Abstract
Using a high-sensitivity radiometer at a frequency of 61.2 GHz (millimeter spectral range), the radio brightness characteristics of aqueous solutions of alkalis (KOH, NaOH) have been studied under laboratory conditions. The radio brightness parameters have been compared with calculated data from dielectric spectra in the millimeter range. Using KOH solutions as an example, a correspondence between the experimental and calculated radio brightness parameters in the initial concentration range, where hydration changes in water in solutions predominate, has been revealed. It has been established that even in the millimeter range it is necessary to consider the spectral contributions of both dipole and ion losses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The microwave radiometric method is widely used for remote studies of the Earth from space [1]. The method is extremely efficient, it allows one to control the thickness of the earth’s surface and ice, is sensitive to roughness and waves on the surface of land and water [2]. Changes in the intrinsic thermal microwave radiation of the surfaces under study can be expressed in radio brightness temperature (Tb). Such changes in Tb can be experimentally obtained using highly sensitive millimeter-range radiometers that have appeared recently. The millimeter spectral range has long attracted the attention of researchers; it has found application in medicine. The effect of EHF exposure on living organisms was discovered about half a century ago. School of Academician Devyatkov occupies a leading position in the development of treatment procedures using the millimeter therapy method [3, 4].
EHF radiometry (61.2 GHz) is used by us in laboratory practice to determine the characteristics of radiation and reflection of water samples and solutions of various compositions and concentrations. The experimentally obtained radiometric parameters can be compared with the calculated dielectric data from the centimeter spectral range. The operability of the calculation scheme for the relationship between dielectric and radio brightness properties was previously proved by us on various model systems, solutions of electrolytes and nonelectrolytes [5–9].
The present work focuses on studying the radio brightness properties of aqueous solutions of alkali metal hydroxides (KOH, NaOH). The question that can be posed in this work is whether the hydroxide anion, as a violator of the water structure, will eliminate differences in the effect of cations with different hydration, potassium and sodium, on water. Earlier, in the series of alkali metal chlorides and nitrates (K+, Na+), radio brightness effects of different signs have been shown, associated with different (opposite in sign) effect of these cations on water [10–12].
EXPERIMENTAL
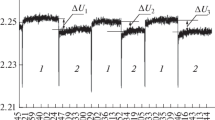
Samples of bidistillled water and KOH and NaOH solutions of various concentrations were studied by the radiometry methods. Studies were carried out with the use of a highly sensitive radiometer with a fixed operating frequency of 61.2 GHz (IRE RAS, NPO Istok, Fryazino [13]). The setup included an antenna (horn) on which the test sample was placed, a receiver, a recording device (PC), and a fan used to prevent overheating of the radiometer. A more detailed description of the radiometer and the measurement and calculation techniques have been reported elsewhere [5, 10, 13, 14]. The instrument readings were digitized and transferred to a PC, where a special program was used to record and further process the signal. The signal was recorded in the following way: the time of measurements (in hours, minutes, seconds) was plotted on the abscissa axis, and the radiophysical response (voltage at the horn output in volts, U) was plotted along the ordinate axis. The instrument scale was calibrated using a highly reflective polished copper plate [5]. The signal level from the metal (copper) plate was taken as zero. The maximum reading for the copper surface corresponded to the minimum radiation (zero signal), and the minimum reading for water and solutions corresponded to the maximum effect. Thus, the radiometer had an inverse scale. As an example, the recorded signals for a copper plate, distilled water, and sodium hydroxide solution are shown in Fig. 1: the radiation increases when passing from a copper plate to water and a solution. When passing from water to a 2 M sodium hydroxide solution, the radiation decreases.
During measurements, the signal from the radiometer periodically fluctuates. The U values for a copper plate, water, and a solution can change even with slight changes in temperature; therefore, it is more expedient to compare the radio brightness parameters for water and solutions using the relative method of analysis. The ∆U = Usolution – Uwater value practically does not depend on the temperature in the room and changes only when the concentration of solutions changes. To minimize measurement errors and improve the accuracy of the results, paired measurements of Usolution and Uwater were carried out at least 5 times. The value ∆Uav = (∆U1 + ∆U2 + ∆U3 + ∆U4 + ∆U5)/5 can be used to calculate its proportional parameters χ (emissivity) and Tb (radio brightness temperature). The ∆Uav, χ, and Tb values of KOH and NaOH solutions, obtained using a radiometer at a frequency of 61.2 GHz, are presented in Table 1.
