Abstract
Pymetrozine is an insecticide that is widely used, causing severe environmental problems. This study determined the pymetrozine degradation by an enrichment culture of microorganisms from paddy soil. The results showed that the enrichment culture utilized 67.3 ± 10.1% of the pure substrate as the sole carbon and nitrogen source in liquid media after 36 h. However, the utilization rates of pymetrozine in herbicide were lower than those of the pure substrate. The maximum utilization rates of pure pymetrozine and pymetrozine in herbicide were 0.051 ± 0.006 and 0.039 ± 0.004 mmol/h, respectively. During the degradation, the metabolites 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one and nicotinic acid were produced and then further degraded. In addition, the inoculation of a fungal strain, Phanerochaete sp. Th1, which degraded rice straw components, increased pymetrozine degradation in rice straw and soil. The study provides essential information on pymetrozine degradation in liquid media, rice straw during solid-state fermentation, and soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pymetrozine (6-methyl-4-[(E)-pyridin-3-ylmethylideneamino]-2,5-dihydro-1,2,4-triazin-3-one) is a superior insecticide that is widely used to control whiteflies, leafhoppers and aphids in crops (Fuog et al., 1998). The compound interferes with neurvous regulation of sucking insects (Shen et al., 2009). Pymetrozine is highly toxic to aquatic organisms (Yu et al., 2018). The compound is also genotoxic and has weak cytotoxic effects on human peripheral lymphocytes (Yildirim et al., 2017). The Environmental Protection Agency (EPA) has deemed pymetrozine a likely human carcinogen (EPA, 2000). The European Union has recently decided to ban insecticides harmful to bees (Butler, 2018).
According to Vela et al. (2019), the intensive use of pesticides in agricultural fields results in the contamination of pesticides in agro-wastewater. Pymetrozine is widely detected in paddy soil, water, and rice tissues (Li et al., 2011; Zhang et al., 2015). The compound accumulates in cucumber (Talebi and Ghazizadeh, 2006) and honey bees (Badawy et al., 2015). Moreover, pymetrozine is found in peaches, citrus, and tomatoes (JMPR, 2022). Therefore, its environmental behavior and the degradation mechanisms of the insecticide merit investigation.
Some previous studies have determined pymetrozine dissipation in flooded paddy soil water (Li et al., 2011; Zhang et al., 2015). The insecticide accumulation in rice straw may interfere with the degradation of straw components in compost and solid fermentation processes: only one pymetrozine-degrading pure culture, Pseudomonas sp. BYT-1 has been isolated (Sun et al., 2019). However, a metabolite, 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one, produced from pymetrozine degradation by this Pseudomonas sp. BYT-1 was not further transformed (Sun et al., 2019). The product triggers a structural alert for genotoxicity (JMPR, 2022), so it should be removed. Moreover, all previous studies evaluated degradation of pymetrozine as pure substrate; however, pesticides contain not only active compounds but also adjuvants, which may affect degradation of the main ingredients.
The present study determined pymetrozine degradation by mixed microorganisms enriched from paddy soil. The degradation metabolites were also analyzed. In addition, the degradation of the compound in contaminated rice straw and the role of a fungal strain, Phanerochaete sp. Th1 were studied. Moreover, the augmentation of pymetrozine degradation in fruit garden soil using the microorganisms from the paddy soil was tested.
MATERIALS AND METHODS
Soil Collection, Enrichment, and Bacterial Diversity Analysis
Soil samples were collected from a fruit garden, a corn field, and a rice field in Dong Thap Province (Vietnam), where pesticides had been extensively applied. Surface soil (upper 10 cm) was collected, placed in plastic bags, and taken to our lab. The soil was pulverized and sieved through a 2 mm mesh before determining its physicochemical properties (Table S1). Next, soil samples, each weighing 500 g, were transferred to plastic containers (length × width × depth = 15 × 25 × 20 cm). Pymetrozine was added at 0.01 mM (2.2 mg/kg of dry soil), and sterile water was sprinkled to a soil moisture of 40%. Each container was capped with a plastic cover and placed in the dark at room temperature (~30°C). Pymetrozine was supplemented at 0.01 mM every 30 days for three months and then 0.1 mM every 30 days for the next three months. Each treatment was conducted in three replicates.
