Abstract
The relationship between the dielectric and radio brightness characteristics of aqueous solutions of (NH4)2[PdCl4] at low concentrations at 298.15 K were considered. The dielectric relaxation parameters representing the disturbance of the H-bond network in the transition from water to solution were calculated from experimental data on the complex permittivity near the maximum of dispersion of water and solutions. These data and the Fresnel equations were used to find the quasi-optical coefficients and radio brightness characteristics of aqueous solutions in the centimeter and millimeter bands. The radio brightness characteristics of model solutions were determined with a high-sensitivity radiometer at a frequency of 61.2 GHz. The radio brightness temperature in the model solutions decreases in the transition from water to solution according to both experimental and calculated data. It was shown that the intrinsic radiation of the solution reflects the contributions of both dipole and ionic losses, which still remain significant in the millimeter band. They may even determine the difference in the signs of the changes in the radio brightness. Basics of a new approach to studying solutions containing complex ions based on their intrinsic radiation in the millimeter band were developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hydration processes in solutions of complex compounds still remain insufficiently studied [1]. Their investigation by microwave dielectric spectroscopy was begun by the example of solutions of K2[PdCl4] and K2[PtCl4] [2]. The changes in the static permittivities and the relaxation dynamics in the transition from water to the salt solutions were determined. At the same time, such changes are also affected by both the cation and the anion. Solutions of ammonium tetrachloropalladate were chosen because they provide the clearest picture of the changes in water under the action of a complex anion since the \({\text{NH}}_{{\text{4}}}^{ + }\) ion scarcely disturbs the initial network of H-bonds of water [3]. The ammonium ion is the only cation that forms substitutional solid solutions in ice [4]. A comprehensive investigation of aqueous solutions of complex compounds (in this case, (NH4)2[PdCl4]) in the centimeter (dielectric spectroscopy) and millimeter (radiometry) spectral bands is important for understanding the changes in the water structure in solutions in which complexation and hydrolysis occur. Moreover, aqueous solutions of ammonium tetrachloropalladate are of practical interest. (NH4)2[PdCl4] is a member of the class of biologically active palladium complexes (AH)n[PdCl4] where AH is a protonated nitrogen-containing anion exhibiting high antitumor, radioprotective, and immunomodulating activities [5], which are absent, e.g., in K2[PdCl4] [5–7].
CALCULATION EXPRESSIONS
The complex permittivity at frequency ν is defined as ε*(ν) = ε'(ν) – iε''(ν), where ε' is the permittivity, ε'' is the loss, and i is the imaginary unit. Its frequency dependence in nonelectrolytes is usually described using the Debye or Cole–Cole model with an additional parameter of the relaxation time distribution:
where ε∞ is the high-frequency limit in the considered dispersion region, ∆ε is the difference between the static permittivity εs and the high-frequency limit ε∞, τ is the relaxation time, and α is the relaxation time distribution parameter.
In electrolyte solutions, there is an additional component in ε''(ν), which is due to the displacement of charges (ions) under the actions of electromagnetic radiation. This component is given by the expression
where σ is the electrical conductivity of solution, S/m.
Such a model was used to describe the frequency dependences of the dielectric properties of solutions of potassium salts with the ions [PdCl4]2– and [PtCl4]2– solutions of ammonium salts with other anions in the centimeter band [8].
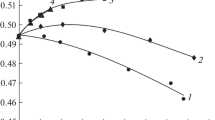
Figure 1 illustrates the approximation of the results of the dielectric experiment that were obtained at frequencies of 7–25 GHz using the chosen relaxation model. Figure 1 also indicates the frequency (61.2 GHz) at which the radiometric measurements were made.
Knowing the complex permittivity of aqueous solutions, one can find their quasi-optical parameters, in particular, the reflection coefficient R(ν) at frequency ν. It is calculated from ε*(ν) using the Fresnel equation
Thus, the extrapolation of the results of the dielectric experiment can give the reflection coefficient, which, under thermodynamic equilibrium conditions, is related to the emissivity χ(ν) measured in the radiometric experiment by a simple expression: χ(ν) = 1 – R(ν). Further, the main radiometric index—radio brightness temperature Tb(ν) at frequency ν—is found from the expression Tb(ν) = χ(ν)*T, where T is the thermodynamic temperature. The radio brightness temperature Tb(water) of water in this work is constant; therefore, sometimes, especially in figures, data are more clearly represented as ∆Tb = Tb(solution) – Tb (water).
The ionic contribution to ε''(ν) rapidly decreases with increasing frequency; therefore, it is often neglected in the millimeter band. In view of this, it was of interest to compare both variants of calculation of the radio brightness characteristics of solutions, in one of which both ionic and dipole losses (χ, Tb) were taken into account, and in the other only the dipole contribution to ε'' (χ(d), Тb(d)) was considered.
Previously [9], for solutions of alkali metal sulfates, R(ν) were calculated from their dielectric characteristics in the millimeter band, and a good agreement with the results of the radiometric experiment was shown. Using a high-sensitivity radiometer by the example of (NH4)2[PdCl4], this approach was used to determine the radio brightness temperature Tb of aqueous solutions in which complexation occurs.
