Abstract
A microbial community, selected by its ability to degrade triazinic herbicides was acclimatized by successive transfers in batch cultures. Initially, its ability to degrade prometryn, was evaluated using free cells or cells attached to fragments of a porous support. As carbon, nitrogen and sulfur sources, prometryn, (98.8 % purity), or Gesagard, a herbicide formulation containing 44.5 % prometryn and 65.5 % of adjuvants, were used. In batch cultures, a considerable delay in the degradation of prometryn, presumptively caused by the elevated concentration of inhibitory adjuvants, occurred. When pure prometryn was used, volumetric removal rates remarkably higher than those obtained with the herbicide formulation were estimated by fitting the raw experimental data to sigmoidal decay models, and differentiating them. When the microbial consortium was immobilized in a continuously operated biofilm reactor, the negative effect of adjuvants on the rate and removal efficiency of prometryn could not be detected. Using the herbicide formulation, the consortium showed volumetric removal rates greater than 20 g m−3 h−1, with prometryn removal efficiencies of 100 %. The predominant bacterial strains isolated from the microbial consortium were Microbacterium sp., Enterobacter sp., Acinetobacter sp., and Flavobacterium sp. Finally, by comparison of the prometryn removal rates with others reported in the literature, it can be concluded that the use of microbial consortia immobilized in a biofilm reactor operated in continuous regime offer better results than batch cultures of pure microbial strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prometryn is a persistent methylthiotriazine herbicide acting as a PSII inhibitor, frequently found in aquatic ecosystems (Schuler and Rand 2008), mainly as a result of terrestrial runoffs or land drainage. Prometryn is listed in the Pesticide Action Network (PAN) Bad Actor Pesticides as a chemical of special concern, in the US EPA Toxics Release Inventory (TRI) List, as a reproductive and developmental toxic compound, and in the prioritization list of the European Union as an endocrine disruptor (PAN Pesticides Database 2013). Because prometryn is a PSII inhibitor, it has a direct impact on photosynthetic aquatic microorganisms. Although microbial communities in freshwater ecosystems are not direct targets, they can be affected by herbicides as a result of the interactions that occur between aquatic microorganisms (Villeneuve et al. 2011). Notwithstanding that herbicides could be found in aquatic environments in low concentrations, a potential hazard of persistent organic pollutants is their bioaccumulation and biomagnification in aquatic organisms essentials in the food web (Geyer et al. 2000; Jin et al. 2012). In addition to pesticides, some adjuvants (solvents, dispersants, surfactants) have been detected in runoffs from irrigated fields. Commonly, these xenobiotics are inappropriately perceived as inert compounds (Pedersen et al. 2003); however, by their chemical characteristics or their potential toxicity, they can have significant effects on the environment. Thus, in remediation processes of soil or water contaminated by pesticides, the removal of adjuvants should also be considered. To reduce the contamination of water bodies by xenobiotic compounds, the use of bio-barriers could be a feasible option to reduce the environmental risk represented by these contaminants. For these reasons, the aims of this work were: (a) to determine the effect that adjuvants have on prometryn biodegradation by free and attached cells of a bacterial consortium growing in batch culture, and (b) to evaluate the performance of a continuously operated biofilm reactor, devised to remove prometryn, together with the adjuvants present in the commercial herbicide Gesagard.
Materials and methods
Chemicals
The herbicide Gesagard was acquired from Syngenta. It contains not less than 44.4 % of prometryn (2,4-Bis(isopropylamino)-6-(methylmercapto)-s-triazine) as the active ingredient; the rest of components are adjuvants (diluents, wetting and dispersing compounds). Standards of prometryn (98.8 %) and cyanuric acid (98.0 %) were acquired from Sigma-Aldrich Inc., Steinheim Germany. The solvents used for HPLC were purchased from J. T. Baker, USA.
Culture media
Along the experimental process, a minimal-mineral-salts medium (MS) was prepared, and its formulation was (in g L−1); K2HPO4, 0.4; MgSO4, 0.1; NaCl, 0.1; CaCl2, 0.02. Five mL of an oligo-elemental solution (in mg L−1, FeSO4·7H2O, 0.55; ZnSO4·7H2O, 0.23; MnSO4·H2O, 0.34; CoCl2·6H2O, 0.065; Na2MoO4·2H2O, 0.34) were added to reach the final concentration.