In analyzing the bacterial diversity and relative abundance in soil, the relative abundances of the bacterial species in the soil slurries were determined by sequencing 16S rRNA genes using an Illumina MiSeq benchtop sequencer. The universal primers 338F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') were used to amplify the 16S rRNA genes at the V3-V4 region. Other procedures were conducted as described by Duc et al. (2021). Rarefaction and α-diversity indices, i.e., abundance-based coverage estimators (ACE), Chao1, and Shannon, were calculated.
Culture Media
Mineral medium (MM) was used for pymetrozine degradation. The components of the medium included (g/L) Na2HPO4 (2.79), KH2PO4 (1.00), MgCl2·6H2O (0.20), and 1.0 mL of trace mineral solution. The trace mineral solution consisted (g/L) of H3BO3 (0.30), FeCl2·6H2O (0.20), ZnCl2·7H2O (0.10), Na2MoO4·2H2O (0.03), MnCl2·4H2O (0.03), and CuCl2·2H2O (0.01). Glucose and ammonium sulfate (0.5 g/L each) were used as additional carbon and nitrogen sources. The pH was adjusted to 7.0 ± 0.1 using NaOH. The media containing glucose were sterilized at 110°C for 20 min, while other media were autoclaved at 121°C for 15 min. Pymetrozine (99% purity), 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one (>99% purity), and nicotinic acid (>99% purity) were purchased from Sigma-Aldrich (USA). These compounds were individually dissolved in absolute ethanol at 0.1 M and used as stock solutions. The stock solutions were pipetted into flasks, and the MM was added after the ethanol evaporated completely.
Pymetrozine Utilization by Enrichment Culture
Two grams of soil (dry weight) from each container after six months of enrichment were put in a 500 mL flask containing 200 mL supplemented with 0.1 mM pymetrozine and incubated for 36 h. The enriched culture (0.5 mL) was transferred to a new MM supplemented with glucose, ammonium sulfate, and 0.1 mM pymetrozine. The culture was incubated to an optical density (OD600) of 0.5, and 0.5 mL was transferred to a fresh MM (100 mL) to determine the degradation of pymetrozine and the growth of microorganisms. Utilization was studied with pymetrozine as a sole carbon and nitrogen source or with the addition of glucose and ammonium sulfate. The incubation was conducted at room temperature (about 30°C) at an agitation rate of 150 rpm.
Degradation was also studied using a commercial insecticide named Chess 50WG (Syngenta Company, Viet Nam), which contains 500 g/kg pymetrozine and adjuvants. In order to determine degradation kinetics, the MM medium was supplemented various thiobencarb concentrations (from 0.01 to 1.5 mM), glucose (0.5 g/L) and ammonium sulfate (0.5 g/L). The obtained results were used to calculate kinetic parameters.
Degradation of Pymetrozine and Rice Straw Components during Solid-State Fermentation
Rice straw was obtained from a rice field from which the soil was collected as described above after the autumn harvest and chopped to 0.5–1.0 cm length. The straw was dried at 50°C for four days. The main components of the rice straw were as follows: 33.5 ± 1.6% cellulose, 19.8 ± 1.8% hemicellulose, 21.5 ± 1.4% lignin, 10.8 ± 1.1% moisture, 42.6 ± 3.8% carbon, and 0.6 ± 0.0% nitrogen. Two hundred grams of rice straw mixture in the dry state was transferred to a plastic box as described above. Pymetrozine was added at 0.01 and 0.1 mmol/kg of dry straw.
The enrichment culture of microorganisms from paddy soil was grown in the MM supplemented with glucose, ammonium sulfate, and 0.1 mM pymetrozine, as described above. The grown biomass was collected by centrifuging for 10 min at 6800 g and resuspended in sterile water to OD600 of 1.5.
