Abstract
The effect anions (hydrocarbonates, chlorides, nitrates, and sulfates) have on the kinetics of oxidation of methyl orange in a combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system is studied using the UV radiation of a KrCl exilamp (222 nm). Adding hydrocarbonates and nitrates to a solution inhibits the mineralization of dissolved organic carbon. Mineralization degree grows from 31 to 60% in the presence of chlorides (10 mM) and sulfates (1–100 mM). Oxidation is inhibited greatly in real aqueous matrices (natural water and waste water from fur dyeing). In natural surface water, this is mainly due to the effect of hydrocarbonates; in waste water, it is caused by the multicomponent pattern of an aqueous matrix containing process impurities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Advanced oxidation processes (AOPs) based on photolysis, sonication, ozonation, catalytic systems, and combinations of them are highly efficient in removing toxic and bioresistant pollutants of different chemical natures [1–5]. When using АОРs, many resistant organic substances are oxidized to nontoxic compounds and ultimately mineralized to CO2 and H2O [1, 2, 6–8]. Hydroxyl radicals are typically the main reactive oxygen species (ROSes) that participate in oxidation [4]. However, the nonselectivity of OH radicals and the high rates of their reactions with both target compounds and components of real aqueous matrices are limiting factors in using AOPs based on them [9, 10]. Researchers are therefore giving more attention to oxidation systems in which highly reactive sulfate radical anions (E0 = 2.5–3.1 V), characterized by selectivity [11, 12] and longer lives in solution (30–40 µs) [11] are the main ROSes.
Sulfate radical anions are produced in solutions upon the activation of persulfates or peroxymonosulfates by ultraviolet (UV) radiation, ultrasonic or heat treatment, reactions with transition metal ions or some organic compounds, and in strongly alkaline media [13, 14]. Photochemical processes are easy to induce and allow the highly efficient degradation of organic compounds. Additional use of transition metal catalysts increases the rate of oxidation of pollutants and their mineralization [15].
Sources of UV radiation are usually low-pressure mercury lamps (λmax = 254 nm). However, the global trend toward reducing the industrial and household use of mercury has stimulated the development of new UV sources, including exilamps and light-emitting diode lamps [16, 17]. Gas-discharge exilamps have spectra with narrow emission bands, and are therefore referred to as quasi-monochromatic.
Analysis of the literature data [9, 10, 18–20] shows that the degree and pattern of the effect the components of real aqueous matrices have on the oxidative destruction of organic compounds in combined systems using persulfates as oxidants is governed not only by the nature of the pollutants to be removed, but by how the persulfate is activated as well. In [21], we demonstrated the great effectiveness of the quasi-monochromatic UV radiation of a KrCl exilamp (λmax = 222 nm) when activating persulfate in the oxidative destruction of azo dyes. This work is a continuation of that study. Our aim was to examine the effect the anions most typical of natural and waste waters (hydrocarbonates, chlorides, nitrates, and sulfates) have on the kinetics of the photochemical destruction of azo dyes in a combined Fenton-like UV/S2O\(_{8}^{{2 - }}\)/Fe2+ oxidation system.
EXPERIMENTAL
This work was performed using solutions of methyl orange (sodium 4-(4-dimethylaminophenylazo)benzenesulfonate) with a concentration of 30 µM prepared in different aqueous matrices:
• distilled water (pH 5.7 ± 0.2; specific conductivity, 2 µS cm−1);
• natural surface water from the Selenga river (pH 8.3 ± 0.2; HCO\(_{3}^{ - }\) 76.25 ± 9.15 mg/L, Cl− 1.30 ± 0.17 mg/L, NO\(_{3}^{ - }\) 0.205 ± 0.037 mg/L, SO\(_{4}^{{2 - }}\) 11.27 ± 1.47 mg/L; Fetot 0.372 ± 0.089 mg/L, COD 31 ± 9.3 mg/L O2, dissolved organic carbon (DOC) 6.25 ± 1.25 mg/L);
• waste water (wash water from dyeing fur) (pH 2.7, COD 420 mg/L O2, DOC 210 mg/L).
