Abstract
The occurrence of bioactive trace pollutants such as pharmaceuticals in natural waters is an emerging issue. Numerous pharmaceuticals are not completely removed in conventional wastewater treatment plants. Advanced oxidation processes may represent an interesting alternative to completely mineralize organic trace pollutants. In this article, we show that sulfate radicals generated from peroxymonosulfate/CoII are more efficient than hydroxyl radicals generated from the Fenton’s reagent (H2O2/FeII) for the degradation of the pharmaceutical compound, carbamazepine. The second-order rate constant for the reaction of SO4 ·− with carbamazepine is 1.92·109 M−1 s−1. In laboratory grade water and in real urban wastewater, SO4 ·− yielded a faster degradation of carbamazepine compared to HO· . Under strongly oxidizing conditions, a nearly complete mineralization of carbamazepine was achieved, while under mildly oxidizing conditions, several intermediates were identified by LC–MS. These results show for the first time in real urban wastewater that sulfate radicals are more selective than hydroxyl radicals for the oxidation of an organic pollutant and may represent an interesting alternative in advanced oxidation processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of urban wastewater is an emerging topic related to the EU water framework directive (EC Directive 2000), particularly to achieve elimination of priority hazardous substances. To achieve viable and economical wastewater treatment plant, effective and affordable technologies are needed. It is well known that many pharmaceutical products are not completely removed in conventional waste-water treatment plants, which are mainly based on biological treatment (Ternes 1998).
One of the consequences is that pharmaceutical products or their metabolites can reach groundwater and drinking water. For example, the anticonvulsant carbamazepine has been detected in groundwater at concentrations up to 615 ng L−1 (Drewes et al. 2002), and clofibric acid, a lipid regulator metabolite, has been detected up to 165 ng L−1 in tap water in Berlin (Stan et al. 1994).
Advanced oxidation processes (Munter 2001) represent promising technologies for the remediation of polluted wastewater, particularly for eliminating refractory organic pollutants. Most advanced oxidation processes are based on the generation of the very reactive hydroxyl radical (HO·), which can oxidize almost all organics with a high second-order rate constant (107–1010 M s−1). The Fenton’s reagent is probably the well-known system able to generate HO· according to the following simplified reaction:
The Fenton system is currently applied in advanced oxidation technologies for the treatment of industrial discharges and for soil and groundwater remediation. However, the non-selectivity of HO· is not an advantage for oxidizing target compounds because it can be readily inactivated by the matrix. To overcome this problem, we propose here the application of the highly reactive and selective oxidant sulfate radical (SO4 ·−) for the elimination of pharmaceuticals. Sulfate radicals represent an interesting alternative to hydroxyl radicals in advanced oxidation processes. Sulfate radicals are known, since the 1950s, to be involved in the mechanism of oxidation by persulfate (PS) (Tsao and Wilmarth 1959; House 1962) and more recently by peroxymonosulfate (PMS) (Warneck et al. 1994; Kim and Edwards 1995). PS and PMS are actually gaining emerging interest for their application in advanced oxidation technologies (Yang et al. 2008). One selective and efficient way to produce sulfate radicals is:
The sulfate radical is a powerful oxidizing agent able to oxidize chloride ion into Cl2 ·− (Huie and Clifton 1990). Consequently, it has a great potential to degrade many pollutants. Similarly to hydroxyl radicals, sulfate radicals react with organics by electron transfer, hydrogen abstraction, or addition mechanisms (Zemel and Fessenden 1978; Khursan et al. 2008; Caregnato et al. 2008). However, SO4 ·− appears to be more selective than HO· since most reactions implying SO4 ·− involve electron transfer.
This study compares the oxidation kinetics of carbamazepine (CBZ) both in laboratory grade water and in real urban wastewater with sulfate or hydroxyl radicals, produced from PMS and the Fenton reagent, respectively. The major intermediates of the oxidation have been identified in the two systems.
Experimental
The chemicals peroxymonopersulfate (Oxone, 2KHSO5, KHSO4, K2SO4, 95%), CBZ (99%), hydrogen peroxide (35% w/v), and methanol were purchased from Sigma–Aldrich (Saint Quentin-Fallavier, FR); Cobalt nitrate (Co(NO3)2, 6 H2O), (CoCl2, 6H2O), and benzene were furnished by Prolabo (Fontenay-sous-Bois, FR). Tert-butyl alcohol was furnished from Carlo Erba (Val de Reuil, FR) All chemicals were used as received with no further purification. All aqueous solutions were prepared with UHQ milliQ water (Millipore, Bedford, USA).