The degradation experiment used a mixture of the culture from paddy soil and fungal strain Phanerochaete sp. Th1 (GenBank under accession numbers OQ592854), earlier isolated by us from soil and stored in our lab. The fungus cannot degrade pymetrozine, but it degrades rice straw components.The fungus was cultivated on potato dextrose agar slants at 30°C for seven days. Spores were gently scraped from the agar surface and mixed with the enrichment culture from paddy soil to 107 spores/mL. The concentrated cell suspension (10 mL) was sprinkled into 100 g of dry rice straw. Sterile water was added to 40% moisture content, maintained during the incubation. The straw was thoroughly mixed. The plastic boxes were kept in dark conditions at room temperature for 25 days. The boxes were manually shaken twice a week, for about 5 min each time.
Pymetrozine Degradation in Soil
The soil was collected from a fruit garden, transferred to the laboratory, and processed as described above. Pymetrozine degradation in the soil was conducted using free and immobilized microorganisms from the paddy soil, with and without inoculating with Phanerochaete sp. Th1.
For the augmentation of degradation using free cells, the enrichment culture of microorganisms from the paddy soil were suspended in 100 mL sterile water to OD600 of 1.5 and added to 1.0 kg dry soil. For the experiment with immobilized cells, 100 mL of the mixture of microorganisms and fungal spores, prepared as described above, was introduced into 500-mL flasks containing 120 g of dry straw. The flask was shaken at 150 rpm for 12 h at room temperature. Most of the liquid media was absorbed by the straw, and the straw was used to inoculate 1.0 kg of soil (on a dry weight basis). The moisture content was set to approximately 40% by mass and was further monitored and adjusted by sprinkling with sterile water during fermentation for 25 days. Each experiment was conducted in three replicates. Control was sterile soil autoclaved at 121°C for 15 min.
Pymetrozine Extraction and Analytical Methods
Pymetrozine was extracted from rice straw and soil with acetonitrile/water (3/1, v/v). 5.0 g of straw, soil, or mixture of straw and soil was crushed and then 10 mL of this mix was put into a 50-mL centrifuge tube, followed by shaking on a shaker at 500 rpm for 12 h and centrifugation. The supernatant was filtrated through a 0.45-µm filter. The extraction efficiencies from the straw and soil were 91.8 and 94.5%, respectively.
Pymetrozine concentration was analyzed using High-Performance Liquid Chromatography (HPLC). The HPLC system (Shimadzu Corporation, Kyoto, Japan) consisted of LC 20AD pumps, a SIL-20A autosampler, and an SPDM20A photodiode array (PDA) detector. A Shimadzu Shim-Pack XR-ODS column was used to separate metabolites. The HPLC was operated as described in a previous study (Duc et al., 2021). The degradation products were analyzed using TripleTOF 5600+ QTOF LC-MS/MS System as described in a previous report (Sun et al., 2019).
Van Soest’s method was used to determine rice straw’s hemicellulose, cellulose, and lignin contents (Van Soest et al., 1991). Hemicellulose was estimated based on the difference between the neutral-detergent and the acid-detergent fibers. Cellulose was determined as the difference between the acid-detergent fiber and the acid-detergent lignin. Lignin was estimated as the difference between the detergent lignin and the ash content.
Statistical Analysis
For β-diversity analysis, the principal coordinate analysis (PCoA) and permutational analysis of variance (PERMANOVA) were performed using PRIMER7 software. OTU abundance table was square-root transformed and used for calculating Bray–Curtis distances. Main test and Pair-wise PERMANOVA were calculated using the Type III method with 999 permutations.
All data obtained from at least three replicate experiments are presented as mean ± standard deviation. In addition, Duncan’s multiple range test (p < 0.05), run on SPSS 22.0, was used to analyze significant differences among means.