The concentration of anions was varied in the range of 1–100 mM.
Our experiments on the effect of anions in model aqueous solutions prepared in distilled water were performed under optimum conditions determined earlier for the destruction of methyl orange (MO) in a combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system with [Fe2+] : [MO] : [S2O\(_{8}^{{2 - }}\)] = 6 : 1 : 6, without adjusting the pH of the medium [21].
The initial pH of the natural water medium was adjusted with 0.1% sulfuric acid.
Our studies were performed using the experimental setup described in [21]. The radiation source was a KrCl exilamp (λmax = 222 nm, 23 W; the KrCl_BD_P model produced by Eksilamps, Russia) with an absorbed radiation intensity of 0.82 mW cm−2, as measured by chemical actinometry with atrazine [22].
The following reagents were used: methyl orange (99.9%, Merck, Germany), potassium persulfate (>99%, Sigma–Aldrich, United States), iron(II) sulfate (100%, Scharlab, Spain), sodium sulfate, sodium chloride, sodium nitrate, and sodium carbonate (Khimreaktivsnab, Russia). The pH of the medium was measured using a portable Multi3410 instrument with a SenTix®940(WTW) electrode. The concentration of the dye in each solution was controlled via HPLC using an Agilent 1260 Infinity unit equipped with a diode-array UV detector, and a Zorbax SB-C18 column (4.6 × 150 mm). The injection volume was 50 µL, the temperature was 35°C, and the eluent was acetonitrile : 75 mM acetic acid (40 : 60). The flow rate was 0.5 mL/min. Prior to analysis, samples were adjusted to pH 7–8 using 0.1% NaOH and run through a FMPTFE-0.45 µm membrane filter (Vladisart, Russia).
The concentration of the dye in the waste water was determined via spectrophotometry at the characteristic wavelength of 463 nm.
The mineralization degree of organic substrates was estimated from the change in the content of dissolved organic carbon as determined on a Shimadzu TOC-L CSN unit (limit of detection, 50 µg L−1). The instrument was calibrated using reference samples of potassium biphthalate and sodium bicarbonate.
The efficiency of oxidation and mineralization degree were determined from the change in the concentration of methyl orange (MO) and DOC in a treated solution, using the formula
where C0 and Сτ are the initial concentrations of MO or DOC and their concentrations at time τ (min), respectively.
The COD value was determined with potassium dichromate. The samples were oxidized in a DRB200 COD reactor (Hach, Germany) with direct recording of the data using a DR-890 portable colorimeter (Hach, Germany).
RESULTS AND DISCUSSION
Effects of Anions
Hydrocarbonates, chlorides, nitrates, and sulfates are typical components of aqueous matrices (natural and waste waters) that affect the kinetics and efficiency of the oxidative destruction of organic compounds.
Hydrocarbonates are known to react at high rates with almost all radicals, and thus have a considerable effect on the efficiency of combined oxidation processes [23]. It has been established experimentally that hydrocarbonates in the considered range of concentrations (1–100 mM) largely inhibits the oxidation of methyl orange in the UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system (Fig. 1). In the presence of hydrocarbonates, the initial rate of oxidation (W0) of the target compound went from 6.1 to 2.3 µM min−1 (by 2.6 times). The efficiency of oxidation of MO fell from 100 to 60%, and no mineralization of DOC was observed (Table 1).
A conjugated radical chain mechanism with the participation of both sulfate radical anions and hydroxyl radicals [21] is observed when MO is oxidized in the UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system. It includes the activation of persulfate with Fe2+ and UV radiation,
the photoreduction of Fe3+ from hydroxo- and organic complexes; and the formation of HO• radicals [24, 25]:
where Fe(OOCR)2+ are organometallic complexes with carboxylic acids that form in a solution upon the oxidation of MO.
The target compound and intermediates also undergo direct photolysis reactions:
At the first step of the reaction between methyl orange and ROS, the azo bond is cleaved and nitro compounds are produced. Subsequent oxidation includes the formation of polyatomic nitrophenols, the opening of aromatic rings, the decarboxylation of carboxylic acids, and the evolution of carbon dioxide [26, 27].