The degradation experiments of CBZ were carried out in deionised water or urban wastewater at ambient temperature in 0.5-L stirred glass reactors. CBZ was tested at the initial concentration of 50 μM except for the competition experiment where 33 μM was selected. The molar ratio [CBZ]0 to [PMS]0 ranged from 1 to 30. Cobalt salts, CoCl2, 6H2O, and Co(NO3)2·6 H2O were tested to assess the role of the counterion on the transformation of CBZ. The concentration of CoII was determined spectrophotometrically, and the molar ratio [CBZ]0 to [Co]0 was set from 0.1 to 10. A blank consisting of CBZ (50 μM) in real wastewater was performed to check the stability of CBZ at room temperature. The results show that no degradation of CBZ was observed within 24 h.
The experiments were carried out at pH 3 in order to compare the PMS/Co system with Fenton’s system conditions which performs best at pH 3. pH adjustments were performed with few drops of H2SO4 1 M. No pH adjustment was made once the reaction had started. At different time intervals, 2-mL sample was withdrawn and quenched with 100 μL of an aqueous solution of NaNO2 (10 M) before HLPC analysis. The presented results are means of three repeated experiments.
The monitoring and quantification of the transformation of CBZ and of benzene, which was used as inhibitor in the competitive kinetic investigations, was performed with HPLC–UV (Hitachi L-2200, L-2130) equipped with a L-2400 UV–Vis detector and a RP-C18 LiChrospher column (Merck, length 250 mm, diameter 4.6 mm, particle size 5 μM). Analytes were separated with a mixture of methanol and water (75:25 v/v) at a flow rate of 0.8 mL min−1 in isocratic mode. The peak of CBZ was detected at 286 nm, and the retention time was 5.6 ± 0.2 min.
The analysis of CBZ and its transformation intermediates were carried out by liquid chromatography multistage mass spectrometry (LC/MS2). Positive electrospray ((+) ESP) was performed on an Esquire 6000 ion trap system (Bruker, Bremen, Germany), with a potential of 4 kV applied to the electrospray needle. Nitrogen was used as drying and nebulizing gas. The capillary temperature was held at 250°C. Full scanning analysis was performed in the range of 100–500 m/z. The relative collision energy was set at 30% of the maximum (1 V). The analytical separation was carried out with a MetaChem C-18 column (150 × 2 mm i.d., 3 μm) at a flow rate of 0.2 mL min−1. The mobile phase consisted of binary mixture of solvents A (methanol) and B (0.1% formic acid in water). The gradient was operated from 5 to 100% A for 30 min and then back to initial conditions in 5 min.
Results and discussion
Cobalt counter ion effect
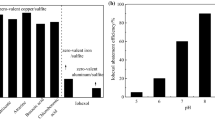
Two sources of CoII were first tested, CoCl2·6H2O and Co(NO3)2·6H2O, in order to assess the effect of the counterions, chloride ion, and nitrate ion. For this purpose, a comparative degradation of CBZ (50 μM) was carried out in the presence of PMS (150 μM) and CoCl2·6H2O (150 μM) or Co(NO3)2·6H2O (150 μM). The results are shown in Fig. 1a. The degradation of CBZ expressed as the ratio of [CBZ]/[CBZ]0 decreased as a function of time from 1 to 0.18 and 1 to 0 within 35 min with the catalyst CoCl2·6H2O and Co(NO3)2·6H2O, respectively. The curves followed a pseudo first-order decay (Fig. 1b) with a rate constant k app = (0.945 ± 0.047)·10−3 s−1 and k app = (1.520 ± 0.076)·10−3 s−1 for the degradation of CBZ in the presence of Cl− and NO3 −, respectively. This result indicated that the source of CoII had an influence on the degradation rate of CBZ. In the presence of Cl−, the transformation rate of CBZ is slower than in the presence of NO3 −. In the case of CoCl2, the slower reaction rate could be explained by the possible scavenging reaction of SO4 ·− by Cl− at a relative high second-order rate constant (k = 2.0·108 M−1 s−1, Anipsitakis et al. 2006). The catalyst salt Co(NO3)2·6H2O was then chosen as source of CoII in the following experiment.