RESULTS
Pymetrozine Degradation by Mixed Microbial Cultures
The pymetrozine degradation abilities of mixed microbial cultures from soil samples collected from three sites were determined. The results showed that pymetrozine degradation by soil microorganisms after collection from a fruit garden, corn field, and rice field was 15.4 ± 3.3, 20.6 ± 3.5, and 18.8 ± 2.1%, respectively, in MM medium without co-substrate at 1.0 mM pymetrozine after 36 h. After six months of enrichment, utilization under the same conditions was 40.5 ± 4.3, 50.6 ± 6.5, and 67.3 ± 10.1%, respectively. The enrichment culture from the rice field showed the highest degradation and was used for further experiments.
The enrichment culture from the rice field utilized pymetrozine as a sole carbon and nitrogen source (Fig. 1a). The addition of glucose and ammonium sulfate to the MM medium increased the utilization by 22.8% on average (Fig. 1b). Moreover, cell growth increased with the presence of the co-substrate in the medium. During the degradation, two metabolites were produced (Fig. 1). The first metabolite had m/z 129.0771 corresponding to 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one (Sun et al., 2019). The second one with m/z 124.0393 was identified as nicotinic acid (Sun et al., 2019). The concentrations of the intermediates were highest after 24 h in the medium without the co-substrates and after 18 h in the medium supplemented with the co-substrates. The results showed that pymetrozine was oxidatively hydrolyzed at the C=N double bond at the initial step.
Pymetrozine utilization (square symbols, solid line) and cell growth rate (square symbols, dashed line) of the enrichment culture from the rice field in MM (a) without and (b) with supplementation with glucose and ammonium sulfate. During the pymetrozine utilization, 4‑amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one (triangle symbols) and nicotinic acid (diamond symbols) were produced. The abiotic control (open symbols) shows no degradation.
The microbial mixture was also used to utilize amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one and nicotinic acid. The results showed that the utilization of these compounds in the MM without any co-substrate was 52.2 ± 6.7 and 54.3±7.3%, respectively. The supplementation with glucose and ammonium sulfate increased the utilization to 66.4 ± 7.0% for 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4] triazine-3-one and to 83.8 ± 6.8% for nicotinic acid (Fig. 2). The addition of the co-substrates also increased cell growth in the medium.
Utilization (solid line) of 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one (triangle symbols) and nicotinic acid (diamond symbols) and the corresponding bacterial growth (dashed lines) of the enrichment culture in the MM (a) without and (b) with glucose and ammonium sulfate. The chemical concentrations in abiotic controls are also shown.
Effects of Pymetrozine on Bacterial Community in Soil
Microorganisms from paddy soil showing highest degradation were further determined in terms of their abundances at genus and phylum levels. After six months of supplementation with pure pymetrozine, some changes in bacterial community structures were found (Fig. 3). At the phylum level, Proteobacteria representatives were the most abundant, with 53.0 ± 2.2% in the original soil slurry, which was 56.8 ± 4.4 and 59.0 ± 5.2% (on average) in soil without pymetrozine and with pymetrozine after six months, respectively. Actinobacteria was the second in abundance, with corresponding data of 12.3 ± 1.1, 12.9 ± 2.0, and 11.4 ± 1.7% on average. Bacteroidetes was 6.6 ± 0.4% at the beginning and relatively stable in soil without the substrate after six months (6.0 ± 0.8%), while reduced to 3.9 ± 0.5% in the sample supplemented with the substrate.
At the genus level, Bacillus was the most abundant initially, 6.8%, which was relatively stable in all treatments after six months. Other genera, i.e., Aeromonas, Enterobacter, Orchrobactrum, Achromobacter, Comamonas, Arthrobacter, Flavobacteriia, Anaerolinea, and Streptococcus, were also relatively stable during the incubation. The relative abundances of Acrobacter, Pseudomonas, Acinetobacter, and Streptomyces increased after six months. Pseudomonas was the most abundant in soil amended with pymetrozine, with 7.1 ± 0.8% on average. Meanwhile, the relative abundances of Cupriavidus, Sphingobacterium, and Rhodococcus decreased in all treatments after that time. The relative abundances of Delftia, Pseudomonas, and Acinetobacter in soil with pymetrozine were higher than those in the samples without the substrate.