Adding hydrocarbonates buffers a solution (Table 1). This is accompanied by the hydrolysis of Fe2+ ions, their deactivation, and chain termination in Fenton-like UV/S2O\(_{8}^{{2 - }}\)/Fe2+ oxidation. At the same time, there is needless consumption of ROSes, due to the high rate of their interaction with hydrocarbonates
Data on the effect chlorides have on the oxidative destruction of aqua pollutants in combined systems based on using persulfates as oxidants are contradictory. Many studies have noted the inhibitory effect of chlorides, especially at high concentrations of them [28, 29]. This is because chlorides can also react at high rates with hydroxyl radicals and sulfate radical anions produced in solutions, after which they act as radical traps (reactions (12) and (13)). Upon the activation of persulfate using Fe2+ ions, chlorides can form stable complexes with Fe3+, thereby terminating the cycle of iron reduction in the catalytic system. It has been confirmed experimentally [30, 31] that upon oxidating a mixture of organic compounds containing benzene, toluene, ethylbenzene, and xylenes with thermally activated persulfate, the effect of chlorides is governed by both the nature of the oxidized substance and the concentration of anions.
It has been established experimentally that chlorides at concentrations of up to 1 mM have no effect on the rate of oxidation of MO or the mineralization degree of DOC in the combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system (Figs. 1 and 2). A further increase in the concentration of chlorides is accompanied by a slight drop in the initial rates of oxidation of MO, but the radiation dose required for the complete conversion of MO is almost unchanged. It should be noted, however, that mineralization degree grows considerably (from 31 to 50%) at a chloride concentration of 10 mM (Fig. 2).
A similar effect has been observed for the oxidation of sulphamethoxazole, carbamazepine, and bisphenol-A upon activating persulfate and peroxomonosulfate with UV radiation [30, 31]. Laser flash photolysis was used to prove experimentally that chlorine-containing radicals \({\text{ClO}}{{{\text{H}}}^{{ \bullet -}}}\), \({\text{C}}{{{\text{l}}}^{ \bullet }}\), and \({\text{Cl}}_{2}^{{ \bullet - }}\) are produced in a solution, due presumably to reactions (12)–(19) [12]. Quantum chemical calculations showed that chlorides lower the energy of activation of the formation of sulfate radical anions from persulfates [32], which can also raise the mineralization degree of DOC:
The effect nitrates have on the oxidation of organic compounds by persulfates depends on how they are activated. For example, it was found that nitrates at concentrations of 10–500 mM have no appreciable effect on the oxidation of a mixture of organic compounds consisting of benzene, toluene, ethylbenzene, and xylenes with thermally activated persulfates [20]. At the same time, it is known that nitrates can have a dual effect on the course of photocatalytic reactions. On the one hand, nitrates can absorb ultraviolet radiation (at λ = 205 nm, ε = 9900 M−1 cm−1 [33]), which prevents light from penetrating to the bulk of a solution. On the other hand, nitrates exposed to UV radiation in an aqueous solution can serve to form hydroxyl and nitrite radicals or superoxide radical anions, which raises the total oxidation potential of the system:
However, the contribution from reactions (20) and (21) to oxidation becomes appreciable only when using vacuum UV radiation (λ < 200 nm) [34].
In our combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system, nitrates had no appreciable effect on the kinetics of MO oxidation (Fig. 1). However, the mineralization of DOC fell considerably (from 31 to 6%) upon raising the concentration of nitrates from 1 to 100 mM, due mainly to an increase in the contribution from the shielding effect that lowered the total oxidation potential of the system (Fig. 2).
We studied the effect sulfates have on the photochemical oxidation of MO in the combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system. It was found experimentally that in the presence of sulfate ions, the radiation dose required for the complete conversion of MO in the considered range of concentrations remained almost unchanged. However, the mineralization degree of DOC rose to 56–61%.