a Time-dependent degradation curve of CBZ (50 μM) in the presence of PMS (150 μM) and (open circle) CoCl2·6H2O (150 μM) and (filled triangle) Co(NO3)2·6H2O (150 μM), pH 3. b Plot of log [CBZ]/[CBZ]0 versus time. c Effect of MeOH and t-BuOH on CBZ oxidation by PMS/CoII. (open circle) without scavenger, (filled triangle) with t-BuOH, (times) with MeOH. Experimental conditions: [CBZ]0 = 50 μM, [PMS]0 = [CoII]0 = 150 μM, [Alcohol] = 225 mM, at initial pH 3
Sulfate radical identification
Metal-catalyzed activation of PMS generates sulfate radicals as the main reactive species, with hydroxyl and peroxymonosulfate radicals as secondary species (Anipsitakis and Dionysiou 2004). Quenching experiments were performed in order to identify and confirm the primary radical species produced in our system. Methanol (MeOH) and tert-butyl alcohol (t-BuOH) were selected as quenching agents since MeOH is a well-known quenching agent for both hydroxyl (k = 9.7·108 M−1 s−1) (Buxton et al. 1988) and sulfate radicals (k = 1.1·107 M−1 s−1) (Clifton and Huie 1989), while t-BuOH reacts at a significantly higher rate with HO· (k = 6.0·108 M−1 s−1) (Buxton et al. 1988) than with SO4 ·− (k = 8.4·105 M−1 s−1) (Clifton and Huie 1989). Hydroxyl radicals react with MeOH approximately 90-times faster than sulfate radicals and around 700-times faster with t-BuOH. The reaction of alcohols and aromatics with peroxymonosulfate radicals can be neglected due to their relatively low rate constant, which is about 10−3 M−1 s−1 (Anipsitakis and Dionysiou 2004). Consequently, the presence of MeOH in the reaction mixture containing CBZ/PMS/CoII would inhibit much more HO· and SO4 ·− than t-BuOH, which will inhibit more selectively HO· than SO4 ·−. Then, if SO4 ·− are the main reactive species, it is expected that the presence of t-BuOH in the reaction mixture would not affect significantly the CBZ transformation.
The results of the experiments carried out with the reaction mixture containing CBZ (50 μM), PMS (150 μM) and CoII (150 μM) in the absence and in the presence of the inhibitors MeOH and t-BuOH are shown in Fig. 1c. The figure shows the relative transformation of normalized CBZ concentration vs. reaction time. The inhibition of CBZ decay was 74% in the presence of MeOH, while it was only 7% in the presence of t-BuOH, indicating that SO4 ·− was the major active free radical species. These results are in accordance with those obtained by Anipsitakis and Dionysiou (Anipsitakis and Dionysiou 2004) for the oxidation of 2,4-dichlorophenol at pH 7. However, the inhibition yields in the presence of MeOH obtained in this work were not as high as those observed by the above mentioned authors, who used CoCl2 as catalyst and ethanol as the inhibitor. The supplementary quenching effect of the chlorine ions as well as the higher pH value and second-order rate constant of ethanol with HO· (k = 1.9·109 M−1 s−1) (Buxton et al. 1988) and SO4 ·− (k = 3.8·107 M−1 s−1) (Clifton and Huie 1989) may explain the difference.
Sulfate radical second-order rate constant with CBZ
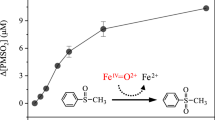
The direct rate constant measurement was determined by competitive kinetics using benzene as the reaction standard. Benzene is known to react with sulfate radicals at the rate constant (k 2) of 2.4·109 M−1 s−1 (Warneck and Ziajka 1995). When benzene, in the 0–20·10−3 M range, was added to a solution containing the substrate CBZ (33 μM) in the presence of PMS (150 μM) and CoII (150 μM), benzene was found to inhibit CBZ oxidation. For example, 20 mM of benzene caused 45% inhibition (Fig. 2a).
