Abstract
Four typical chloroaluminate ionic liquids (ILs) with different cations, namely 1-butyl-3-methylimidazoliumchloride [Bmim]Cl/AlCl3 (33.3/66.7 mol%), 1-butyl-3-methylpyridinium chloride [BMPyri]Cl/AlCl3 (33.3/66.7 mol%), 1-butyl-1-methylpyrrolidiniumchloride [Py1,4]Cl/AlCl3 (33.3/66.7 mol%), and trimethylphenylammonium chloride [TMPA]Cl/AlCl3 (33.3/66.7 mol%), were employed to deposit Al. The viscosity and ionic conductivity of these ILs were measured. The results showed that [Bmim]Cl/AlCl3 (33.3/66.7 mol%) had the lowest viscosity and the highest conductivity. Raman spectra showed that Al2Cl7 − was the main anion in the four systems. Cyclic voltammetry indicated that [Bmim]Cl/AlCl3 (33.3/66.7 mol%) had the highest reduction current for Al deposition in the four ILs. By comparing the quality of the Al coatings prepared at the same current density and temperature, it was found that compact and smooth Al deposits could be obtained from [Bmim]Cl/AlCl3 (33.3/66.7 mol%) at 303 K, while the temperature needed was higher than 333 K for the other three ILs to obtain Al deposits with the same quality. Based on the density functional theory (DFT) calculations, the differences in these properties were attributed to the different molecular structure and cation-anion interaction induced by the cations of these ILs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been paid to ionic liquids (ILs) due to their attractive physico-chemical properties such as extremely low volatility, nonflammable, and good solvents for both organics and inorganics [1]. Moreover, some ILs have wide electrochemical window, which gives access to electrodeposition of active elements at moderate temperatures [2]. Therefore, ILs are considered as suitable alternatives to many conventional plating baths [3].
Al coatings are well known for corrosion resistance. In addition, they have a very high reflectivity of light and heat, which makes them highly promising from the point view of solar energy utilization [4]; hence, electrodeposition of Al coatings from ILs has been extensively studied [5–15]. Chloroaluminate ILs are the most used ILs, and it is believed that they have a bright future in electrodeposition of Al due to their relatively high conductivity, wide electrochemical window, and tunable Lewis acidity [16].
Chloroaluminate ILs are the mixtures of organic chlorides ([R]Cl) with AlCl3, and their Lewis acidity can be adjusted by varying the molar ratio of organic chlorides to AlCl3 (Eq. 1). So far, there are mainly four types of chloroaluminate ILs used to deposit Al, and their corresponding cations are N-alkylpyridinium [17], N,N′-dialkylimidazolium [18], N-alkylpyrrolidinium [19], and trialkyl-arylammonium [20], respectively. Researchers have investigated the cyclic voltammetric behavior of the ILs and the effect of electrodeposition conditions on the morphologies of the obtained Al deposits.
However, ILs are entirely composed of anions and cations. The electrochemical active species in chloroaluminate ILs is Al2Cl7 −, which is just the anion of these systems. Furthermore, there is no solvent in chloroaluminate ILs. From the above discussion, it can be concluded that the chloroaluminate ILs are quite different from aqueous solutions, and the specificity of the ILs might produce influence on the deposits. Actually, it has been reported that the types of cations affect the morphologies of Al deposits [21]. Recently, Endres et al. found that microcrystalline Al deposits were obtained in [Emim]Cl/AlCl3 (40/60 mol%), whereas nanocrystalline Al deposits were obtained from both [Py1,4]Cl/AlCl3 (40/60 mol%) and its mixtures with no more than 20 vol% of [Emim]Cl/AlCl3 (40/60 mol%) [19].
However, researchers have not associated the physico-chemical properties that result from different cations, with the quality of the obtained Al deposits in chloroaluminate ILs. Therefore, in this study, we chose four typical chloroaluminate ILs, the structures of their cations shown in Fig. 1, to study systematically the effect of cations on the electrodeposition of Al coatings. Initially, we made the property comparison of the four ILs. After that, we varied the deposition conditions, temperature and current density, to prepare Al deposits. Finally, DFT calculations were utilized to analyze how the cations affected the properties of the ILs and consequently affected the quality of the obtained Al coatings.
