Abstract
The conductivity of the mixture of 1-butyl-3-methylimidazolium chloride (BMIC) ionic liquid with aluminum chloride (AlCl3) and titanium chloride (TiCl4) are systematically investigated over a range of temperature (70–110 °C) using the electrochemical impedance spectroscopy (EIS) method. The molar ratios of the components are changed to study the effect of molar ratio on the conductivity. The conductivity data are plotted against temperature to check whether it obeys the Arrhenius law. The activation energy and the density are calculated. The conductivity of the solution increases with increasing temperature for every composition. For varying molar ratio, conductivity increases with increasing TiCl4 content up to a certain composition then starts to decrease for each temperature. At room temperature, density of the solution increases with increasing TiCl4 content in the solution.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Ionic liquids are gaining interest day by day due to their functional physical properties, for example, low vapor pressure, good electrical conductivity, exceptional thermal stability, and wide electrochemical window [1,2,3,4]. Apart from these properties, ionic liquids are eco-friendly, which makes them a potential candidate as an electrolyte in green electrodeposition and energy storage materials. The conductivity of the electrolyte plays a vital role in the deposition thickness, deposition morphology, and current efficiency during the electrodeposition process [5]. Conductivity also represents the existence and concentration of the conducting ions. Several research works have already been done on measuring the conductivity of various ionic liquid systems [6,7,8,9,10]. Though many experiments have been done on titanium alloy electrodeposition using titanium tetrachloride (TiCl4) and ionic liquid solutions, there is no report on the conductivity of these electrolyte systems. Electrochemical impedance spectroscopy (EIS) has been applied widely for characterizing corrosion, electroplating, and energy storage materials [11, 12]. A sinusoidal signal is passed through the system, and the resulting Nyquist plot (imaginary versus real impedance) is obtained. Resistance can be calculated from the following simple mathematical expression.
A is the effective current collector area, R is the solution resistivity, which can be calculated from the Nyquist plot, l is the current carrier length, and ρ is the resistivity. Conductivity (κ) is the reciprocal of this resistivity value. EIS method has been used to measure the solid-state electrolyte [13, 14]. The conductivity of aluminum chloride (AlCl3) and 1-butyl-3-methylimidazolium (BMIC) solution has been determined as well by using conductivity meter and EIS. The data from these two sources are comparable to each other. Lu et al. obtained the conductivity of 2.3 S/m for the AlCl3 and BMIC solution at 2:1 molar ratio at 70 °C using a conductivity meter. Whereas, Shinde et al. applied the EIS method to quantify it as 2.36 S/m for the same temperature and molar ratio [10, 15]. The conductivity for other compositions of AlCl3 and BMIC solution at different temperatures are also similarly reported.
Reddy et al. have studied the AlCl3-BMIC system extensively as an electrolyte [16, 17]. This electrolyte shows great prospects in metal deposition. However, Ti-Al alloys were obtained with relatively higher Ti content than other studies, an energy-efficient way of pure Ti electrodeposition is still out of reach. The addition of TiCl4 in the AlCl3-BMIC system can be used for the electrowinning study of titanium. Characterization of the physical and chemical properties of electrolytes displays a wider view of the electrolysis system. This data can be used to not only resolve the existing problems, but also for numerical modeling. In this article, we studied the response of conductivity for variation in the temperature and molar ratio for BMIC with AlCl3 and (TiCl4) system. The TiCl4 molar ratio in the solution was varied as 0.08, 0.12, 0.16, and 0.32, whereas the molar ratio of BMIC to AlCl3 is fixed at 1:2. The temperature was varied from 70 to 110 °C at 10 °C intervals. The activation energy for each solution composition was measured by applying the Arrhenius equation. Physical properties such as density are the essential factors of the 3-D modeling of the electrolysis process as well as calculating the ion concentration. We also reported the relationship between the density and the molar ratio of the solution in this paper.