The richness and community structure of soil bacteria were also evaluated using the α-diversity indices. Table 1 demonstrates that only sequence numbers in the treatment without pymetrozine significantly increased during the incubation. Other indices, i.e., OTUs, ACE, Chao1, and Shannon were not statistically different among treatments.
The effects of pymetrozine on β-diversity were investigated. The structures of bacterial communities in terms of the OTU composition of paddy, corn and fruit garden soils were significantly different (Pseudo-F = 114.14, p = 0.01). PCoA revealed the formation of distinct clusters among three soil types (Fig. 4). The main test showed that bacterial communities from fruit garden soil were significantly different, depending on whether it was the original sample, soil without treatment, or soil treated with pymetrozine (Table 1). However, the OTU compositions of soils collected from the rice field and the corn field, with and without pymetrozine amendment, were not statistically different. Pairwise PERMANOVA analysis indicated that the effects of pymetrozine on bacterial communities in soil collected from the fruit garden were marginally significant, while no significant difference was observed in other soil types (Table S2).
Utilization of Pure Pymetrozine and Pymetrozine in a Pesticide at Different Concentrations
The utilization of pure pymetrozine and pymetrozine in a pesticide at different concentrations was studied in the MM supplemented with glucose and ammonium sulfate. At low concentrations (from 0.01 to 0.8 mM), the utilization rates of the enrichment culture were not statistically different in the medium with pure substrate and in the medium with pesticide (Fig. 5). However, concentrations higher than 0.8 mM, the utilization rates of the pure substrate were higher than those of pymetrozine in the pesticide. The curves of utilization rates of the pure substrate at various concentrations [S] followed the saturation kinetics of a Michaelis–Menten model (Fig. 5) given by the equation V = Vmax[S]/(Ks + [S]). The calculation of kinetics parameters showed that the maximum utilization rate (Vmax) and apparent half-saturation coefficient (Ks) were 0.051 ± 0.006 mM/h and 0.51 ± 0.05 mM, respectively. In contrast, the utilization and cell growth rates on the substrate in the Chess 50WG insecticide fitted well the Edwards model given by the equation V = Vmax[exp(–S/Ki) – exp(–S/Ki)]. The values of Vmax and Ks were 0.039 ± 0.004 mM/h and 0.45 ± 0.05 mM, respectively, and the inhibition coefficient (Ki) was 0.93 ± 0.10 mM.
Degradation of Pymetrozine in Rice Straw during Solid-State Fermentation
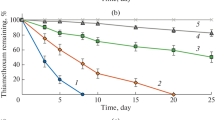
Table 2 shows degradation performances of straw components and pymetrozine in straw by the microbial culture and Phanerochaete sp. Th1. In the control, only small amounts of hemicellulose, cellulose, lignin, and pymetrozine in the rice straw were reduced. The fungus could not degrade the insecticide, and the mixture of microorganisms could not significantly degrade hemicellulose, cellulose, and lignin in most treatments. The presence of the fungus increased the degradation of pymetrozine in the straw by the enrichment microbial culture at both 0.01 and 0.1 mM. The degradation of the straw components by the fungus and the microbial mixture and the fungus was not statistically different at 0.01 mM; however, the degradation of the straw components by Phanerochaete sp. Th1 decreased with the addition of 0.1 mM pymetrozine (Table 2). The microbial mixture increased straw components degradation at 0.1 mM pymetrozine.
Degradation of Pymetrozine in Soil
Fresh soil collected from the fruit garden showed the slowest degradation, as described above. It was used to determine augmentation of pymetrozine degradation by the enrichment culture from the rice field. Pymetrozine content decreased in both sterile and nonsterile soil without augmentation. The amount of pymetrozine dissipated in nonsterile soil was always significantly higher than in the sterile soil. The degradation percentages of pymetrozine at 0.01 mM were significantly higher than those at 0.1 mM in most treatments (Table 3). Adding microorganisms from the paddy soil enhanced the insecticide degradation in both sterile and nonsterile fruit garden soil. However, the degradation rates in the soil augmentated using free microorganisms and microorganisms immobilized in rice straw were not statistically different (Table 3). Even though Phanerochaete sp. Th1 could not degrade the pesticide, the presence of the fungus significantly increased pymetrozine degradation in most treatments.