Material and methods
Chemicals
The [R]Cl (99%), [Bmim]Cl, [BMpyri]Cl, [Py1,4]Cl, and [TMPA]Cl were purchased from Linzhou Keneng Material Technology Co., China. Prior to use, they were dried under vacuum at 328 K for 48 h. Anhydrous AlCl3 (AR) was purchased from Sinopharm Chemical Reagent Co. and used as received. All the chloroaluminate ILs were prepared by slowly adding anhydrous AlCl3 into dried [R]Cl until the apparent molar ratio of AlCl3 to [R]Cl was 2:1 in an argon-filled glove box (Universal, MIKROUNA Co., China) where water and oxygen content was both kept below 1 ppm. The ILs were purified by constant-potential electrolysis between two aluminum electrodes (99.99%).
Experimental
Electrochemical experiments
All the electrochemical experiments were carried out in the above mentioned argon-filled glove box. Cyclic voltammograms were tested using an electrochemical work station (CHI660D, CH Instrument, USA) in a three-electrode cell. An Al wire (Alfa, 99.99%) placed in a separate fritted glass tube containing the [R]Cl/AlCl3 (33.3/66.7 mol%) IL was used as the reference electrode. A Teflon-sheathed Pt disk electrode was used as working electrodes and a Pt foil was used as the counter electrode. Prior to each experiment, the Pt disk electrode was polished with aqueous slurry of 0.3 and 0.05 μm alumina, and then dried with N2.
The electrodeposition of Al on Cu substrates was conducted by galvano static method using a constant current meter purchased from Xiamen University, China. The anode was pure Al sheet (99.95%) and the cathode was copper foil (99.95%). Prior to use, the Al plate and the copper foil were processed as per the previous publication [14], and a square area (10 mm × 10 mm) was exposed for the copper substrate and the electrodeposition time was 1800 s. All the temperatures were kept at a certain value (±0.5) K (RET basic, IKA, Germany). After electrodeposition, all the deposits were washed with acetonitrile, ethanol, deionized water and finally dried under N2.
Viscosity and electrical conductivity measurements
An automated microviscometer (Anton PaarAMVn, Anton Paar Co., Austria) was used to measure the viscosity of the four ILs at 303 K. The electrical conductivities of them were tested by a conductivity meter (FE30, Mettler Toledo Int. Inc., Switzerland) equipped with a conductivity probe (InLab 710, Mettler Toledo Int. Inc., Switzerland) in the argon-filled glovebox. The conductivity values were recorded in the temperature ranging from 303 to 363 K. The experimental temperature was controlled by a thermostat with an accuracy of ±0.05 K. At each given temperature, the time for attaining thermal equilibrium was more than 0.5 h. The relative expanded uncertainty U r for these measurements is also 0.005(0.95 level of confidence).
Raman spectroscopy
The Raman spectroscopy were recorded by a LabRam HR 800 spectrometer (Jobin Yvon-Horiba) with 785 nm of an Ar-Kr 2018 RM laser (Spectra Physics) as the excitation source. The electrolyte was sealed in a quartz cuvette inside the glove box.
Computational methods
All calculations were performed with the Gaussian09. The structural optimizations were carried out at the B3LYP/6-31++G(d,p) level. The 6-31++G(d,p) basis set is considered as the double-ξquality for valence electrons with the diffuse functions. Before optimization, the initial configurations for the ion pairs mainly referred to the charge distribution and electrostatic potential of the isolated cation and anion. During the optimization process, the ions and ion pairs were relaxed the symmetry constraint. After optimization, all of the obtained geometries were confirmed by frequency confirmation to ensure no imaginary frequencies. A basic deficiency of this density functional theory (DFT) functional is attractive dispersion interaction and the perturbation correction is only 25%. Thus, for the interaction energy, the empirical dispersion correction was adopted [22].