Experimental Procedure
Materials
The AlCl3 (95%, HPLC), TiCl4 (99%) and BMIC (98%, HPLC) salt were collected from Alfa Aesar, Beantown Chemical and Sigma-Aldrich respectively. The AlCl3 and BMIC (which were in powder form) were taken into a 50 ml beaker and sealed with parafilm tape. After this, the solution was heated at a fixed temperature for 40 min. After 40 min, the required amount of liquid TiCl4 was added quickly to the AlCl3 and BMIC mixture under argon gas. Again, this solution was stirred at a set temperature by a magnetic stirrer for 20 min for homogenous mixing. In this way, solutions with different molar ratios were prepared. The molar ratios of the AlCl3: BMIC: TiCl4 was 2:1:0.08, 2:1:0.12, 2:1:0.16 and 2:1:0.32.
Electrical Conductivity Measurement
The electrical resistance of the solution was carried out using a VersaSTAT 3 (M-100) potentiostat. Two nickel electrodes of identical dimensions were used in this experiment. Here, the effective current collector surface area, which we considered in our calculation, is the area of the working electrode in contact with the electrolyte. Both the electrodes were polished with 600 grit silicon carbide abrasive paper, washed with water, and then air-dried just before every experiment. These electrodes were placed in a quartz cuvette. Electrodes were attached to the opposite wall of the cuvette. This cell was filled with the ionic liquid solution, and argon gas was purged to get rid of air from the top surface of the solution. Subsequently, the cell was sealed by Teflon tape. The whole system was put in an oil bath for uniform heating. The thermometer was inserted in the oil bath to record the temperature. The cell was connected to the potentiostat. The schematic representation of the experimental setup is shown in Fig. 1.
After reaching the desired temperature, the temperature was fine-tuned. The temperature of the ionic liquid was stabilized for at least 10–15 min. The applied DC potential and the area of the electrode were fixed at 0.2 V and 144 mm2, respectively. The amplitude of the sinusoidal wave was 10 mA, where the start frequency was 100,000 Hz, and the end frequency was 1 Hz. The density of the solution was measured by a labeled bottle. The weight of the dry bottle was recorded, and then 1.5 ml of the solution was poured into the bottle. The weight of the solution and subsequently, the density of the solution was estimated at room temperature.
Results and Discussion
All the conductivity data mentioned in this paper were taken during the heating of the solution. The temperature of the solution was increased to 70 °C from room temperature and after measuring the impedance value, the temperature was raised to the next data point. Electrical conductivity data as a function of temperature (in Kelvin scale) for the different molar ratios of the solute is given in Table 1.
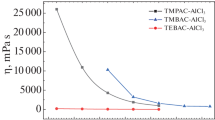
The variation of electrical conductivity versus temperature for the different molar ratios of AlCl3: BMIC: TiCl4 solutions are presented in Fig. 2. For comparison, the conductivity data of AlCl3: BMIC (the molar ratio is 2:1) is also plotted from literature data [10].
With increasing temperature, the charge transfer rate increases and the viscosity decreases. Viscosity has a direct effect on transport properties [18]. The mobility of the ions increases with temperature in the liquid solution. Apart from this, high temperature may be a probable cause of dissociation of molecules. Several reports also find this behavior [18,19,20].
The plot of electrical conductivity versus molar ratio of the TiCl4 at different temperatures is shown in Fig. 3. Conductivity at zero molar ratio of TiCl4 is taken from the conductivity data of AlCl3: BMIC (2:1), which is equivalent to the AlCl3: BMIC: TiCl4 system which has the molar ratio of 2:1:0, respectively.
From Fig. 3, the conductivity value increases up to the composition of 2:1:0.12 (AlCl3: BMIC: TiCl4) and decreases as the molar ratio increases for all temperatures. This is likely due to the decrease in mobility of the ions causing significant decrement in the viscosity [21]. Because of this, conductivity goes to the maximum where the mobility of the charge carriers is maximum. After this threshold level, mobility decreases and thus conductivity decreases. Tong et al. found that the self-diffusion coefficient in lithium-ion-based ionic liquid solution increases up to a certain concentration of lithium-ion and then drops [22]. The Arrhenius plot of the conductivity is shown in Fig. 4.
The activation energy of each system was calculated from this plot, which is given in Table 2.