DISCUSSION
After enrichment for six months, the degradation by native microorganisms increased for all soils collected from the fruit garden, corn field, and rice field. The mixture of microorganisms from the paddy soil showed the highest degradation ability after enrichment, probably due to their higher adaptation. Moreover, the higher contents of silt, total C and total N in the paddy soil might support microorganisms involved in higher degradation. Pairwise comparisons showed that most bacterial soil communities were not statistically different, whether at the beginning or after six months, either with or without soil treatment with pymetrozine. However, after prolonged amendment with pymetrozine, the bacterial community structure was changed in tems pf both phyla and genera. Some genera that were more abundant in soil with pymetrozine than in the sample without the substrate are probably involved in the degradation. The analysis of α- and β-diversity showed that there was no detectable negative effect of the insecticide at 0.1 mM on the bacterial community of soil collected from the rice field. The mixed culture from the paddy soil could utilize pymetrozine and grow on the substrate as a sole carbon and nitrogen source.
During the pymetrozine degradation, two metabolites, 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one and nicotinic acid, were found. A previous study showed that these metabolites were produced in the pesticide degradation by Pseudomonas sp. BYT-1 (Sun et al., 2019). BYT-1 could degrade 2.3 mM pymetrozine within 20 h under optimum conditions. However, the intermediate 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one was not further transformed by Pseudomonas sp. BYT-1 (Sun et al., 2019). In this study, 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one and nicotinic acid were further degraded. The enrichment culture from the rice field utilized the intermediates as the sole carbon and nitrogen source at a similar rate after 36 h. However, the supplementation of co-substrates resulted in higher degradation of nicotinic acid than of the other one. This result suggested that 4-amino-6-methyl-4,5-dihydro-2H-[1,2,4]triazine-3-one was more persistent than nicotinic acid, but low nitrogen content in nicotinic acid reduced its utilization as a sole source of carbon and nitrogen.
The degradation rates for pure pymetrozine were not statistically changed at the higher 0.8 mM concentration. In contrast, the rates for pymetrozine in the pesticide Chess 50WG decreased faster than the concentration of this chemical in the liquid medium. The low water solubility of pure pymetrozine probably resulted in the saturation of degradation, while adjuvants in the pesticide caused the degradation reduction at high concentrations. In previous studies on other herbicides, adjuvants caused the reduction of prometryn degradation by free cells (Pérez-Bárcena et al., 2014) and suppressed the degradation of butachlor and propanil by Pseudomonas sp. But2 and Acinetobacter baumannii DT in liquid media but not in soil (Duc et al., 2020).
A small amount of pure pymetrozine in rice straw was dissipated after 25 days of solid-state fermentation. A previous study showed that the concentration of pure pymetrozine in live rice straw was reduced to an undetectable level after 21 days of application (Li et al., 2011; Zhang et al., 2015). The uninoculated control showed poor degradation of straw components and the pesticide, indicating that microorganisms which might be present in the straw did not play important roles in the degradation. The inoculation of the fungus in the rice straw increased pymetrozine degradation probably because the straw components were degraded to provide nutrients for the microbial culture. Moreover, pymetrozine at 0.1 mM inhibited the degradation of straw components by the fungus. The inoculation of the enrichment culture resulted in no inhibition of rice straw degradation by Phanerochaete sp. Th1 due to the pymetrozine degradation by the mixed bacterial culture. The application of pymetrozine has the potential to contaminate rice straw, rice husk, and brown rice (Xu et al., 2018), which may reduce the degradation of hemicellulose, cellulose, and lignin in solid-state fermentation. The immobilization of microorganisms in rice plants to degrade an insecticide has been reported (Ruiz-Hidalgo et al., 2014; Duc, 2022).