Results and discussion
Measurement of the viscosity and conductivity of the four ILs
Figure 2 shows the digital photograph of the four ILs, namely [Bmim]Cl/AlCl3 (33.3/66.7 mol%), [BMPyri]Cl/AlCl3 (33.3/66.7 mol%), [Py1,4]Cl/AlCl3 (33.3/66.7 mol%), and [TMPA]Cl/AlCl3 (33.3/66.7 mol%). They are limpid. [Bmim]Cl/AlCl3 (33.3/66.7 mol%) and [TMPA]Cl/AlCl3 (33.3/66.7 mol%) are colorless, while [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) and [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) are yellowish. This might be because of different visible-light absorption of the cations of the four ILs under investigation.
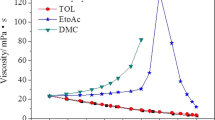
As we know, a good electrolyte should have lower viscosity and higher conductivity. At 303 K, the viscosities of these ILs are [Bmim]Cl/AlCl3 (33.3/66.7 mol%) 18.1262 mPa s, [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) 26.8728 mPa s, [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) 37.6965 mPa s, [TMPA]Cl/AlCl3 (33.3/66.7 mol%) 37.7386 mPa s, respectively. It can be seen that the viscosity of the four ILs follows the order: [Bmim]Cl/AlCl3 (33.3/66.7 mol%) < [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) < [Py1,4]Cl/AlCl3(33.3/66.7 mol%) ≈ [TMPA]Cl/AlCl3 (33.3/66.7 mol%).
Figure 3 shows the change of the conductivities of the four ILs with increasing the temperature from 303 to 363 K. At 303 K, their conductivity value is only several mS cm−1. When the temperature rises to 363 K, the conductivity is no more than 35 mS cm−1. Apparently, [Bmim]Cl/AlCl3(33.3/66.7 mol%) has the highest conductivity at each temperature, and the sequence of conductivity is [Bmim]Cl/AlCl3 (33.3/66.7 mol%) > [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) > [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) > [TMPA]Cl/AlCl3 (33.3/66.7 mol%). Moreover, the temperature does have a significant influence on the conductivity for each system. Among the four systems, the variation in the conductivity of [Bmim]Cl/AlCl3 (33.3/66.7 mol%) with temperature is the most obvious, while that of [TMPA]Cl/AlCl3 (33.3/66.7 mol%) is the least notable.
Determination of the electro-active species of the ILs
The apparent molar ratio of [R]Cl to AlCl3 for all the four systems is 1:2, so all their anions should be Al2Cl7 − based on Eq. 1. The Raman spectra shown in Fig. 4 confirms the existence of Al2Cl7 − in all the four ILs, because the Raman spectra displays only a peak at around 310 cm−1, which is assigned to the most intense peak of Al2Cl7 − [16]. Furthermore, it is reported that Al2Cl7 − is the electro-active species of chloroaluminate ILs. Therefore, chloroaluminate ILs have a rich Al(III) source to be reduced, which is another reason why chloroaluminate ILs are popular to produce Al coatings [13].
Figure 5 records the cyclic voltammetry curves of the four ILs on a Pt disk electrode at 303 K. The electrode potential was scanned from open circuit potential (OCP) to the point of forming a complete cathode peak with a scan rate of 50 mV s−1. It can be seen that the deposition of Al starts at ca. 0 V vs. Al in all cases. The [Bmim]Cl/AlCl3 (33.3/66.7 mol%) has the highest cathodic current at each potential, followed by [BMPyri]Cl/AlCl3 (33.3/66.7 mol%), [Py1,4]Cl/AlCl3 (33.3/66.7 mol%), and [TMPA]Cl/AlCl3 (33.3/66.7 mol%). The same order applies to the stripping current density, and the shape of the stripping peaks is nearly similar to each other. This should be due to the above sequence of the viscosity and conductivity of the four electrolytes.