The activation energy is the lowest energy required to occur a reaction. For electronic conductivity, it indicates the needed energy to jump an ion to a hole [23]. From the table, it can be found that the activation energy of AlCl3: BMIC: TiCl4 (2:1:0) solution is much higher than the other systems. As a result, the electrical conductivity of AlCl3: BMIC: TiCl4 (2:1:0) solution is relatively lower. In the other compositions, activations energies are very close and overlap with each other within the standard deviation range. The plot of density versus different TiCl4 molar ratios of the solution is shown in Fig. 5.
The density of 2:1 molar ratio of AlCl3: BMIC solution is 1.347 g/cc [24]. A density of AlCl3:BMIC system increases with addition of TiCl4, and is higher (1.387 g/cc) for TiCl4 mole ratio of 0.32. So, with increasing the TiCl4 molar ratio in the AlCl3: BMIC: TiCl4 solution, the density also increases, which agrees with our findings.
Conclusions
The conductivity response with varying temperature and molar ratio of AlCl3: BMIC: TiCl4 solution is reported. The effect of molar ratio on density is also presented. Conductivity for each molar composition of the solution increased with temperature from 70 to 110 °C. However, the conductivity increased up to 2:1:0.12 molar ratio of AlCl3: BMIC: TiCl4 solution. After this composition molar ratio, the conductivity decreases with each temperature. Effect of TiCl4 on the electrical conductivity of the AlCl3: BMIC mixture is remarkably higher compared to the conductivity data of AlCl3: BMIC solution without TiCl4. There is a little difference between the activation energy for a different composition. Nevertheless, the addition of TiCl4 reduces the activation energy for electrical conduction. The density of AlCl3: BMIC: TiCl4 system increased with increasing TiCl4.
References
Galiński M, Lewandowski A, Stępniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51:5567–5580. https://doi.org/10.1016/j.electacta.2006.03.016
Huddleston JG, Visser AE, Reichert WM et al (2001) Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem 3:156–164. https://doi.org/10.1039/b103275p
Sakaebe H, Matsumoto H, Tatsumi K (2007) Application of room temperature ionic liquids to Li batteries. Electrochim Acta 53:1048–1054. https://doi.org/10.1016/j.electacta.2007.02.054
Andriyko YO, Reischl W, Nauer GE (2009) Trialkyl-substituted imidazolium-based ionic liquids for electrochemical applications: basic physicochemical properties. J Chem Eng Data 54:855–860. https://doi.org/10.1021/je800636k
Mahapatro A, Suggu SK (2018) Modeling and simulation of electrodeposition: effect of electrolyte current density and conductivity on electroplating thickness. Adv Mater Sci. https://doi.org/10.15761/ams.1000143
Vila J, Ginés P, Rilo E et al (2006) Great increase of the electrical conductivity of ionic liquids in aqueous solutions. Fluid Phase Equilib 247:32–39. https://doi.org/10.1016/j.fluid.2006.05.028
Zhang Q-G, Sun S-S, Pitula S et al (2011) Electrical conductivity of solutions of ionic liquids with methanol, ethanol, acetonitrile, and propylene carbonate. J Chem Eng Data 56:4659–4664. https://doi.org/10.1021/je200616t
Wileńska D, Anusiewicz I, Freza S et al (2014) Predicting the viscosity and electrical conductivity of ionic liquids on the basis of theoretically calculated ionic volumes. Mol Phys 113:630–639. https://doi.org/10.1080/00268976.2014.964344
Leys J, Wübbenhorst M, Menon CP et al (2008) Temperature dependence of the electrical conductivity of imidazolium ionic liquids. J Chem Phys 128:064509. https://doi.org/10.1063/1.2827462
Shinde PS, Ahmed AN, Nahian MK, Peng Y, Reddy RG (2020) Conductivity of 1-Ethyl-3-Methylimidazolium Chloride (EMIC) and Aluminum Chloride (AlCl3) Ionic Liquids at Different Temperatures and AlCl3 Mole Fractions. ECS Trans 98:129–139. https://doi.org/10.1149/09810.0129ecst