CALCULATIONS
The optical and dielectric properties of solutions are related to each other through the relationship between the reflection coefficient R(ν) and the complex permittivity ε*(ν) (Fresnel formula, the case of a normally incident wave) [15, 16]:
The complex permittivity ε*(ν) is determined using the experimentally measured permittivity ε'(ν) and dielectric loss ε"(ν) [17]:
To determine the ε' and ε" values for a different frequency ν (in this case, for ν = 61.2 GHz), it is required to approximate the dielectric data obtained for other frequencies within the given relaxation model of the complex dielectric permittivity spectrum. In the present study, the dielectric data ε' and ε" in the region of the water dispersion maximum in the frequency range of 7–25 GHz for aqueous solutions of alkali metal hydroxides [18, 19] were approximated by the following functions:
where ε∞ is the high-frequency limit for the dispersion range under consideration, εs is the static dielectric permittivity, τ is the relaxation time, and α is the relaxation time distribution parameter [20].
The dielectric losses of electrolytes (ε") are made up of two components: dipole and ionic losses:
Dipole losses are associated with the absorption of radiation caused by the reorientation of dipole molecules. In electrolyte solutions, in addition to the dipole component of losses, there are movements of charged ions under the action of radiation (ionic contribution to losses). The frequency dependence of the ionic contribution is expressed by the formula [21]:
where ε0 is the electric constant, and σ is the electrical conductivity of the solution, S m–1.
The values of the parameters of the models of dielectric spectra used in the extrapolation of dielectric data from the frequency range of 7–25 GHz to a frequency of 61.2 GHz for KOH solutions [18] are given in Table 2. For NaOH solutions, data on the dielectric properties for only one concentration (0.4 M) in a different frequency range are available [19].
The reflection coefficient R is related to the emissivity χ measured in a radiometric experiment by a simple relationship:
The radio brightness temperature Тb is found by the equation:
(here, the thermodynamic temperature is Т = 298.15 K).
The χ and Тb values of aqueous KOH and NaOH solutions at a frequency of 61.2 GHz determined by extrapolation of dielectric data from the frequency range 7–25 GHz are presented in Table 2. Two variants of the calculated parameters are compared. In one case, both ionic and dipole dielectric losses (χ, Тb) are considered, and in the other case, only the dipole contribution to ε" (χ(d), Тb(d)) is considered.
RESULTS AND DISSCUSSION
A review of the available literature on the measurement characteristics of aqueous solutions of alkalis and acids shows that these compounds are a special case in which the proton and hydroxide anion have anomalous conductivity. Therefore, in order to consider the losses introduced by through conduction, it is necessary to measure the low-frequency electrical conductivity. The radiometric method, which considers both components, makes it possible to characterize the intrinsic radiation parameters of solutions with different ions in the millimeter range of the spectrum and opens up opportunities for a more accurate selection of a relaxation model for describing spectra in the centimeter range.
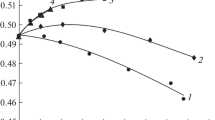
It can be seen from Fig. 2 that the radio brightness parameters for the systems under consideration decrease when going from water to a solution. This follows from both experimental and calculated data. In the case of salt solutions, the decrease in the radio brightness parameters is primarily associated with weak hydration of the ions (the K+ ion). The Na+ ion, which is more strongly hydrated than the potassium ion, gives the opposite change. For example, this is seen for solutions of alkali metal chlorides [11, 15, 16]. However, the abnormally mobile hydroxide ion introduces a predominant effect. Figure 2 shows that the radio brightness effects for both sodium hydroxide and potassium hydroxide give changes of the same sign.
We have compared the calculated and measured radio brightness parameters. For KOH solutions, there is agreement in the data in the initial concentration range, where hydration changes in water in solutions predominate. For NaOH solutions, this comparison is only qualitative. The dielectric parameters for calculation are available only for one concentration and are obtained for a different set of frequencies.
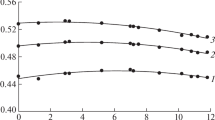
In the case of KOH solutions, the ∆Tb values related only to dipole dielectric losses, were also calculated. As can be seen from Fig. 3, there is no agreement between the experimental and calculated values. This follows even from the sign of the changes. In the millimeter range, it is necessary to consider the spectral contributions of both dipole and ion losses.