The dissipation percentages of pymetrozine in nonsterile soil from the fruit garden were significantly higher than those in sterile soil due to the activities of indigenous microorganisms. Previous studies reported various rates of pymetrozine dissipation in soils. Li et al. (2011) showed that no pymetrozine was detected in flooded paddy soil 42 days after applying 600 g a.i./hm2. Meanwhile, Xu et al. (2018) reported that the insecticide remained in the soil after 60 days of applying 450 and 675 g a.i./hm. The half-life of pymetrozine in sandy roams was about one month (Wyss et al., 2015). In this study, pymetrozine was quite persistent in soil, and the dissipation depended on the insecticide concentrations. The inoculation of enrichment of microorganisms using free and immobilized cells enhanced pymetrozine degradation. The inoculation of the fungus also increased the degradation in soil. The results of this study indicate that both the fungus and microorganisms from the paddy soil played essential roles in the degradation of straw components and pymetrozine.
In a nutshell, the enrichment culture of microorganisms from paddy soil utilized pymetrozine at higher rates than the enrichment cultures from soils collected in fruit garden and corn field. The supplementation with the enrichment culture from the paddy soil increased pymetrozine degradation in rice straw and soil, and the inoculation of Phanerochaete sp. Th1 into rice straw increased the degradation of straw components and pymetrozine. The application of pymetrozine at 0.1 mM did not cause a statistically negative effect on bacterial structure in the soil collected from a fruit garden. Some microorganisms, namely, Acrobacter, Pseudomonas, Acinetobacter, and Streptomyces, increased their relative abundances after six months. However, no pure culture showing effective degradation of the insecticide was isolated. The experiment is ongoing.
REFERENCES
Badawy, M.E., Nasr, H.M., and Rabea, E.I., Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions, Apidologie, 2015, vol. 46, pp. 177–193. https://doi.org/10.1007/s13592-014-0315-0
Butler, D., Scientists hail European ban on bee-harming pesticides, Nature, 2018.
Duc, H.D., Thuy, N.T.D., Thanh, L.U., Tuong, T.D., and Oanh, N.T., Degradation of diuron by a bacterial mixture and shifts in the bacterial community during bioremediation of contaminated soil, Curr. Microbiol., 2021, vol. 79, no. 1, p. 11. https://doi.org/10.1007/s00284-021-02685-5
Duc, H.D., Thuy, N.T.D., Truc, H.T.T., Nhu, N.T.H., and Oanh, N.T., Degradation of butachlor and propanil by Pseudomonas sp. strain But2 and Acinetobacter baumannii strain DT, FEMS Microbiol. Lett., 2020, vol. 367, no. 18, p. fnaa151. https://doi.org/10.1093/femsle/fnaa151
Duc, H.D., Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1, Environ. Eng. Res., 2022, vol. 27, no. 4, p. 210158.https://doi.org/10.4491/eer.2021.158
EPA., Pesticides-Fact Sheet for Pymetrozine. https:// www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-101103_01-Aug-00.pdf (accessed August, 2014), 2000.
Fuog, D., Fergusson, S., and Flückiger, C., Pymetrozine: a novel insecticide affecting aphids and whiteflies, in Insecticides with Novel Modes of Action, 1998, pp. 40–49.
JMPR. (The Joint FAO/WHO Meeting on Pesticide Residues) JMPR Report: Pymetrozine. http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/lpe/lpe.
