, it can be said that the viscometer measured data is reliable, accurate and can be used for the measurement of system viscosity.
(1) To examine the effect of temperature change on the viscosity of different ionic liquids.
TMPAC-AlCl3 and TMBAC-AlCl3 ionic liquids were tested at 20, 30, 40, 50, and 60°C, respectively, for changes in temperature and ionic liquid viscosity. TEBAC-AlCl3 ionic liquids were tested at 40, 50, 60, 70, and 80°C, respectively, for changes in temperature and ionic liquid viscosity. This is because due to the high melting point of the system, the ionic liquid tends to solidify at lower temperatures, making it inconvenient to measure viscosity values.
(2) Examining the effect of organic solvents such as benzene (benzene, toluene and xylene) on the electrical conductivity of ionic liquids.
TMPAC-AlCl3 ionic liquid was used as an example of the change in conductivity of the ionic liquid when benzene, toluene and xylene were added at 0, 10, 20, 30, 40, 50, and 60 vol %, respectively. Other ionic liquids were tested as above.
Determination of the Conductivity of ILs
The conductivity of the ILs was measured using a conductivity meter (DDSJ-308F Shanghai Vidian Science Instrument Co., China). Under the same conditions, the time for measuring the conductivity was longer than the 60 s. Each group is sampled three times, and the average value is considered to be the ultimate conductivity.
The influence of temperature on the conductivity of the ILs system was studied. The conductivity of the ILs is measured in the temperature range from 20–110°C by using an oil bath heating system to heat the electrolytes.
To investigate the influence of benzene on the electrical conductivity of the ionic system, benzene was introduced into the ILs in the amounts of 10, 20, 30, 40, 50, and 60 vol %, respectively. The neat IL was also tested for comparison. All the mixtures are homogenized by magnetic agitation.
The initial temperature for conductivity measurements for TMPAC-AlCl3 and TMBAC-AlCl3 systems is set at 20°C since the systems are liquid at this temperature. Because the TEBAC-AlCl3 and TBBAC-AlCl3 systems were solid when the temperature was lower than the above ones, thereby the starting temperature for conductivity measurements was 30 and 60°C, respectively.
To investigate the effect of alkali halides on the conductivity of TMPAC-AlCl3, various electrolytes were individually added to 1 L of TMPAC-AlCl3 electrolytes (including 20 g NaCl, 20 g KCl, 20 g KI, 20 g LiCl, 40 g LiCl, 20 g LiF) with continuously stirring of the mixtures to enhance the dissolution of the salts. Upon completion of the salts, the conductivity of the system at different temperatures is measured.
RESULTS AND DISCUSSION
Effect of Temperature on the Viscosity of ILs
Figure 1 shows the results of viscosity measurements for three AlCl3-quaternary ammonium salt ionic liquids at different temperatures. The TMPAC-AlCl3 ionic liquid and TMBAC-AlCl3 ionic liquid were tested at temperatures ranging from 283.15 to 323.15 K because the viscosity of the system is relatively small if the temperature is too high, which is outside the precise range of viscosity testing (20–85%). This is because the system has a relatively high melting point and when the temperature is too low, it tends to solidify and prevent the rotor from turning for viscosity testing, so a higher temperature range is chosen for testing.
When the temperature increases, the viscosity of ionic liquids is decreasing, this is because the viscosity of ionic liquids is mainly generated by the internal friction between the molecules of the system, macroscopically expressed as the resistance to fluid movement, when the temperature increases, the van der Waals forces between molecules weaken, the rate of molecular movement accelerates, the electrostatic gravitational force between anions and cations weaken, the resistance to ionic movement decreases, so the viscosity also decreases. We can further investigate the effect of temperature on viscosity using the Arrhenius equation (Fig. 2)
To further investigate the effect of temperature on viscosity, the viscosity (η) was taken as the natural logarithm and plotted as lnη against 1/T. The results are shown in Fig. 2. The fit was found to satisfy a linear relationship between lnƞ and 1/T, and the relationship between viscosity and temperature can be used in the Arrhenius equation description:
where Eη is the activation energy for viscous flow, A is the frequency factor or finger front factor, R is the gas constant, and η is the viscosity of the electrolyte solution. The values of the viscosity of the systems TMPAC-AlCl3 ionic liquid, TMBAC-AlCl3 ionic liquid and TEBAC-AlCl3 ionic liquid are fitted as a function of temperature by lnη to 1/T.