Middlemiss LA, Rennie AJ, Sayers R, West AR (2020) Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep 6:232–241. https://doi.org/10.1016/j.egyr.2020.03.029
Encinas-Sánchez V, Miguel MD, Lasanta M et al (2019) Electrochemical impedance spectroscopy (EIS): an efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol Energy Mater Sol Cells 191:157–163. https://doi.org/10.1016/j.solmat.2018.11.007
Uddin M-J, Cho S-J (2018) Reassessing the bulk ionic conductivity of solid-state electrolytes. Sustain Energy Fuels 2:1458–1462. https://doi.org/10.1039/c8se00139a
Wei Z, Ren Y, Wang M et al (2020) Improving the conductivity of solid polymer electrolyte by grain reforming. Nanoscale Res Lett 15:122. https://doi.org/10.21203/rs.3.rs-17250/v1
Lu J, Dreisinger D (2003) Electrochemistry: ionic liquid electroprocessing of reactive metals. ACS Symp Ser 495–508. https://doi.org/10.1021/bk-2003-0856.ch039
Shinde PS, Peng Y, Reddy RG (2020) Electrodeposition of titanium aluminide (TiAl) alloy from AlCl3–BMIC ionic liquid at low temperature. In: TMS 2020 149th annual meeting & exhibition supplemental proceedings. The minerals, metals & materials series, pp 1659–1667. https://doi.org/10.1007/978-3-030-36296-6_153
Pradhan D, Reddy R, Lahiri A (2009) Low-temperature production of Ti-Al alloys using ionic liquid electrolytes: effect of process variables on current density, current efficiency, and deposit morphology. Metall Mater Trans B 40:114–122. https://doi.org/10.1007/s11663-008-9214-y
Yuan W-L, Yang X, He L et al (2018) Viscosity, conductivity, and electrochemical property of dicyanamide ionic liquids. Front Chem. https://doi.org/10.3389/fchem.2018.00059
Zheng Y, Dong K, Wang Q et al (2012) Density, viscosity, and conductivity of Lewis acidic 1-butyl- and 1-hydrogen-3-methylimidazolium chloroaluminate ionic liquids. J Chem Eng Data 58:32–42. https://doi.org/10.1021/je3004904
Ferrara C, Dall’Asta V, Berbenni V et al (2017) Physicochemical characterization of AlCl3–1-ethyl-3-methylimidazolium chloride ionic liquid electrolytes for aluminum rechargeable batteries. J Phys Chem C 121:26607–26614. https://doi.org/10.1021/acs.jpcc.7b07562
Rosol ZP, German NJ, Gross SM (2009) Solubility, ionic conductivity and viscosity of lithium salts in room temperature ionic liquids. Green Chem 11:1453. https://doi.org/10.1039/b818176d
Tong J, Wu S, Solms NV et al (2020) The effect of concentration of lithium salt on the structural and transport properties of ionic liquid-based electrolytes. Front Chem. https://doi.org/10.3389/fchem.2019.00945
Vila J, Ginés P, Pico J et al (2006) Temperature dependence of the electrical conductivity in EMIM-based ionic liquids. Fluid Phase Equilib 242:141–146. https://doi.org/10.1016/j.fluid.2006.01.022
Kamavaram V (2004) Novel electrochemical refining of aluminum based materials in low temperature ionic liquid electrolytes. Ph.D. thesis, The University of Alabama
Acknowledgements
The authors acknowledge the financial support from the National Science Foundation (NSF) and ACIPCO for this research project. The authors also thank the Department of Metallurgical and Materials Engineering, The University of Alabama, for providing the experimental and analytical facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Nahian, M.K., Ahmed, A.N., Shinde, P.S., Reddy, R.G. (2021). Conductivity of AlCl3-BMIC Ionic Liquid Mixtures Containing TiCl4 at Different Temperatures and Molar Ratios. In: TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-65261-6_90
Download citation
DOI: https://doi.org/10.1007/978-3-030-65261-6_90
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65260-9
Online ISBN: 978-3-030-65261-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)