EXPERIMENTAL
A comprehensive investigation of hydration processes in solutions of complex compounds (this investigation can be complicated by the occurring hydrolysis) was performed by two experimental methods: microwave dielectric spectroscopy in the frequency range 16–25 GHz at 298.15 K and radiometry at a frequency of 61.2 GHz at 298.15 K. The object of investigation was ammonium tetrachloropalladate. Synthesized ammonium tetrachloropalladate is water-soluble dark-green crystals. Test solutions were prepared gravimetrically using double distilled water. The maximum concentration of the (NH4)2[PdCl4] solution prepared for measurements was 0.86 mol/kg. This quite low value is explained by the solubility limit of the salt. Saturated and supersaturated solutions are unsuitable for these investigations.
The high-sensitivity permittivity ε' and the total loss ε'' of aqueous solutions of ammonium tetrachloropalladate (m = 0.3, 0.5, 0.75, and 0.86 mol/kg) at frequencies of ν = 16, 19, 22, and 25 GHz at a temperature of 298.15 K were studied by microwave dielectric spectroscopy. The complex permittivity of the aqueous solutions in the centimeter band was measured by the cylindrical-rod-in-waveguide method [10]. The measurement procedure and equipment were described in detail previously [11–14]. The relative errors of measuring ε' and ε'' are within the ranges ±(1.5–2) and ±(2–2.5)%, respectively.
Table 1 presents the dielectric relaxation parameters and the static permittivities that were obtained by the above method. These data were used to find the quasi-optical parameters of the solution (extrapolation to a frequency of 61.2 GHz). Table 2 presents the results.
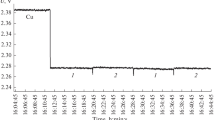
The radio brightness characteristics of the solutions were experimentally studied with a high-sensitivity Dicke radiometer with a constant operating frequency of 61.2 GHz (λ = 4.9 mm) (NPO Istok, Kotel’nikov Institute of Radio Engineering and Electronics, Russian Academy of Sciences, Fryazino, Moscow oblast, Russia) [15]. The design of the radiometer and the measurement procedure were described before [16]. However, in these works, the calibration of the signal for calculating the radio brightness parameters of solutions was not performed. The readings from the instrument were digitized and recorded as plots of the radiophysical response U (the voltage at the radiometer outlet, V) versus time. Figure 2 shows a part of such a plot for the results of simultaneous measurements of samples of pure water and the 0.5 mol/kg (NH4)2[PdCl4] solution. A constant portion no less than 3–5 min long with a voltage drift of no more than 0.1 mV/min was used for determining the statistically averaged value of U. Consecutive measurements of water and the solution of the complex compound gave the difference ΔU = U(solution) – U(water). Such consecutive measurements were repeated several times to increase the accuracy and reliability of the results, and also to minimize the effect of the possible change in the instrument status and in the state of the solution in the course of the experiment. The measurement results referred to a certain time interval. The changes due to the hydrolysis in concentrated solutions were not considered. Then, the average values of ΔU were found from the obtained sets of increments ΔU1, ΔU2, …, ΔUn. The instrument scale calibration was described before [17]. The ΔU values were converted to the emissivities χ of the solutions using the data on water, potassium chloride solution, and copper plate. Table 2 presents the χ and Td for the tests solutions, which were found from the experimental ΔU values.
RESULTS AND DISCUSSION
Figure 1 and Table 1 show that the changes in the complex permittivity of water in ammonium tetrachloropalladate solutions at low concentrations were described by the Cole–Cole relaxation model with a small relaxation time distribution parameter (α < 0.1). The static permittivity εs and the permittivity times τ decrease in the transition from water to solution. This corresponds to a typical hydrophilic hydration. As for solutions of KCl and other ammonium salts, the changes in the parameters suggest a weak hydration of the tetrachloropalladate ion.
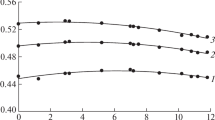
Figure 3 compares the calculated ∆Tb values for solutions of KCl [18], LiCl [12, 19], and (NH4)2[PdCl4]. In the transition from water to solution, ∆Tb for the solutions of potassium chloride and ammonium tetrachloropalladate decreases, unlike the lithium chloride solutions. This follows not only from the calculated data, but also from the measurement results. At the same time, the calculated and measured radio brightness parameters of the ammonium tetrachloropalladate solutions agree only in the very first approximation. The discrepancies are likely to be due to hydrolysis observed in ammonium tetrachloropalladate solutions [20, 21]. The calculations in the cases of the total and dipole losses showed that the ionic contributions still remain significant in the millimeter band. This is seen in Fig. 4, which presents both the total and the dipole components of the radio brightness temperature in comparison with water. Comparison of the obtained characteristics with the data for the other salt solutions showed that the considered case corresponds to weak hydration of the ion, as in solutions of potassium chloride or cesium sulfate, unlike lithium chloride solutions.