We can also investigate the effect of temperature on viscosity using the Vogel–Tamman–Fulcher (VTF) equation as follows:
The Arrhenius equation and VTF equation were used to fit the viscosity data versus temperature for TMPAC-AlCl3 ionic liquid, TMBAC-AlCl3 ionic liquid, and TEBAC-AlCl3 ionic liquid, respectively, and it can be seen from Fig. 2 that both the Arrhenius equation and the Vogel–Tamman–Fulcher (VTF), the fitted correlation coefficient results for TMPAC-AlCl3 ionic liquid, TMBAC-AlCl3 ionic liquid, and TEBAC-AlCl3 ionic liquid all had r2 ≥ 0.9960, with the TMPAC-AlCl3 system having r2 ≥ 0.9980. This suggests that the two equations used to describe the temperature dependence of the viscosity of the TMPAC-AlCl3 system are better than the other two systems.
The Effect of the Benzene Series on the Viscosity of Mixed ILs
For AlCl3-quaternary ionic liquids, the addition of a suitable solvent is an effective way to reduce the viscosity. As shown in Figs. 3–5, when benzene, toluene and xylene were added at 0, 10, 20, 30, 40, 50, and 60 vol % and the temperature was room temperature (25°C), the concentrations of different benzene systems (benzene, toluene and xylene) were measured at ±5 K intervals in the quaternary ammonium salt ionic liquids.
The viscosities of TMPAC-AlCl3 and TMBAC-AlCl3 were 9.33, 6.39, and 8.2 mPa s when benzene, toluene and xylene were present at 50% by volume, respectively. TMPAC-AlCl3 system and TMBAC-AlCl3 system had the lowest viscosity, which was caused by the fact that benzene (boiling point 80.1°C) evaporates more easily in air than toluene (boiling point 110.4°C), causing the viscosity of the electrolyte to rise. The overall viscosity values are all relatively lower compared to the absence of the benzene addition. The high viscosity and low conductivity of the liquid is caused by the large volume and interaction of the anions/cations in the ionic liquid. As some aromatic hydrocarbon compounds (including benzene, toluene and xylene) can be used as co-solvents to reduce the viscosity of the system. In the experiment, with the addition of benzene, toluene and xylene, the interaction between ions is weakened and the resistance to ion migration is becoming smaller, making it easier for the ionic liquid to move, thus making the viscosity of the ionic liquid lower. In addition, in aqueous ionic liquid solutions, the hydrogen bonding interactions between the water molecules and the anions play a dominant role in the structure of the ionic liquid solution. Therefore, in addition to water having a high dielectric constant, the hydrogen bonding interaction formed between the water molecules and the anions in the ionic liquid also contributes to the lower viscosity of the aqueous ionic liquid solution.
Effect of Temperature on the Conductivity of ILs
It was found in previous studies that temperature has a significant influence on the conductivity of ILs [22]. The temperature dependence of the conductivity of TMPAC-AlCl3, TMBAC-AlCl3, TEBAC-AlCl3, and TBBAC-AlCl3 ionic liquid systems was investigated, and the conductivity-temperature relationship curves were obtained (plotted in Fig. 6). It could be described by the Arrhenius equation (see Fig. 7) in the relationship calculated and obtained by the fitted parameters (see Table 1).
The increase in temperature reduces the viscosity of ionic liquids and their mixtures, facilitating the migration of ions. Herein, the Arrhenius equation is well suited to describe the temperature-dependent behavior of the ionic electrolytes:
The above Eq. (1) can be equally transferred to the following Eq. (5)
where \(a = \ln A\), \(b = - \frac{E}{R}\), σ is the conductivity, A is the pre-exponential factor, E is the apparent activation energy, R is the molar gas constant, and T is the absolute temperature. The relevant reference data for the linear Arrhenius are detailed shown in Fig. 7 and Table 1. As is widely acknowledged, the ionic conductors produce current by the migration of ions. In the present work, as the temperature increases, the activation energy of the ions increases significantly and the activation energy decreases, making it easier to overcome the forces between the ions and facilitate movement under the action of the electric field.
Influence of Benzene Series on the Conductivity of Mixed ILs
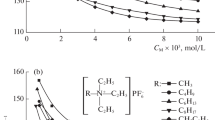
Figure 8 shows the variation of conductivity of each ILs after the addition of benzene at different temperatures, while Figs. 9 and 10 show the change in conductivity of TMPAC-AlCl3 ILs and TMBAC-AlCl3 ILs after the addition of toluene and ethylbenzene, respectively, at the same temperature.