A similar intensifying effect of sulfates has been observed during the oxidative destruction of chloramphenicol with photoactivated persulfate [35]. The authors assumed that when there are sulfates in a solution, the probability of needless reactions that lead to the consumption of ROSes (including ones between sulfate radical anions and persulfate) falls
Even though sulfates do not react with sulfate radical anions [20], they can indirectly generate additional amounts of \({\text{SO}}_{4}^{{ \bullet - }}\) via the reaction [36]
Effect of Components of Real Aqueous Matrices
The destruction of micropollutants in combined oxidation systems in aqueous matrices of multicomponent natural and waste waters proceed much more slowly, and depend not only on the concentration of anions but on their mutual effect as well [37].
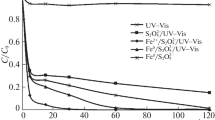
The components of two aqueous matrices (natural surface water of the Selenga River and the wash water from dyeing fur black) were found experimentally to affect the kinetics of oxidation of MO in the combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system. The initial rate of oxidation of MO in the natural water fell from 6.1 to 1.5 µM min−1, the efficiency of dye oxidation at a radiation dose of 1.48 J cm−2 was only 50%, and no mineralization of DOC was observed (Fig. 3).
We assume the reduced efficiency of MO oxidation in this matrix was mainly due to the presence of hydrocarbonates (76 mg/L), since the pattern of the kinetic curve correlates well with the above data on the oxidation of MO in model solutions (distilled water) in the presence of hydrocarbonates.
Experiments with the initial pH of the medium adjusted to 4.5 were performed to mitigate the inhibitory effect of \({\text{HCO}}_{3}^{ - }\). The initial rate of MO oxidation rose to 4.43 µM min−1, the efficiency of oxidation was 99%, and the mineralization degree of DOC reached 35%.
Wash waters from fur dyeing have a complex multicomponent composition that includes (in addition to dyes) residual concentrations of process impurities and reagents used in the production of dye baths (e.g., levelers, organic acids, and surfactants). This results in high contents of oxidation-resistant impurities (COD = 420 mg/L O2) and DOC (210 mg/L).
The efficiency of using the combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ oxidation system to purify fur dyeing wash waters was estimated from the change in the characteristic indices of water quality, the COD, and the concentration of DOC. Under the optimum conditions determined for model solutions, the efficiency of MO oxidation was 83% at an absorbed radiation dose of 5.9 J cm−2. Mineralization degree was 24%, and COD fell by 7% (Figs. 4 and 5). Doubling the concentration of the oxidizer intensified the destruction of reaction products, as is seen from the considerable drop in the COD (60%) (Fig. 6). The specific consumption of the oxidizer was in this case 0.7 µmol of S2O\(_{8}^{{2 - }}\) per gram of removed COD. We do not advise raising the concentration of persulfate further, since this results in its nontargeted consumption (Fig. 5).
CONCLUSIONS
We studied the effect the anions (hydrocarbonates, chlorides, nitrates, and sulfates) most typical of natural and waste waters have on the kinetics of oxidation of methyl orange in a combined UV/S2O\(_{8}^{{2 - }}\)/Fe2+ system using a KrCl exilamp (222 nm) as the UV source. Adding hydrocarbonates and nitrates to a solution was found to inhibit the mineralization of dissolved organic carbon. The mineralization degree rose from 31 to 60% using chlorides (10 mM) and sulfates (1–100 mM). Oxidation is inhibited considerably in real aqueous matrices (natural water and waste water from fur dyeing). In natural surface water, this effect is due to the predominance of hydrocarbonates. In waste water, it is associated with the multicomponent pattern of an aqueous matrix containing process impurities.
REFERENCES
A. Babuponnusami and K. Muthukumar, Chem. Eng. J. 183, 1 (2012).
A. Sharma, J. Ahmad, and S. J. S. Flora, Environ. Res. 167, 223 (2018).
D. S. Babu, V. Srivastava, P. V. Nidheesh, and M. S. Kumar, Sci. Total Environ. 696, 133961 (2019).
D. B. Miklos, Ch. Remy, M. Jekel, et al., Water Res. 139, 118 (2018).
M.-H. Zhang, H. Dong, L. Zhao, et al., Sci. Total Environ. 670, 110 (2019).