Li, C., Yang, T., Huangfu, W., and Wu, Y., Residues and dynamics of pymetrozine in rice field ecosystem, Chemosphere, 2011, vol. 82, no. 6, pp. 901–904. https://doi.org/10.1016/j.chemosphere.2010.10.053
Pérez-Bárcena, J.F., Ahuatzi-Chacón, D., Castillo-Martínez, K.L., Ruiz-Ordaz, N., Galíndez-Mayer, J., Juárez-Ramírez, C., and Ramos-Monroy, O., Effect of herbicide adjuvants on the biodegradation rate of the methylthiotriazine herbicide prometryn, Biodegradation, 2014, vol. 25, pp. 405–415. https://doi.org/10.1007/s10532-013-9669-7
Ruiz-Hidalgo, K., Chin-Pampillo, J.S., Masís-Mora, M., Carazo, E.R., and Rodriguez-Rodriguez, C.E., Degradation of carbofuran by Trametes versicolor in rice husk as a potential lignocellulosic substrate for biomixtures from mineralization to toxicity reduction, Process Biochem., 2014, vol. 49, pp. 2266–2271. https://doi.org/10.1016/j.procbio.2014.10.006
Shen, G., Hu, X., and Hu, Y., Kinetic study of the degradation of the insecticide pymetrozine in a vegetable-field ecosystem, J. Hazard. Mater., 2009, vol. 164, nos. 2−37, pp. 497–501. https://doi.org/10.1016/j.jhazmat.2008.08.020
Sun, G., Zhang, M., Liu, X., Gao, Q, Jiang, W., Zhou, Y., Wang, H., Cui, M., Qiu, J., Xu, J., and Hong, Q., Isolation and characterization of the pymetrozine-degrading strain Pseudomonas sp. BYT-1, J. Agric. Food Chem., 2019, vol. 67, no. 15, pp. 4170–4176. https://doi.org/10.1021/acs.jafc.8b06155
Talebi, K. and Ghazizadeh, H.A., Determination of pymetrozine residues in cucumber, Commun. Agric. Appl. Biol. Sci., 2006, vol. 71, pp. 75–78.
Van Soest, P.J., Rovertson, J.B., and Lewis, B.A., Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition, J. Dairy Sci., 1991, vol. 74, pp. 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Vela, N., Fenoll, J., Garrido, I., Perez-Lucas, G., Flores, P., Hellin, P., and Navarro, S., Reclamation of agro-wastewater polluted with pesticide residues using sunlight activated persulfate for agricultural reuse, Sci. Total Environ., 2019, vol. 660, pp. 923–930. https://doi.org/10.1016/j.scitotenv.2019.01.060
Wyss, P. and Bolsinger, M., Plant-mediated effects on pymetrozine efficacy against Aphids, Pest. Manag. Sci. 2015, vol. 50, pp. 203–210. https://doi.org/10.1002/(SICI)1096-9063(199707)50:3<203::AID-PS584>3.0.CO;2-W
Xu, W.M., Zhang, M., Wei, K., Chen,Y., Liu, Q., Xue, W., Jin, L.-H., He, M., Chen, Z., and Zeng, S., Development and evaluation of pymetrozine controlled-release formulation to control paddy planthopper, RSC Adv., 2018, vol. 8, no. 40, pp. 22687–22693. https://doi.org/10.1039/c8ra03516d
Yildirim, Z., Kayraldiz, A., Donbak, L., et al., Analysis of genotoxicity of pymetrozine in human peripheral lymphocytes, Case Study and Case Report., 2017, vol. 7, pp. 30–42. https://doi.org/10.1515/aiht-2015-66-2584
Yu, J., Xu, E.G., Li, W., Jin, S., Yuan, T., Liu, J., Li, Z., and Zhang, T., Acute toxicity of an emerging insecticide pymetrozine to Procambarus clarkii associated with rice-crayfish culture (RCIS), Int. J. Environ. Res. Public Health. 2018, vol. 15, p. 984. https://doi.org/10.3390/ijerph15050984
Zhang, Y., Zhang, L., Xu, P., Li, J., and Wang, H., Dissipation and residue of pymetrozine in rice field ecosystem, Environ. Monit. Assess., 2015, vol. 187, no. 3, p. 78. https://doi.org/10.1007/s10661-014-4256-x
ACKNOWLEDGMENTS
The authors are grateful to the anonymous reviewers and editors, whose suggestions helped to improve this manuscript.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duc, H.D., Oanh, N.T. Pymetrozine Degradation by an Enrichment Culture from Paddy Soil. Microbiology 93, 324–332 (2024). https://doi.org/10.1134/S002626172360249X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002626172360249X