MS medium plus 32 mg L−1 of equivalent prometryn contained in the commercial herbicide Gesagard (MSG) was used for the selection and batch tests of the microbial consortium able to grow on prometryn. The same medium was fed to the biofilm reactor. The MS medium containing 32 mg L−1 of pure prometryn (MSP) was utilized exclusively for batch tests of the microbial consortium. MSG-Agar (2 %) was used as selective medium for maintenance of the bacterial consortium and the isolated bacterial strains. To assess the growing ability of the bacterial isolates on cyanuric acid, MS medium, plus 50 mg L−1 of the catabolic intermediary was used (MSC). Peptone-yeast extract-agar medium (PYA) was chosen for viable cell counting, and for observation of the morphological diversity of the cultivable microorganisms. For DNA extraction from isolated bacterial strains, they were cultivated in Luria–Bertani medium.
Microorganisms
By successive transfers of soil samples incubated on mineral salt medium complemented with an amount of Gesagard equivalent to 32 mg prometryn L−1, a microbial community, able to grow on this herbicide, was obtained from agricultural soil samples collected at the surroundings of Mexico City. Erlenmeyer flasks containing 50 mL of MSG medium and fragments of volcanic rock were incubated under stirring at room temperature for 96 h. After that time, 45 mL of the medium were drained and substituted with fresh MSG medium. The rock fragments were maintained in the flasks. To verify the herbicide degradation, flasks were periodically sampled, and the remaining prometryn was spectrophotometrically measured. When the removal of the herbicide was evident, suspended cells and cells detached from a sample of the rock fragments were harvested by centrifugation at 13,000 rpm for 5 min. To preserve the microbial community, the pellets obtained after decanting the culture medium were resuspended on 200 μL of glycerol and cryopreserved at −70 °C in a Revco ultra-low freezer (General Signal Laboratory Equipment, Inc., USA). To define the amount of cells to be used as inoculum in batch cultures, the viable cell counting of the colonized porous support and the suspended culture was determined (Macías-Flores et al. 2009).
Prometryn biodegradation in batch culture
To observe the effect of herbicide adjuvants on the prometryn removal kinetics, using free or biofilm-forming microorganisms, the selected consortium was inoculated in 500 mL Erlenmeyer flasks containing 100 mL of MSG or MSP media (for growing free cells). For growing of biofilm-forming microorganisms, the same volume of medium was used, but, fragments of colonized porous support were added to the Erlenmeyer flasks. The flasks were incubated at room temperature under constant agitation in a rotatory shaker (60 rpm). Flasks were periodically sampled for determination of prometryn concentration, using UV spectrophotometry and liquid chromatography (HPLC).
Biofilm reactor
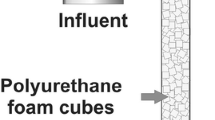
Figure 1 shows a column with a porous glass base (pore diameter of 40–100 μm) packed with fragments of volcanic rock (ϕ = 12.16 ± 4.8 mm) that was utilized as biofilm reactor. The total operating capacity of the bioreactor was 1,670 cm3, and the drained liquid volume was 570 cm3. The column has ports for sampling, input of liquid medium, and output of exhausted air and liquid medium. The column has a glass cover sealed with a neoprene gasket for hermetic operation. Air and liquid medium were concurrently supplied at the base of the packed column. To avoid channeling and redistribute the air along the reactor, a porous plate was inserted in the middle of the column.