As can be seen from Fig. 8 that the conductivity of ILs increases as the benzene content of the system increases, but in the present work it was experimentally found that the solutions tend to stratify when benzene exceeds 50%. The conductivity of TMPAC-AlCl3 ionic liquid is the highest when the benzene content is 50%. Figures 9 and 10 show the relationship curves between the conductivity of TMPAC-AlCl3 and TMBAC-AlCl3 with the volume fraction of toluene and ethylbenzene respectively at 25°C. The results show that the conductivity of the systems in TMPAC-AlCl3 and TMBAC-AlCl3 showed a linear increase with the continuous addition of toluene or ethylbenzene. When the volume fraction of toluene and ethylbenzene in TMPAC-AlCl3 and TMBAC-AlCl3 reached 50%, the conductivity of the mixture reached a great value. After the extreme value point, the conductivity of the mixture changes little or even decreases. With the addition of benzene, the viscosity of the ionic liquid decreases, the resistance to ion migration becomes smaller and the conductivity increases. The conductivity of 14.5, 11.7, and 4.65 mS/cm in TMPAC-AlCl3 containing 50 vol % of benzene, toluene and ethylbenzene at 25°C, respectively. At 25°C, the conductivity of TMPAC-AlCl3 with 50 vol % benzene exceeded that of the imidazole-like ionic liquid [EMIm]Cl-AlCl3 by 12.1 mS/cm [28]. The conductivity of TMPAC-AlCl3 and TMBAC-AlCl3 decreased when the benzene content exceeded the extreme point.
The experimental phenomena showed that the mixture at this point showed liquid stratification after sufficient shaking, with the upper layer being the low-density benzene and the lower layer being the high-density ionic liquid mixture, indicating that the benzene was saturated in the ionic liquid and the conductivity no longer increased further with increasing benzene content. According to the structure (Table 2), toluene and ethylbenzene contain more methyl groups than benzene, which affects its intercalation and is not conducive to improving the conductivity of ionic liquids. Benzene, due to its relative symmetry, can be well inserted between ions, thus reducing viscosity and increasing ionic mobility [29].
Effect of Halides on the Electrical Conductivity of TMPAC-AlCl3
In addition to temperature and organic solvents, solid salts also improve the electrical conductivity of TMPAC-AlCl3 ILs. Certain content of alkali halides (including 20 g NaCl, 20 g KCl, 20 g KI, 20 g LiCl, 40 g LiCl, and 20 g LiF) was dissolved in 1 L of TMPAC-AlCl3 ILs, respectively. The experimental phenomena of different alkali halides during the dissolution process are shown in Table 3. After 20 h of full dissolution of the electrolytes of the halides, the effect of temperature change on the conductivity of the ILs under dissolution equilibrium of the electrolytes at this time was observed and the influence rule curve is shown in Fig. 11. When the electrolyte containing halide was fully dissolved for 20 h, the variation of conductivity of the acquired ionic electrolytes with different addition of alkali halides is shown in Fig. 6 and Table 3.
As summarized in Table 3 and Fig. 11, an increase in viscosity but a decrease in conductivity of the electrolytes are recognized. In particular, it is experimentally observed that it yields a deep red solution for the ILs with additional KI with its original coloration of bright yellow. For the case of LiF, although it has not yet been fully dissolved, Liquid electrolytes at room temperature with a higher viscosity than the neat IL is obtained. Within our expectation, the electrolyte conductivity increases with the experimental temperatures. Regarding the case of LiCl, the highest conductivity is achieved with 40 g additional LiCl. According to Table 3, the electrolyte is close to being saturated. Notably, the dissolution rate of LiCl is much slower than that of KI.
Alkali halides were dissolved in 1 L of TMPAC-AlCl3. When the electrolyte containing the halide was fully dissolved for 20 h, without considering the amount of halide dissolved or precipitated in the electrolyte and the degree of dissolution, only the effect of temperature change on the conductivity of the electrolyte at dissolution equilibrium at this time was examined. Figure 12 shows the curve of the effect of temperature change on the conductivity of the electrolyte. Figure 12 shows the Arrhenius linear fit curve for the conductivity of the ionic liquid TMPAC-AlCl3 with the addition of alkali halides as a function of temperature, where the specific activation energy values are shown in Table 4. Activation energy of original is the lowest (21.86 kJ mol–1).
CONCLUSIONS
The conductivity of the four kinds of quaternary ammonium salt-type AlCl3 ionic liquids was studied to seek some suitable baths for electrochemical preparation of Al and its alloys deposition, the following conclusions can be drawn.
(1) With the increasing temperature, the electric conductivity of the ILs rises. Through the calculation and linear-fitting analysis from Arrhenius equation, the TMPAC-AlCl3 ILs has the lowest value of apparent activation energy (19.29 kJ/mol) and the highest value of electric conductivity (14.5 mS/cm) among all the tested quaternary ammonium salt ILs.
