Abstract
X2Sn=Sn: (X = H, Me, F, Cl, Br, Ph, Ar, …) are new species of chemistry. The cycloaddition reaction of X2Sn=Sn: are new study field of stannylene chemistry. To explore the rules of cycloaddition reaction between X2Sn=Sn: and the symmetric π-bonded compounds, the cycloaddition reactions of H2Sn=Sn: and ethylene were selected as model reactions in this paper. The mechanism of cycloaddition reaction between singlet H2Sn=Sn: and ethylene has been first investigated with the MP2/GENECP (C, H in 6-311++G**; Sn in LanL2dz) method in this paper. From the potential energy profile, it could be predicted that the reaction has one dominant reaction channel. The reaction rule presented is that the two reactants firstly form a four-membered Sn-heterocyclic ring stannylene through the [2 + 2] cycloaddition reaction. Because of the 5p unoccupied orbital of Sn: atom in the four-membered Sn-heterocyclic ring stannylene and the π orbital of ethylene forming a π → p donor-acceptor bond, the four-membered Sn-heterocyclic ring stannylene further combines with ethylene to form an intermediate. Because the Sn: atom in intermediate happens sp3 hybridization after transition state, then, intermediate isomerizes to a spiro-Sn-heterocyclic ring compound. The research result indicates the laws of cycloaddition reaction between X2Sn=Sn: and the symmetric π-bonded compounds. The study opens up a new research field for stannylene chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
The unsaturated olefins of the main group IV elements (C, Si, Ge) are all active intermediates [1–6]. Their cycloaddition reaction has been studied [7–11], With the progress of these studies, research on unsaturated stannylene has been mentioned on the agenda. At present, Bundhun et al. have conducted very good research on unsaturated stannylene [12]. But there has been no published reports about cycloaddition reactions of unsaturated stannylene till now. The unsaturated stannylene (e.g., X2Sn=Sn: (X = H, Me, F, Cl, Br, Ph, Ar, …)) is a new chemical species and new study field of stannylene chemistry. In particular, the study of mechanism of their cycloaddition reaction. To explore the rules of cycloaddition reaction between X2Sn=Sn: and the symmetric π-bonded compounds, H2Sn=Sn: and ethylene were selected as model molecules in this paper, and the cycloaddition reaction mechanism was investigated and analyzed theoretically. The research result indicates the laws of cycloaddition reaction between X2Sn=Sn: and the symmetric π-bonded compounds, which are significant for the synthesis of small-ring with Sn, and spiro-Sn-heterocyclic ring compound. The study extends the research area and enriched the research content of stannylene chemistry.
2 CALCULATION METHODS
We used the method of Second-order perturbation theory (MP2) [13] and Gaussian 09 package to optimize the structure of H2Sn=Sn: and its cycloaddition reaction with ethylene, its transition state form at the MP2/GENECP (C, H in 6-311++G**; Sn in LanL2dz) theory level. In order to further confirm the correctness of the relevant species and get the thermodynamic function for the species, vibration analysis are included. Finally, the intrinsic reaction coordinate (IRC) [14, 15] is also calculated for all the transition states to determine the reaction paths and directions.
3 RESULTS AND DISCUSSIONS
Theoretical calculation show that the ground state of H2Sn=Sn: (R1) is a singlet state, its cycloaddition reaction with ethylene (R2) has the following three possible ways:



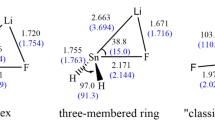
The geometrical parameters of the intermediates (INT1, INT3), transition states (TS1, TS3) and products (P1, P2, P3) which appear in the above three reactions are given in Fig. 1, the energies are listed in Table 1, and the entropy, enthalpy and Gibbs free energy are listed in Table 2, and the potential energy profile of the above three reactions is shown in Fig. 2.
The unique imaginary frequency of the transition states TS1 and TS3 through vibrational analysis are 63.1i and 53.2i cm–1, therefore, these transition states can be affirmed as the real ones. IRC (with the stepsize of 0.1 amu–1/2 Bohr) analysis confirms that TS1 connects INT1 and P1, TS3 connects INT3 and P3.