A. Karci, I. Arslan-Alaton, T. Olmez-Hanci, and M. Bekbölet, J. Photochem. Photobiol. A 230, 65 (2012).
J. Méndez-Díaz, M. Sánchez-Polo, J. Rivera-Utrilla, et al., Chem. Eng. J. 163, 300 (2010).
H. Mestankova, K. Schirmer, S. Canonica, and U. von Gunten, Water Res. 66, 399 (2014).
A. R. Ribeiro, N. F. F. Moreira, G. L. Puma, and A. M. T. Silva, Chem. Eng. J. 363, 155 (2019).
R. Matta, S. Tlili, S. Chiron, et al., Environ. Chem. Lett. 9, 347 (2011).
F. Ghanbari and M. Moradi, J. Hazard. Mater. 310, 41 (2017).
W. Huang, A. Bianco, M. Brigante, and G. Mailhot, J. Hazard. Mater. 347, 279 (2018).
L. Matzek and K. E. Carter, Chemosphere 151, 178 (2016).
I. A. Ike, K. G. Linden, J. D. Orbell, and M. Duke, Chem. Eng. J. 338, 651 (2018).
Q. Yang, Y. Ma, F. Chen, et al., Chem. Eng. J. 378, 1221499 (2019).
G. Matafonova and V. Batoev, Water Res. 132, 177 (2018).
G. Matafonova and V. Batoev, Chemosphere 89, 637 (2012).
Y. Fu, G. Wu, J. Geng, et al., Water Res. 150, 12e2013 (2019).
C. Luo, J. Ma, J. Jiang, et al., Water Res. 80, 99 (2015).
J. Ma, Y. Yang, X. Jiang, et al., Chemosphere 190, 296 (2018).
M. Sizykh and A. Batoeva, Russ. J. Phys. Chem. A 93, 2349 (2019). https://doi.org/10.1134/S003602441912029X
S. Canonica, L. Meunier, and U. Gunten, Water Res. 42, 121 (2008).
J. Ma and N. J. D. Graham, Water Res. 34, 3822 (2000).
E. Brillas and C. A. Martínez, Appl. Catal. B 166–167, 603 (2015).
F. C. Moreira, S. Garcia-Segura, V. J. P. Vilar, et al., Appl. Catal. B 142–143, 877 (2013).
Q. Qin, J. Xu, T. Sun, et al., Res. Chem. Intermed. 45, 3541 (2019).
J. M. Joseph, H. Destaillats, H.-M. Hung, and M. R. Hoffmann, J. Phys. Chem. A 104, 301 (2000).
W. L. Bi, Y. L. Wu, X. N. Wang, et al., Chem. Eng. J. 302, 811 (2016).
J. Sharma, I. M. Mishra, D. D. Dionysiou, and V. Kumar, Chem. Eng. J. 276, 193 (2015).
X. W. Ao and W. J. Liu, Chem. Eng. J. 313, 629 (2017).
J. Deng, Y. S. Shao, N. Y. Gao, et al., Chem. Eng. J. 222, 150 (2013).
R. Zhang, X. Wang, L. Zhou, and D. Crump, Chem. Eng. J. 361, 960 (2019).
J. Mack and J. R. Bolton, J. Photochem. Photobiol. A 128, 1 (1999).
M. Han and M. Mohseni, Water Res. 168, 115169 (2020).
A. Ghauch, A. Baalbaki, M. Amasha, E. R. Asmar, et al., Chem. Eng. J. 317, 1012 (2017).
M. G. Antoniou, A. A. de la Gruz, and D. D. Dionysiou, Appl. Catal. B 96, 290 (2010).
S. Al. Hakim, S. Jaber, N. Z. Eddine, and J. Xu, Chem. Eng. J. 380, 1224782 (2020).
Funding
This work was performed as a part of a State Task for the Baikal Institute of Nature Management, Siberian Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Utegenov
Rights and permissions
About this article
Cite this article
Sizykh, M.R., Batoeva, A.A. & Munkoeva, V.A. Effect of Inorganic Anions on the Photochemical Destruction of Azo Dyes. Russ. J. Phys. Chem. 95, 1230–1237 (2021). https://doi.org/10.1134/S0036024421060236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421060236