Degradation kinetics of CBZ (33 μM) in the presence of PMS/Co (150 μM/150 μM) and benzene at pH 3: a versus reaction time (filled circle) [Benzene] = 0; (filled square) [Benzene] = 1·10−3 M; (filled diamond) [Benzene] = 5·10−3 M; (filled triangle) [Benzene] = 10·10−3 M; (filled star) [Benzene] = 20·10−3 M. b Stern–Volmer-type plot: (r 1 0 − r 1)/r 1 vs. [benzene]
Since CBZ and benzene compete for the available sulfate radicals, the rate constant of CBZ in the above system can be obtained. In a typical reaction, the PMS/Co system generates SO4 ·−, which in turn reacts competitively with CBZ and benzene to form oxidized CBZ derivatives and phenol, respectively. Assuming that SO4 ·− reacts as follow:
The reaction rate of sulfate radical (r) is given by:
Then,
In the absence of the inhibitor (benzene), the reaction rate of CBZ (r 1 0) is given by the following equation:
Assuming that the initial SO4 ·− radical yield is the same in both solutions, the standard competition kinetics Eq. 8 predicts that plot of (r 1 0 − r 1 )/r 1 vs. [benzene] would give a straight line with the slope k 2 /(k 1 [CBZ]).
The oxidation of CBZ has been performed at pH 3. The plots of [CBZ]/[CBZ]0 vs. time for the different concentrations of benzene showed an exponential decay (Fig. 2a), which was fitted following the equation y = a exp(−bx). The coefficients a and b were determined for each curve. The slope of the tangent (y′ = −ab) to the fitting curve at the origin was determined for each curve to give r 1 0 and r 1 . Then, plotting Eq. 8 as a function of [benzene] showed a simple Stern–Volmer behavior for the scavenging of SO4 ·− by benzene with a correlation coefficient of 0.985 (Fig. 2b). The ratio of (r 1 0 − r 1 )/r 1 to [benzene] can be directly obtained from the slope of this plot (slope = 37.86·103 M−1). The second-order rate constant k 1 was then deduced and found to be (1.92 ± 0.01)·109 M−1 s−1. This value is in good agreement with those reported by (Neta et al. 1977).
Degradation of carbamazepine in the presence of PMS/Co compared to the Fenton reagent
The oxidation of CBZ (50 μM) was performed in Milli-Q water and in wastewater (Major chemical parameters are reported in Table 1) in the presence of PMS (250 μM) and CoII (250 μM) at pH 3, and compared with the degradation of CBZ in the same conditions in the presence of H2O2/FeII. The results are shown in Fig. 3. Under our experimental conditions, the curves indicated that no evolution was observed after 30 min. If we compare the capacity PMS/CoII and H2O2/FeII, it clearly appeared that these two oxidation systems had different efficiencies to convert CBZ in both milli-Q water and urban wastewater. In milli-Q water, the transformation of CBZ reached 100% in 20 min in the presence of PMS/CoII while it only reached 83% after 60 min in the presence of H2O2/FeII. The apparent first-order kinetics of CBZ degradation were determined to be 4.13·10−3 s−1 and 3.15·10−3 s−1 for PMS/Co and H2O2/Fe systems, respectively. The difference of CBZ degradation kinetics for the two oxidative systems may be explained by the probable difference of the initial radical concentration, which is assumed to be higher in the case of SO4 ·− (2.15·10−12 M) than HO· (1.64·10−12 M). The radical concentration was estimated as follow: [radical] = k 1st /k 2nd where k 1st is the apparent first-order constant, and k 2nd is the second-order rate constant of sulfate and hydroxyl with CBZ (1.92·109 M−1 s−1 (this work) and 8.8·109 M−1 s−1 (Huber et al. 2003), respectively). The figure also shows that the wastewater had an important inhibition effect on the oxidation of CBZ for the two oxidation systems. In the presence of PMS/Co, 50% of CBZ conversion was inhibited in urban wastewater, while in the presence of H2O2/Fe, only 7% of CBZ was transformed. The better lability of dissolved Cobalt than Iron in the presence of organic matter (Warnken et al. 2008) could explain the observed difference and consequently the probable higher initial [SO4 ·−] than [HO·].