Preparation of Al coatings at different conditions
Al coatings were deposited at a wider range of experiment conditions to determine the electrochemical property of each IL. Table 1 shows the statistical results of the quality of the Al coatings obtained from the four ILs at different experimental conditions. For each electrolyte, the effects of two main experimental parameters, current density and temperature, on the morphologies of Al coatings have been investigated. The conditions under which uniform and smooth Al coatings can be obtained are marked with —, whereas the condition where only poor quality Al coatings and even no coatings can be prepared are represented by ○. It can be seen that the system of [Bmim]Cl/AlCl3 (33.3/66.7 mol%) has the widest working range, [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) comes second, and [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) and [TMPA]Cl/AlCl3 (33.3/66.7 mol%) can only be used to electrodeposit Al coatings at higher temperature (333 and 363 K). When the temperature is lower (303 K), no Al is deposited on substrates in both [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) and [TMPA]Cl/AlCl3 (33.3/66.7 mol%). Moreover, the two elelctrolytes cannot bear too high current density. This can be attributed to the higher viscosities and lower conductivities of the two ILs.
However, the variation trend of the morphologies of Al coatings with current density and temperature is consistent in the four ILs. The conclusion is that the grain size of the Al deposits decreased with the increase of current density but increased with the increasing temperature. Two examples were presented here. Figure 6 shows the SEM images of the Al coatings obtained at 363 K from [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) with increasing current density. Clearly, the grain size of the Al deposits decreases with the increase of current density. The Al coating (Fig. 6a) deposited at 5 mA cm−1 is composed of large particles. A smooth deposit with a smaller grain size can be obtained at 10 mA cm−1 (Fig. 6b). When the current density increases to 15 mA cm−1, an Al coating with fine grains (Fig. 6c) is obtained. These results suggest that increasing the current density can refine the grain, which is consistent with our previous report [12].
SEM images of the Al deposits obtained from [Bmim]Cl/AlCl3 (33.3/66.7 mol%) at 5 mA cm−1 with different temperatures are shown in Fig. 7. As can be seen, the grain size of Al coatings gradually increases with the temperature rising from 303 to 363 K. It is reported that the grain size of the deposit is determined by the competition between the nucleation rate and the growth rate of nuclei. With the increase of temperature, the nucleation rate decreases and the growth rate increases, resulting in the formation of the coatings with coarse particles [12]. Based on the results obtained from cyclic voltammetry and the quality of the obtained Al coatings, it can be concluded that performance of the four ILs for Al deposition decreases in the following order: [Bmim]Cl/AlCl3 (33.3/66.7 mol%) > [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) > [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) ≈ [TMPA]Cl/AlCl3 (33.3/66.7 mol%).
The effect of cations on the morphologies of Al coatings
As shown, the only difference of the four electrolytes is the cations, so it can be inferred that cations have an important effect on the property of ILs, which leads to different quality of the Al coatings obtained at the same deposition condition. To illustrate the relationship between molecular structure and properties of these ILs, the optimized geometries and interaction energy (ΔE) of four ion pairs have been calculated by DFT (see Fig. 8 and Table 2). The results show that the interaction energy of the ion pairs increases in the order, [Bmim]Cl/AlCl3 (33.3/66.7 mol%) < [TMPA]Cl/AlCl3 (33.3/66.7 mol%) ≈ [BMPyri]Cl/AlCl3 (33.3/66.7 mol%) < [Py1,4]Cl/AlCl3 (33.3/66.7 mol%). Therefore, it is easy to understand that [Bmim]Cl/AlCl3 (33.3/66.7 mol%) has the lowest viscosity and highest conductivity due to the weakest interaction between [Bmim]+ and Al2Cl7 −. For the system of [Py1,4]Cl/AlCl3 (33.3/66.7 mol%), the result is just opposite because of the strongest interaction between [Py1,4]+ and Al2Cl7 − [23, 24].