According to Fig. 2, it can be seen that reaction (1) consists of two steps: the first one is that the two reactants (R1, R2) form an intermediate (INT1). According to Fig. 2 and Table 2, the reaction is a barrier-free exothermic reaction, and the molar constant volume heat of reaction (ΔrUm) and molar heat of reaction (ΔrHm) at normal temperature and pressure are –94.7 and –88.7 kJ/mol, and the molar Gibbs free energy of reaction (ΔrGm) is –44.4 kJ/mol. The second is that the INT1 isomerizes to a three-membered Sn-heterocyclic ring product P1 via a transition state TS1 with an energy barrier of 63.4 kJ/mol. According to Fig. 2 and Table 2, the reaction is an endothermic reaction, and the ΔrUm and ΔrHm at normal temperature and pressure are 47.2 and 42.1 kJ/mol, and the ΔrGm is 46.8 kJ/mol. So, R1 + R2 → P1 is prohibited in thermodynamics at 298 K and 101.325 kPa, and reaction (1) will end in INT1.
According to Fig. 2, the two reactants (R1, R2) form a four-membered Sn-heterocyclic ring stannylene (P2) in reaction (2), which is a barrier-free exothermic reaction. According to Fig. 2 and Table 2, the ΔrUm and ΔrHm at normal temperature and pressure are –141.6 and –135.9 kJ/mol, and the ΔrGm is ‒84.6 kJ/mol. Comparing reaction (2) with reaction (1), because of the ΔrGm of R1 + R2 → INT1 and R1 + R2 → P2 are –44.4 and –84.6 kJ/mol, respectively. So reaction (2) will be the dominant reaction channel.
In reaction (3), the four-membered Sn-heterocyclic ring stannylene (P2) further reacts with ethylene (R2) to form a spiro-Sn-heterocyclic ring compound (P3). According to Fig. 2, it can be seen that the process of reaction (3) is as follows: on the basis of P2 formed in the reaction (2), it further reacts with ethylene (R2) to form an intermediate (INT3). According to Fig. 2 and Table 2, the reaction is a barrier-free exothermic reaction, and the ΔrUm and ΔrHm at normal temperature and pressure are –66.2 and ‒58.6 kJ/mol, and the ΔrGm is –10.1 kJ/mol. Then intermediate (INT3) isomerizes to a spiro-Sn-heterocyclic ring compound (P3) via a transition state (TS3) with an energy barrier of 42.8 kJ/mol. According to Fig. 2 and Table 2, the reaction is an endothermic reaction, and the ΔrUm and ΔrHm at normal temperature and pressure are 39.6 and 37.9 kJ/mol, and the ΔrGm is 36.6 kJ/mol.
Considering that the ΔrGm of INT3 → P3 is 36.6 kJ/mol and the ΔrGm of P2 + R2 → P3 is 26.5 kJ/mol and P2 + R2 → INT3 → P3 is a continuous reaction. According to the following thermodynamic formula:
If the reaction is carried out under liquid phase conditions, at a temperature of 298 K and liquid phase conditions, the reaction P2 + R2 → P3 must be carried out, and the pressure of the reaction system must be greater than 137 925 Pa (1.4 atm).
According to all analyses, reaction (3) should be the dominant reaction channel of the cycloaddition reaction between singlet H2Sn=Sn: and ethylene namely:
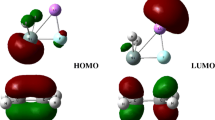
In this reaction, the frontier molecular orbitals of R2 and P2 are shown in Fig. 3. According to Fig. 3, the mechanism of reaction (3) could be explained with the frontier molecular orbital diagrams (see Fig. 4) and Fig. 1. According to Fig. 1, as H2Sn=Sn: initially interacts with ethylene, the [2+2] cycloaddition of two bonding π-orbitals firstly results in a four-membered Sn-heterocyclic ring stannylene (P2). Because P2 is still an active molecular, P2 may further react with ethylene to form a spiro-Sn-heterocyclic ring compound (P3). The mechanism of the reaction could be explained with Figs. 1 and 4, according to orbital symmetry matching condition, when P2 initially interacts with ethylene (R2), the 5p unoccupied orbital of the Sn: atom in P2 insert the π orbital of ethylene forms a π → p donor-acceptor bond, leading to the formation of a intermediate (INT3). As the reaction goes on, the ∠Sn(1)Sn(2)C(4) (INT3: 61.6°, TS3: 117.1°, P3: 145.5°) gradually increases, and the Sn(2)–C(3) and Sn(2)–C(4) bond (INT3: 2.295 Å, 2.553 Å; TS3: 2.152 Å, 2.202 Å; P3: 2.140 Å, 2.143 Å) gradually decreases, the C(3)–C(4) bond (INT3: 1.430 Å, TS3: 1.504 Å, P3: 1.532 Å) gradually lengthens. Before the transition state (TS3), the covalent bond are formed between Sn(2) and C(3), Sn(2) and C(4). After the transition state (TS3), the Sn(2) atom hybridizes to a sp3 hybrid orbital. Thus, the INT3 isomerizes to a spiro-Sn-heterocyclic ring compound (P3) via transition state (TS3).
