Abstract
A new approach to analyzing hydration in electrolyte solutions is developed that explores the relationship between their dielectric, quasi-optical, and radio brightness parameters in the cm and mm ranges of the spectrum. An interesting property is discovered in the region of low concentrations. The characteristic radiation of some electrolyte solutions at mm wavelengths is stronger than that of pure water, while that of other electrolyte solutions are weaker. Contrasts in radio brightness are associated with two contributions determined by the ratio of ion and dipole losses in the dielectric spectrum. This pattern is considered using solutions of alkali metal formiates and a number of other systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The development of dielectric microwave spectroscopy made the millimeter (mm) region of the spectrum available for study. It is of special theoretical and practical interest in the case of aqueous solutions [1–5], where there is a frequency limit to collective relaxation processes in water and solutions. This region is characterized by biological effects of mm wavelengths [1–3]. The initial section of the mm band (60–100 GHz) has been shown to be the high-frequency slope of the main maximum of the dispersion of the complex permittivity aqueous electrolyte solutions. In the initial range of concentrations, it can be described by choosing a fairly simple Debye-type model of relaxation or a Cole–Cole equation with a small parameter of the distribution of periods of relaxation obtained in the region of the maximum dispersion of aqueous solutions. In agreement with experiments, this was shown using solutions of alkali metal sulfates and a number of other systems as an example [6–8]. We can therefore estimate from calculations the quasi-optical coefficients, emissivity, and radio brightness contrast of different solutions in the mm region. In this work, we compared solutions of alkali metal formiates using data obtained earlier on complex permittivity in the region of the maximum dispersion of water and solutions in the cm region of the spectrum [9–11].
CALCULATIONS
Temperature Tb of radio brightness is a characteristic of substances and their state that can be measured remotely. It is associated with thermodynamic temperature T of a body by relation Тb(ν) = χ(ν)*T, where ν is the frequency at which the radiation from the body is measured, and χ(ν) is the emissivity at a given frequency. With complete absorption of radiation by a sample under conditions of thermodynamic equilibrium, χ(ν) = 1 − R(ν), where R(ν) is the coefficient of reflection, which can be obtained from the data on the complex permittivity ε*(ν) of aqueous solutions using the Fresnel formula for a normally incident wave:
The above relations allow us to obtain temperatures of radio brightness based on the existing set of literature data on the frequency dependences of the dielectric properties of aqueous solutions at different concentrations and at temperatures. Unfortunately, it is technically difficult to measure directly the dielectric parameters of solutions at mm frequencies [12–14]. We therefore extrapolated known 7–25 GHz dielectric data [9–11] for a frequency of 61.2 GHz, along with corresponding data on electrical conductivity [15, 16].
The complex permittivity at frequency ν is determined as ε*(ν) = ε'(ν) – iε"(ν), where ε' is permittivity, ε'' denotes dielectric losses, and i is the imaginary unity. The Debye function modified by Cole and Cole in [17, 18] is usually used to reproduce the spectrum of ε*(ν) for non-electrolytes in the range of frequencies
where ε∞ is the high-frequency limit for the considered region of dispersion, εs is static permittivity, τ is the period of relaxation, and α is the distribution of periods of relaxation.
The value of ε''(ν) for water is determined by the dipole relaxation of molecules and can be obtained from expression (2). In solutions of electrolyte, absorption proceeds by two mechanisms associated with reorientations of dipole water molecules and displacements of charged ions in an alternating electromagnetic field. The contribution from ion losses is calculated using experimental data on low-frequency electrical conductivity [18]:
where ε0 is the electrical constant (8.854 × 10−12 F/m), and σ is the specified electrical conductivity of the solution, S/m. The total dielectric losses for solutions of electrolyte are thus found as the sum of losses calculated according to (1) and (2):
where \(\varepsilon _{{\text{d}}}^{{''}}(\nu )\) and \(\varepsilon _{{\text{i}}}^{{''}}(\nu )\) are dipole ionic dielectric losses, respectively.
The ionic contribution to ε"(ν) is reduced rapidly as the frequency rises, so it is often ignored in the mm range of frequencies. It is therefore of interest to compare both ways of calculating the radio brightness of solutions. In one version, we consider both ionic and dipole dielectric losses (R, χ, Тb); in the other, only the dipole contribution to ε" (R(d), χ(d), Тb(d)).
In many cases, the dependences of concentration and temperature Tb are represented more clearly using the quantity
In this work, we consider data at T = 283, 298, and 313 K.
RESULTS AND DISCUSSION
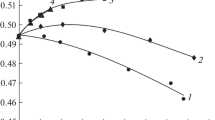
Table 1 shows components of the coefficient of reflection that convey specific features of water–electrolyte systems. They are associated with the comparable contributions from dipole and ion losses at mm wavelengths (4). When the hydration of ions is increased, the effect of dipole losses determines the overall change in the coefficients of reflection relative to that of water. The effect of ion losses arises upon weak (negative) hydration of potassium and cesium ions in the region of low concentrations. This creates the possibility of multi-sign changes in the coefficients of reflection and related parameters, relative to those in water in the initial range of concentrations. A typical example of changes in R due to concentration upon the weak (negative) hydration of cations is shown in Fig. 1. A change in temperature affects the considered concentration dependences, where a small extremum disappears upon a minor increase in temperature.