Comparative degradation of CBZ in PMS/CoII and H2O2/FeII systems, in milliQ (mQ) water and in wastewater (WW). (filled circle) PMS/Co in mQ, (times) PMS/Co in WW, (filled triangle) Fenton in mQ, (filled square) Fenton in WW. Exp. conditions: [CBZ]0 = 50 μM, [PMS]0 = [H2O2]0 = 250 μM, [CoII]0 = [FeII]0 = 250 μM, initial pH 3.0
The presence of chloride ions in wastewater (Table 1) may contribute to the inhibition of the oxidation reaction as chloride ion reacts with SO4 ·− and HO· with a relatively elevated second-order rate constant (2.6·108 and 3.0·108 M−1 s−1, respectively) (Grigor’ev et al. 1987; Padmaja et al. 1993). The oxidation of CBZ (50 μM) was performed in the presence of sodium chloride (100 mg L−1), and the results (not shown) indicated that the apparent degradation kinetic decreased by a factor 1.8 compared to the blank, similarly to the curves shown in Fig. 1a, supporting that chloride ions in wastewater act as a quencher of the oxidation.
Considering our acidic experimental condition and the relatively low second-order rate constant for the reaction of SO4 ·− with bicarbonate, HCO3 − may certainly not be considered as a quenching ion. Additionally, nitrate ions may not influence the oxidation reaction either because of the low second-order rate constant with SO4 ·− (5·104 M−1 s−1) (Logager et al. 1993).
By-products identification
Various similar intermediates were identified at pH 3 by HPLC–MSn in both oxidation systems (PMS/CoII and H2O2/FeII). Intermediates were identified by comparing their MS data with those previously reported in the literature. Figure 4 reports the Total Ion Chromatograms concerning CBZ degradation upon the two oxidation systems. Table 2 reports molecular ions and fragment ions used for the identification of CBZ by-products in LC-ESI(+) MS2. Four common compounds were identified in both oxidation systems (I–IV), and an additional compound (V) was detected in the H2O2/FeII system only. The presence of the initial CBZ (I) indicated that its degradation was incomplete, due to the use of low concentration of PMS or H2O2 in the experimental setup (ratio CBZ/oxidant = 1/3). The major identified by-product structure was 10,11-dihydro-10,11-epoxycarbamazepine (II), known as a metabolite of CBZ and also identified in a sewage treatment plant (Miao and Metcalfe 2003). The compounds III, IV, and V were identified as quinone derivatives, acridine, and chloro-hydroxy acridone, which were also identified during the photodegradation of CBZ into estuarine waters (Chiron et al. 2006). When high concentration of PMS (1.5 mM) was used, HPLC and HPLC–MS analyses (not shown here) demonstrated that the by-products further mineralized into more polar compounds.
Conclusion
The oxidation system consisting of PMS/CoII to generate sulfate radicals has been demonstrated to be a more effective oxidant reagent to degrade CBZ both in laboratory grade water and wastewater, in acidic conditions, compared to the Fenton’s reagent system generating hydroxyl radical. The second-order rate constant of SO4 ·− with CBZ has been determined (1.92·109 M−1 s−1) on the basis of competitive kinetic in the presence of benzene. The wastewater matrix seemed to play an important role on the inhibition of both oxidation PMS/CoII and H2O2/FeII systems. It has been demonstrated that chloride ions were involved in the inhibition effect, and it is also suggested that organic matter could have the preponderant effect on the oxidation kinetic decrease. Concerning the different apparent degradation kinetics of CBZ induced by either the PMS/CoII or the H2O2/FeII oxidation system in laboratory grade water, it was suggested that metal complex exchange kinetics may be responsible of the better lability of Cobalt in solution than Iron, inducing a better initial production of SO4 ·−. The identification of the same intermediates in the two different oxidation systems support that the reactivity of SO4 ·− and HO· could be similar and that the efficiency of a system would only depend on initial concentration of free reactive radicals.