4 CONCLUSION
According to the potential energy profile, the cycloaddition reaction between singlet H2Sn=Sn: and ethylene obtained with the MP2/GENECP (C, H in 6-311++G**; Sn in LanL2dz) method can be predicted. This reaction has one dominant channel. It consists of three steps: (1) the two reactants first form a four-membered Sn-heterocyclic ring stannylene (P2) through a barrier-free exothermic reaction of 141.6 kJ/mol; (2) the four-membered Sn-heterocyclic ring stannylene (P2) further reacts with ethylene (R2) to form an intermediate (INT3) through a barrier-free exothermic reaction of 66.2 kJ/mol; (3) intermediate (INT3) isomerizes to a spiro-Sn-heterocyclic ring compound (P3) via a transition state (TS3) with an energy barrier of 42.8 kJ/mol. At a temperature of 298 K and liquid phase conditions, the reaction is carried out, and the pressure of the reaction system needs to be greater than 137 925 Pa (1.4 atm).
The π orbital and the 5p unoccupied orbital of Sn: in X2Sn=Sn: are the object in cycloaddition reaction of X2Sn=Sn: and the symmetric π-bonded compounds. Two reactants firstly form a four-membered Sn-heterocyclic ring stannylene through the [2+2] cycloaddition reaction. Since the 5p unoccupied orbital of Sn: atom in the four-membered Sn-heterocyclic ring stannylene and the π orbital of symmetric π-bonded compounds form a π → p donor–acceptor bond, resulting in the formation of an intermediate. Because the Sn atom in the intermediate happens sp3 hybridization, the intermediate isomerizes to a spiro-Sn-heterocyclic ring compound.
REFERENCES
P. J. Stang, Chem. Rev. 78, 383 (1978).
P. J. Stang, Acc. Chem. Res. 15, 348 (1982).
H. Leclercq and I. Dubois, J. Mol. Spectrosc. 76, 39 (1979).
R. Srinivas, D. Sulzle, and H. Schwarz, J. Am. Chem. Soc. 113, 52 (1991).
W. H. Harper, E. A. Ferrall, R. K. Hilliard, S. M. Stogner, R. S. Grev, and D. J. Clouthier, J. Am. Chem. Soc. 119, 8361 (1997).
D. A. Hostutler, T. C. Smith, H. Y. Li, and D. J. Clouthier, J. Chem. Phys. 111, 50 (1999).
W. J. Bao, Y. q. Li, and X. H. Lu, Struct. Chem. 24, 1615 (2013).
X. H. Lu, Z. X. Lian, P. P. Xiang, and Y. Q. Li, Russ. J. Phys. Chem. A 85, 76 (2011).
X. H. Lu, X. Che, Y. Q. Li, and Z. N. Wang, Russ. J. Phys. Chem. A 87, 208 (2013).
X. H. Lu and H. Ji, Russ. J. Phys. Chem. A 87, 603 (2013).
X. H. Lu, J. F. Han, Y. H. Xu, L. Y. Shi, and Z. X. Lian, Russ. J. Phys. Chem. A 84, 1090 (2010).
A. Bundhun, M. R. Momeni, F. A. Shakib, P. Ramasami, P. P. Gaspar, and H. F. Schaefer III, RSC Adv. 6, 53749 (2016).
L. A. Curtis, K. Raghavachari, and J. A. Pople, Chem. Phys. 98, 1293 (1993).
K. Fukui, J. Phys. Chem. 74, 4161 (1970).
K. Ishida, K. Morokuma, and A. Komornicki, J. Chem. Phys. 66, 2153 (1977).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiaojun Tan, Xiuhui Lu Features of Mechanism of Cycloaddition Reaction between H2Sn=Sn: and Ethylene. Russ. J. Phys. Chem. 93, 2182–2186 (2019). https://doi.org/10.1134/S0036024419110311
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419110311