(2) Both benzene (or toluene/ethylbenzene) solvent and each kind of ILs can be mutually dissolved tested quaternary ammonium salt ILs can be mutually dissolved in all proportions, and the addition of the solvent into ILs, especially benzene, substantially increase electric conductivity. The electric conductivity of TMPAC-AlCl3 approaches the highest value of 14.5 mS/cm as the benzene volume fraction reaches 50 vol %.
(3) According to the analysis results on the dissolution behavior of different kinds of alkali halides in TMPAC-AlCl3 ILs, the maximum mass of LiCl dissolved in TMPAC-AlCl3 is higher than other tested halides.An electrolyte containing 2 g LiCl has the highest conductivity of 7.45 mS/cm when the temperature is above 60°C. It was experimentally found that NaCl and KCl can raise the melting point of the electrolyte. The addition of KI obviously decreases apparent activation energy compared with other tested halides.
REFERENCES
G. A. Elia, K. V. Kravchyk, and M. V. Kovalenko, J. Power Sources 481, 228870 (2021).
T. T. Wei, P. Peng, and S. Y. Qi, J. Energy. Chem. 57, 169 (2021).
Q. Wang, Q. Zhang, and B. Chen, J. Electrochem. Soc. 162, D320 (2015).
K. Kim, C. Lang, and R. Moulton, J. Electrochem. Soc. 151, A1168 (2004).
A. P. Abbott, G. Capper, and D. L. Davies, Chem. Commun. 19, 2010 (2001).
G. C. Tian, Mater. Res. Found. 54, 249 (2019).
M. Li, B. Gao, C. Liu, et al., Electrochim. Acta 180, 811 (2015).
E. L. Smith, A. P. Abbott, and K. S. Ryder, Chem. Rev. 114, 11060 (2014).
F. Endres and Z. Sherif, Phys. Chem. Chem. Phys. 8, 2101 (2006).
K. R. Liu, Q. Liu, and Q. Han, Trans. Nonferr. Met. Soc. China 21, 2104 (2011).
N. Zhu, K. Zhang, and F. Wu, Adv. Energy Mater. 1, 233 (2021).
K. Fujii, Y. Soejima, and Y. Kyoshoin, J. Phys. Chem. B 112, 4329 (2008).
K. Kanai, T. Nishi, and T. Iwahashi, J. Electron. Spectrosc. 174, 110 (2009).
Q. Wang, Q. Zhang, and B. Chen, J. Electrochem. Soc. 162, D320 (2015).
T. Jiang, M. C. Brym, and G. Dubé, Surf. Coat. Technol. 201, 10 (2006).
V. Kamavaram, D. Mantha, and R. Reddy, Electrochim. Acta 50, 3286 (2005).
S. Bulut, N. P. Ei De, and W. Beichel, ChemPhysChem. 12, 2296 (2011).
T. Jiang, M. C. Brym, and G. Dubé, Surf. Coat. Technol. 201, 1 (2006).
Q. Wang, Q. Zhang, and X. Lu, Ionics 23, 2449 (2017).
Q. Liu, K. R. Liu, and G. F. Tu, J. Northeast. Univ. 31, 1149 (2010).
S. Liu, H. Yu, and Y. J. Ma, Environ. Chem. Lett. 19, 1839 (2021).
Q. Liu, K Liu, and Q. Han, Chin. Surf. Eng. 23, 34 (2010).
X. Yin, L. Xu, and N. Shan, CIESC J. 64, 1022 (2013).
X. M. Yin, L. B. Xu, and J. F. Chen, Chin. J. Nonferr. Met. 23, 2316 (2013).
X. R. Wang, Y. X. Hua, and Q. Zhao, Chin. J. Nonferr. Met. 16, 2138 (2006).
Q. Liu Q, K. R. Liu, and Q. Han, J. Mater. Metall. 1, 1671 (2009).
R. Gupta, T. R. Kaptha, and B. S. Mallik, ACS Omega 4, 19556 (2019).
R. Moy and F.-P. Emmenegger, Electrochim. Acta 37, 1061 (1992).
S. Xia, X.-M. Zhang, and K. Huang, J. Electroanal. Chem. 757, 167 (2015).
ACKNOWLEDGMENTS
Supported by the Basic Research Project of Education Department of Liaoning Province of China (no. LG202020), the Natural Science Foundation of Liaoning Province of China (no. 2019-ZD-0264), High-level Talents Introduction Plan from Shenyang Ligong University (no. 1010147000902), Optical-Selection Growth Plan and Optical-Selection Team Plan from Shenyang Ligong University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Liu, Q., Zhou, X., Yang, SR. et al. Study on Electric Conductivity of Quaternary Ammonium Ionic Liquids. Russ. J. Phys. Chem. 97, 1466–1474 (2023). https://doi.org/10.1134/S0036024423070233
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423070233