It seems that the sequence of electrochemical property of the four ILs cannot be explained using the order of the interaction energy of the four ion pairs. Actually, it is not like that. As far as we know, Al2Cl7 − is both the anion and the electro-active specie in chloroaluminate ILs (33.3/66.7 mol%). When the ILs are used to deposit Al, negative potential is applied to the substrate and the cathodic charge is compensated in the first layer of cations, following by a serial layers of counterions [14]. Therefore, the quality of Al coatings depend not only on the interaction energy of the cations and anions, but also on the size of the cations. In this case, [Bmim]Cl/AlCl3 (33.3/66.7 mol%) has the best electrochemical property not only because of its weakest interaction between [Bmim]+ and Al2Cl7 − but also due to the small size of [Bmim]+, making Al2Cl7 − easiest to close substrates to be reduced. The worst performance of [TMPA]Cl/AlCl3 (33.3/66.7 mol%) should be attributed to the largest volume of [TMPA]+. However, the size of [Py1,4]+ is relatively small, offsetting the biggest interaction between [Py1,4]+ and [Al2Cl7]−, so the deposition performance of [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) is a little better than that of [TMPA]Cl/AlCl3 (33.3/66.7 mol%).
Conclusions
The quality of Al coatings electrodeposited from four typical chloroaluminate ILs with different cations, namely [Bmim]Cl/AlCl3 (33.3/66.7 mol%), [BMPyri]Cl/AlCl3 (33.3/66.7 mol%), [Py1,4]Cl/AlCl3 (33.3/66.7 mol%), and [TMPA]Cl/AlCl3 (33.3/66.7 mol%) alongside the physico-chemical properties of the four ILs was studied. Raman spectra and cyclic voltammetry proved that Al2Cl7 − was both the anion and the electro-active specie in these chloroaluminate ILs (33.3/66.7 mol%). The data of viscosity, conductivity and DFT calculation indicated that the weaker interaction between the cation and Al2Cl7 − caused lower viscosity and higher conductivity, which was the reason for obtaining Al coatings with good quality. On the other hand, the size of cations also had an impact on it because the cathodic charge of the substrate was compensated in the first layer of cations, followed by a serial layers of counter ions when electrodeposition potential was applied. Therefore, the quality performance of the four ILs for Al deposition decreased in the following order: [Bmim]Cl/AlCl3 (33.3/66.7 mol%) > [BMpyri]Cl/AlCl3 (33.3/66.7 mol%) > [Py1,4]Cl/AlCl3 (33.3/66.7 mol%) ≈ [TMPA]Cl/AlCl3 (33.3/66.7 mol%), although the interaction energy of the four corresponding ion pairs increased in the order: [Bmim]Cl/AlCl3 (33.3/66.7 mol%) < [TMPA]Cl/AlCl3 (33.3/66.7 mol%) ≈ [BMpyri]Cl/AlCl3 (33.3/66.7 mol%) < [Py1,4]Cl/AlCl3 (33.3/66.7 mol%).
References
Zhang S, Sun J, Zhang X, Xin J, Miao Q, Wang J (2014) Ionic liquid-based green processes for energy production. Chem Soc Rev 43:7838–7869
Abbott AP, Frisch G, Ryder KS (2013) Electroplating using ionic liquids. Annu Rev Mater Res 43:335–358
SZE A, Polleth M, Meiss SA, Janek J, Endres F (2007) Ionic liquids as green electrolytes for the electrodeposition of nanomaterials. Green Chem 9:549–553
Zhao Y, Vander Noot TJ (1997) Review: electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts. Electrochim Acta 42:3–13
Endo A, Miyake M, Hirato T (2014) Electrodeposition of aluminum from 1,3-dimethyl-2-imidazolidinone/AlCl3 baths. Electrochim Acta 137:470–475
Abbott AP, KJ MK (2006) Application of ionic liquids to the electrodeposition of metals. Phys Chem Chem Phys 8:4265–4279
Abbott AP, Qiu F, Abood HM, Ali MR, Ryder KS (2010) Double layer, diluent and anode effects upon the electrodeposition of aluminium from chloroaluminate based ionic liquids. Phys ChemChem Phys 12:1862–1872