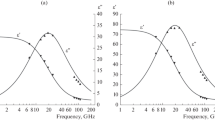
It is convenient to express the studied solutions’ characteristic intensity of radiation at mm wavelengths in terms of their intrinsic coefficients of emission. They can be found easily using the calculated coefficients of reflection. An interesting consequence is then observed. The two contributions to the coefficients of reflection determines the total changes in emissivity, relative to water. This can be seen in Figs. 2 and 3. When hydration of the cation was raised (Fig. 2), the contribution from dipole losses determined the increase in emissivity, relative to water. At weak hydration of potassium and cesium ions, we observe the contribution from ion losses that determines the total value (Fig. 3). No increase in emissivity is observed in a fairly wide range of concentrations, so a small minimum may appear in the concentration dependence of emissivity.
It should be noted that the considered calculation scheme is associated specifically with the initial range of concentrations, in which hydration processes predominate. The values of the coefficients of reflection are calculated here using those of complex permittivity and ones found using a simple model of Debye-type relaxation. This cannot, of course, be extended to the entire range of our concentrations, where Cole–Cole equations with high parameters of the distribution of periods of relaxation, the maximum specific electrical conductivity of solutions [19], and other factors are used. In cases like these, one accepted model of a relaxation spectrum or another must be verified experimentally.
CONCLUSIONS
Changes in the radio brightness temperature of solutions can be found using their calculated emissivity. Figure 4 shows changes in this effective temperature. For solutions with increased hydration of sodium and lithium ions, this value differs in sign from that of negative hydration of potassium and cesium ions. Contrasts in radio brightness radiation can be seen for solutions of alkali metal ions with positive and negative hydration of cations in solutions of alkali metal formiates. Additional radio brightness contrast must therefore be observed in aqueous media partially separated in space. This could be of importance in technological practice.
REFERENCES
O. V. Betskii, V. V. Kislov, and N. N. Lebedeva, Millimeter Waves and Living Systems (Sains-Press, Moscow, 2004) [in Russian].
A. Kh. Tambiev, N. N. Kirkorov, O. V. Betskii, and Yu. V. Gulyaev, Millimeter Waves and Photosynthetic Organisms (Radiotekhnika, Moscow, 2003) [in Russian].
A. K. Lyashchenko, Biomed. Radioelektron., Nos. 8–9, 62 (2007).
A. M. Shutko, Microwave Radiometry of the Water Surface (Nauka, Moscow, 1986) [in Russian].
I. N. Sadovskii, E. A. Sharkov, A. V. Kuz’min, et al., Issled. Zemli Kosmosa, No. 6, 79 (2014). https://doi.org/10.7868/S0205961414060050
A. K. Lyashchenko and V. S. Dunyashev, Phys. Wave Phenom. 29, 169 (2021). https://doi.org/10.3103/S1541308X21020096
A. K. Lyashchenko, I. M. Karataeva, and V. S. Dunyashev, Russ. J. Phys. Chem. A 93, 682 (2019). https://doi.org/10.1134/S0036024419040204
A. K. Lyashchenko, A. Yu. Efimov, V. S. Dunyashev, and I. M. Karataeva, Russ. J. Inorg. Chem. 65, 241 (2020). https://doi.org/10.1134/S0036023620020096
A. S. Lileev, D. V. Loginova, A. K. Lyashchenko, Mendeleev Commun., No. 17, 364 (2007). https://doi.org/10.1016/j.mencom.2007.11.024
A. K. Lyashchenko and A. S. Lileev, J. Chem. Eng. Data 55, 2008 (2010). https://doi.org/10.1021/je900961m
K. S. Ivanova, Cand. Sci. (Chem.) Dissertation (Inst. Gen. Inorg. Chem., Moscow, 1990).
A. K. Lyashchenko, I. M. Karataeva, A. S. Koz’min, and O. V. Betskii, Dokl. Phys. Chem. 462, 127 (2015). https://doi.org/10.1134/S0012501615060032
V. I. Krivoruchko, Radiophys. Quantum Electron. 46, 703 (2003).
A. S. Koz’min, Cand. Sci. (Phys. Math.) Dissertation (Volgogr. State Tech. Univ., Volgograd, 2011).
K. C. Ivanova, A. K. Lyashchenko, and A. S. Lileev, Zh. Neorg. Khim. (1991).
K. S. Ivanova, A. F. Borina, and A. K. Lyashchenko, Zh. Neorg. Khim. 37, 2540 (1992).
K. S. Cole and R. H. Cole, J. Chem. Phys. 10, 98 (1942). https://doi.org/10.1063/1.1723677
J. B. Hasted, Aqueous Dielectrics (Chapman and Hall, London, 1973).
A. K. Lyashchenko and A. A. Ivanov, Zh. Strukt. Khim. 22 (5), 69 (1981).
Funding
This work was supported by the RF Ministry of Science and Higher Education as part of a State Task for the Kurnakov Institute of General and Inorganic Chemistry.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare they have no conflicts of interest.
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Lyashchenko, A.K., Dunyashev, V.S. Reflection and Radio Brightness of Aqueous Solutions of Alkali Metal Formiates at Millimeter Wavelengths. Russ. J. Phys. Chem. 96, 2400–2403 (2022). https://doi.org/10.1134/S0036024422110164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422110164