CONCLUSIONS
As the analysis performed in this work showed, this and other works showed that the changes in the radio brightness characteristics of solutions of different compositions and concentrations differ. There is a significant and differential change in the signal in comparison with water. Here, this was demonstrated for solutions containing complex ions. Correspondingly, one can consider the development of an additional method of experimental investigation of complex compounds in solutions. In many cases, a remote measurement method has undoubted advantages for, e.g., aggressive media, radioactive waters, etc. Continuous monitoring in time also becomes real. The existence of radio brightness contrasts in complex multicomponent systems and microheterogeneous media can probably be important in body fluids and be used in chemical engineering processes. These issues require further investigations.
REFERENCES
J. M. G. Barthel, H. Krienke, and W. Kunz, Physical Chemistry of Electrolyte Solutions: Modern Aspects (Springer, New York, 1998).
A. K. Lyashchenko, D. V. Loginova, A. S. Lileev, et al., Russ. J. Coord. Chem. 35, 633 (2009). https://doi.org/10.1134/S1070328409090012
A. Lileev and A. Lyashchenko, J. Mol. Liq. 150, 4 (2009). https://doi.org/10.1016/j.molliq.2009.08.008
L. Labowitz and E. Westrum, J. Phys. Chem. 65, 403 (1961). https://doi.org/10.1021/j100821a004
T. M. Buslaeva, D. S. Umreiko, G. G. Novitskii, et al., Chemistry and Spectroscopy of Platinum Metal Halides (Universitetskoe, Minsk, 1990), p. 279 [in Russian].
I. A. Efimenko, A. V. Churakov, N. A. Ivanova, et al., Russ. J. Inorg. Chem. 62, 1469 (2017). https://doi.org/10.1134/S003602361711
V. G. Barchukov, I. A. Efimenko, O. S. Erofeeva, et al., RF Patent No. 269 809 (2019).
D. V. Loginova, Candidate’s Dissertation in Chemistry (IONKh RAN, Moscow, 2007).
A. K. Lyashchenko and V. S. Dunyashev, Russ. J. Inorg. Chem. 63, 1656 (2018). https://doi.org/10.1134/S0036023618120136
A. K. Lyashenko and A. S. Lileev, J. Chem. Eng. Data 55, 2008 (2010). https://doi.org/10.1021/je900961m
Broadband Dielectric Spectroscopy, Ed by F. Kremer and A. Schönhals (Springer, Berlin, 2003).
A. Yu. Zasetskii and A. K. Lyashchenko, Quasi-Optical Method for Measuring the Complex Permittivity of Aqueous Solutions of Electrolytes in the Millimeter Band and Relaxation Characteristics of Solutions, Available from VINITI, No. 2181-V99 (06.07.1999) [in Russian].
J. Le Bot, C. R. Acad. Sci. 236, 469 (1953).
A. K. Lyashchenko, A. S. Lileev, and I. M. Karataeva, in Modern Problems of General and Inorganic Chemistry: Proceedings of the II International Conference, Moscow, May 19–21,2009 (InterGrafika, Moscow, 2009), p. 316 [in Russian].
V. I. Krivoruchko, Izv. Vyssh. Uchebn. Zaved., Radiofiz. 46, 782 (2003).
A. K. Lyashchenko, I. M. Karataeva, A. S. Kozmin, and O. V. Betskii, Dokl. Phys. Chem. 462 (2), 127 (2015). https://doi.org/10.1134/S0012501615060032
A. K. Lyashchenko, I. M. Karataeva, and V. S. Dunyashev, Russ. J. Phys. Chem. A 93, 682 (2019). https://doi.org/10.1134/S0036024419040204
A. K. Lyashchenko, A. Yu. Efimov, V. S. Dunyashev, and I. M. Karataeva, Russ. J. Inorg. Chem. 65, 241 (2020). https://doi.org/10.1134/S0036023620020096
W. Wachter, S. Fernandez, R. Buchner, and G. Hefter, J. Phys. Chem. B 111, 9010 (2007). https://doi.org/10.1021/jp072425e
O. B. Bel’skaya, T. I. Gulyaeva, A. B. Arbuzov, et al., Kinet. Catal. 51, 105 (2010). https://doi.org/10.1134/S0023158410010179
Yu. A. Zolotov, G. M. Varshal, and V. M. Ivanov, in Analytical Chemistry of Platinum Group Metals: A Collect ion of Review Articles (KomKniga, Moscow, 2005) [in Russian].
Funding
This work was performed under state assignment on basic scientific research for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow, Russia, and was supported by the Russian Foundation for Basic Research (project no. 19-03-00033a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Lyashchenko, A.K., Efimov, A.Y., Dunyashev, V.S. et al. Dielectric and Radio Brightness Characteristics of Aqueous Solutions of Ammonium Tetrachloropalladate according to the SHF and EHF Data. Russ. J. Inorg. Chem. 65, 1776–1780 (2020). https://doi.org/10.1134/S003602362011011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362011011X