CONCLUSIONS
The method of measuring radiothermal radiation in the millimeter wavelength range turns out to be informative for determining changes in hydration and ion dynamics, which is observed for solutions of alkali metal hydroxides. Previously, this fact has been demonstrated for aqueous solutions of salts and acids. Therefore, the approach is quite general for water–electrolyte media of different composition and concentration. It requires a more detailed study at other frequencies in the millimeter spectral range.
REFERENCES
I. N. Sadovskii, E. A. Sharkov, A. V. Kuz’min, et al., Issled. Zemli Kosm. 6, 79 (2014). https://doi.org/10.7868/S0205961414060050
A. M. Shutko, Microwave Radiometry of the Water Surface (Nauka, Moscow, 1986) [in Russian].
N. D. Devyatkov, Usp. Fiz. Nauk 10, 453 (1973).
N. D. Devyatkov, Elektr. SVCh 4, 130 (1970).
A. K. Lyashchenko, I. M. Karataeva, and V. S. Dunyashev, Russ. J. Phys. Chem. 93, 682 (2019). https://doi.org/10.1134/S0036024419040204
A. K. Lyashchenko, A. Yu. Efimov, V. S. Dunyashev, and I. M. Karataeva, Russ. J. Inorg. Chem. 65, 241 (2020). https://doi.org/10.1134/S0036023620020096
A. K. Lyashchenko, A. Yu. Efimov, V. S. Dunyashev, and I. A. Efimenko, Russ. J. Inorg. Chem. 65, 1776 (2020). https://doi.org/10.1134/S003602362011011X]
A. K. Lyashchenko, A. Y. Efimov, and V. S. Dunyashev, Russ. J. Phys. Chem. A 95, 713 (2021). https://doi.org/10.1134/S0036024421040154
A. K. Lyashchenko, I. M. Karataeva, V. S. Dunyashev, and A. Y. Efimov, Russ. J. Phys. Chem. A 95, 1601 (2021). https://doi.org/10.1134/S0036024421080197
A. K. Lyashchenko, I. M. Karataeva, A. S. Kozmin, and O. V. Betskii, Dokl. Phys. Chem. 462, 127 (2015). https://doi.org/10.1134/S0012501615060032
A. K. Lyashchenko, Proceedings of the 3rd All-Russian Conference “Physics of aqueous solutions,” Moscow, 2020.
A. K. Lyashchenko, Zh. RENSIT 12, 81 (2020). https://doi.org/10.17725/rensit.2020.12.081
V. I. Krivoruchko, Izv. Vuzov. Radiofiz. 46, 782 (2003).
A. S. Koz’min, Cand. Sci. (Phys.-Math.) Dissertation, Volgograd, 2011.
A. Yu. Zasetskii and A. K. Lyashchenko, Dep. VINITI July 6, 1999 (Moscow, 1999, No. 2181-V99).
A. K. Lyashchenko and A. Yu. Zasetsky, J. Mol. Liq. 77, 61 (1998). https://doi.org/10.1016/S0167-7322(98)00068-3
Yu. M. Poplavko, Physics of Dielectrics (Vishcha shkola, Kiev, 1980) [in Russian].
A. S. Lileev, D. V. Loginova, and A. K. Lyashchenko, Russ. J. Inorg. Chem. 56, 961 (2011). https://doi.org/10.1134/S0036023611060167
V. U. Kaatze, J. Chem. Phys. 77, 447 (1973).
K. S. Cole and R. H. Cole, J. Chem. Phys. 10, 98 (1942). https://doi.org/10.1063/1.1723677
J. Barthel, R. Buchner, and M. Munsterer, Electrolyte data collection. Part 2: Dielectric properties of water and aqueous electrolyte solutions (DECHEMA Chemistry Data Series, 1995, vol. 12).
Funding
The work was performed in the framework of the State assignment of the Kurnakov Institute of General and Inorganic Chemistry RAS in the field of basic research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Lyashchenko, A.K., Efimov, A.Y. & Karataeva, I.M. Radio Brightness Contrasts of Aqueous Solutions of Alkali Metal Hydroxides in the Millimeter Spectral Range. Russ. J. Inorg. Chem. 67, 1293–1297 (2022). https://doi.org/10.1134/S0036023622080204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622080204