Barchi L, Bardi U, Caporali S, Fantini M, Scriv Scrivani A (2010) Electroplated bright aluminium coatings for anticorrosion and decorative purposes. Prog Org Coat 67:146–151
Chang JK, Chen SY, Tsai WT, Deng MJ, Sun IW (2007) Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride (EMIC) ionic liquid and its corrosion behavior. Electrochem Commun 9:1602–1606
Endres F, Bukowski M, Hempelmann R, Natter H (2003) Electrodeposition of nanocrystalline metals and alloys from ionic liquids. Angew Chem Int Edit 42:3428–3430
Yue G, Zhang S, Zhu Y, Lu X, Li C, Li Z (2009) A promising method for electrodeposition of aluminium on stainless steel in ionic liquid. AIChE 55:783–796
Zhang Q, Wang Q, Zhang S, Lu X (2014) Effect of nicotinamide on electrodeposition of Al from aluminium chloride (AlCl3)-1-butyl-3-methylimidazolium chloride (BmimCl) ionic liquids. J Solid State Electr 18:257–267
Wang Q, Chen B, Zhang Q, Lu X, Zhang S (2015) Aluminum deposition from lewis acidic 1-butyl-3-methylimidazolium chloroaluminate ionic liquid (BmimCl/AlCl3) modified with methyl nicotinate. Chem Electro Chem 2:1794–1798
Wang Q, Zhang Q, Chen B, Lu X, Zhang S (2015) Electrodeposition of bright Al coatings from 1-butyl-3-methylimidazolium chloroaluminate ionic liquids with specific additives. J Electrochem Soc 162:D320–D324
Abbott AP, Harris RC, Hsieh YT, Ryder KS, Sun IW (2014) Aluminium electrodeposition under ambient conditions. Phys ChemChem Phys 16:14675–14681
Estager J, Holbrey JD, Swadzba Kwasny M (2014) Halometallate ionic liquids. Chem Soc Rev 43:847–886
Robinson J, Osteryoung RA (1980) The electrochemical behavior of aluminum in the low temperature molten salt system n-butyl pyridinium chloride: aluminum chloride and mixtures of this molten salt with benzene. J Electrochem Soc 127:122–128
Chang JK, Chen SY, Tsai WT, Deng MJ, Sun IW (2008) Improved corrosion resistance of magnesium alloy with a surface aluminum coating electrodeposited in ionic liquid. J Electrochem Soc 155:C112–C116
Giridhar P, Zein El AS, Endres F (2012) Electrodeposition of aluminium from 1-butyl-1-methylpyrrolidinium chloride/AlCl3 and mixtures with 1-ethyl-3-methylimidazolium chloride/AlCl3. Electrochim Acta 70:210–214
Jiang T, Chollier Brym MJ, Dubé G, Lasia A, Brisard GM (2006) Electrodeposition of aluminium from ionic liquids: part II—studies on the electrodeposition of aluminum from aluminum chloride (AlCl3)-trimethylphenylammonium chloride (TMPAC) ionic liquids. Surf Coat Tech 201:10–18
Eiden P, Liu Q, SZE A, Endres F, Krossing I (2009) An experimental and theoretical study of the aluminium species present in mixtures of AlCl3 with the ionic liquids BMPTf2N and EMImTf2N. Chem-Eur J 15:3426–3434
Schwabe T, Grimme S (2007) Double-hybrid density functionals with long-range dispersion corrections: higher accuracy and extended applicability. Phys Chem Chem Phys 9:3397–3406
Zheng Y, Dong K, Wang Q, Zhang J, Lu X (2013) Density, viscosity, and conductivity of lewis acidic 1-butyl- and 1-hydrogen-3-methylimidazolium chloroaluminate ionic liquids. J Chem Eng Data 58:32–42
Zhang X, Huo F, Liu X, Dong K, He H, Yao X, Zhang S (2015) Influence of microstructure and interaction on viscosity of ionic liquids. Ind Eng Chem Res 54:3505–3514
Acknowledgements
The authors gratefully acknowledge the financial support from the General Program Youth of National Natural Science Foundation of China (51404230, 21406002), National Basic Research Program of China (2013CB632606), and CAS Province Cooperation Program (2014JZ0012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Zhang, Q., Lu, X. et al. Electrodeposition of Al from chloroaluminate ionic liquids with different cations. Ionics 23, 2449–2455 (2017). https://doi.org/10.1007/s11581-017-2074-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2074-1