References
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Anipsitakis GP, Dionysiou DD, Gonzallez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constant for reaction of hydrated electrons, hydrogen atoms and hydroxyl radicals (HO·/O·−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Caregnato P, David Gara PM, Bosio GN, Gonzalez MC, Russo N, del Carmen Michelini M, Mártire DO (2008) Theoretical and experimental investigation on the oxidation of gallic acid by sulfate radical anions. J Phys Chem A 112:1188–1194
Chiron S, Minero C, Vione D (2006) Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters. Environ Sci Technol 40:5977–5983
Clifton CL, Huie RE (1989) Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4 ·−alcohols. Int J Chem Kinet 21:677–687
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000
Drewes JE, Heberer T, Reddersen K (2002) Fate of pharmaceuticals during indirect potable reuse. Water Sci Technol 46:73–80
Grigor’ev AE, Makarov IE, Pikaev AK (1987) Formation of Cl2 − in the bulk solution during the radiolysis of concentrated aqueous-solutions of chlorides. High Energy Chem 21:99–102
House DA (1962) Kinetics and mechanism of oxidations by peroxydisulfate. Chem Rev 62:185–203
Huie RE, Clifton CL (1990) Temperature-dependence of the rate constants for reactions of the sulfate radical, SO4 −, with anions. J Phys Chem 94:8561–8567
Huber MM, Canonica S, Park GY, von Gunten U (2003) Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ Sci Technol 37:1016–1024
Khursan SL, Semes’ko DG, Teregulova AN, Safiullin RL (2008) Analysis of the reactivities of organic compounds in hydrogen atom abstraction from their C-H bonds by the sulfate radical anion SO4 ·−. Kinet Catal 49:202–211
Kim J, Edwards JO (1995) A study of cobalt catalysis and copper modification in the coupled decompositions of hydrogen-peroxide and peroxomonosulfate ion. Inorg Chim Acta 235:9–13
Logager T, Sehested K, Holcman J (1993) Rate constants of the equilibrium reactions SO4 ·− + HNO3 ⇆ HSO4 − + NO3 · and SO4 ·− + NO3 − ⇆ SO4 2− + NO3 ·. J Radiat Phys Chem 41:539–543
Miao XS, Metcalfe CD (2003) Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography-electrospray tandem mass spectrometry. Anal Chem 75:3731–3788
Munter R (2001) Advanced oxidation processes–current status and prospects. Proc Estonien Acad Sci Chem 50:59–80
Neta P, Madhavan V, Zemel H, Fessenden RW (1977) Rate constants and mechanism of reaction of SO4 ·− with aromatic-compounds. J Am Chem Soc 99:163–164
Padmaja S, Neta P, Huie RE (1993) Rate constants for some reactions of inorganic radicals with inorganic-ions—temperature and solvent dependence. Int J Chem Kinet 25:447–455
Stan HJ, Heberer T, Linkerhaegner M (1994) Occurrence of clofibric acid in the aquatic system—is the use in human medical care the source of the contamination of surface, ground and drinking water? Vom Wasser 83:57–68
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Tsao MS, Wilmarth WK (1959) The aqueous chemistry of inorganic free radicals 1. The mechanism of the photolytic decomposition of aqueous persulfate ion and evidence regarding the sulfatehydroxyl radical interconversion equilibrium. J Phys Chem 63:346–353
Warneck P, Ziajka J (1995) Reaction-mechanism of the iron(III)-catalyzed autoxidation of bisulfite in aqueous-solution—steady-state description for benzene as radical scavenger. Ber Bunsenges Phys Chem 99:59–65
Warneck P, Ziajka J, Pasiuk-Bronikowska W (1994) Scavengers of SO4 ·− in S(IV) autoxidation catalyzed by Fe. In: G. Angeletti, G. Restelli (eds) Proceedings of the Sixth European Symposium Physico-Chemical Behaviour of Atmospheric Pollutants. Varese 18–22 October 1993, vol 2. Report EUR 15609/2 EN, Office of Official Publications of the European Communities, Luxemburg 1994, pp 901–906
Warnken KW, Davison W, Zhang H, Galceran J, Puy J (2008) Interpretation of in situ speciation measurements of inorganic and organically complexed trace metals in freshwater by DGT. Environ Sci Technol 41:3179–3185
Yang Q, Choi H, Chen Y, Dionysiou D (2008) Heterogeneous activation of peroxymonosulfate by supported cobalt catalysts for the degradation of 2, 4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation. Appl Cat B 77:300–307
Zemel H, Fessenden W (1978) Mechanism of reaction of so4 − with some derivatives of benzoic-acid. J Phys Chem 82:2670–2676
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matta, R., Tlili, S., Chiron, S. et al. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ Chem Lett 9, 347–353 (2011). https://doi.org/10.1007/s10311-010-0285-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-010-0285-z