Continuous biodegradation of prometryn in the biofilm reactor
To assess the degradation of the herbicide prometryn, the packed bed column was used. Prior to the inoculation, an abiotic test was conducted. To saturate the support material with the herbicide, the reactor was fed with MSG medium at a flow rate of 0.013 L h−1; concurrently, the reactor was aerated at a gas flow rate of 0.15 L min−1. Changes in prometryn concentration, in the outflowing liquid, were spectrophotometrically measured at λ = 222 nm. It was considered that the support was saturated when the value of absorbance in the effluent reached the value of the MSG medium supplied. At this moment, the reactor was inoculated with the microbial consortium. After 4 days of batch culture, it was considered that the porous support was colonized by the microbial consortium, and the bioreactor begun to operate in continuous regime at different herbicide loading rates (R V = F C i /V L ). In this expression, C i is the input concentration of prometryn or COD, and V L is the liquid volume of the reactor. Along the reactor operation, the outflowing liquid was periodically sampled to measure the chemical oxygen demand (COD) and the concentrations of prometryn and cyanuric acid (1,3,5-triazine-2,4,6-triol; [OOOT]), which usually is the main intermediary of the catabolism of prometryn. Once a steady state was reached, samples of liquid medium were taken, and a new loading rate was probed.
Identification of bacterial isolates
Once finished the operation of the reactor, it was dismantled, and samples recovered from colonized fragments of the porous support were used for the isolation of the main constituents of the biofilm-forming microorganisms. After recovering the attached cells, they were resuspended in distilled water. The cell suspension obtained was diluted, and the higher decimal dilutions were plated in PYA medium. Once growth was observed, the isolated colonies showing morphological differences were selected and conserved in agar slants of MSG medium. To obtain a cell package suitable for DNA extraction, the bacterial isolates were propagated in Luria–Bertani liquid culture medium. The extracted and purified DNA was used for PCR amplification of the 16S rDNA, using the 8FPL and 149RPL primers (Relman 1993). The amplicons were purified (Wizard Genomic DNA Purification Kit, Promega Co., USA). After amplicons sequencing (Macrogen Inc., Seoul, South Korea), and comparison with the sequences deposited in the NCBI gene bank, the isolated strains were identified. 16S rDNA sequences were registered at the NCBI GenBank. The assigned accession numbers were KF619443 for Enterobacter sp., KF619444 for Acinetobacter sp., KF619445 for Microbacterium sp. and KF619446 for the Flavobacteriaceae bacterium. The partial sequences of the 16S rDNA genes for these bacterial isolates were aligned using Clustal X (Larkin et al. 2007), and the corresponding phylogenetic trees were constructed using the neighbor-joining method (Tamura et al. 2011).
Analytical methods
Spectrophotometric determination of prometryn
For rapid evaluation of transient changes in the bioreactor after a loading rate shift, the concentrations of prometryn were determined by measuring the absorbance of the outflowing medium at λ = 222 nm in a Beckman DU650 spectrophotometer.
Determination of cyanuric acid
A turbidimetric Hach method 8139 (Hach 2012) was used for rapid determination of cyanuric acid. The reactive kit used could determine cyanuric acid levels of 5–50 mg L−1.
Determination of prometryn and cyanuric acid by HPLC
From sample filtrates, prometryn and cyanuric acid were determined by liquid chromatography using a Shimadzu HPLC System (Shimadzu LC-10AT). For prometryn determination, the system was equipped with a LiChrospher C18 column (5 μm, 150 × 4.6 mm), and a UV detector (222 nm). The flow rate of the mobile phase was 1.0 mL min−1. The chromatographic separation was performed using a linear gradient of 10 mM phosphate buffer (pH 7.0) with acetonitrile, increasing from 30 to 70 % in 12 min (Marja et al. 2008). For cyanuric acid determination, the system was equipped with an an Inerstil column (5 μm, 150 × 4.6 mm) and a UV detector (280 nm). An isocratic mobile phase (octan-sodium sulfonate 5 mmol L−1 in 0.05 % H3PO4, pH 2.8) was fed at a flow rate of 1.0 mL min−1 (Galíndez-Nájera et al. 2009).
Chemical oxygen demand (COD)
A closed reflux method 8000 (Hach 2012) was used for COD determination. The reactive kit used could determine COD levels from 0.7 to 40 mg L−1.
Results and discussion
Prometryn biodegradation in batch culture
Figure 2 shows the removal kinetics of prometryn in submerged batch cultures using free cells or cells attached to fragments of the porous support immersed in the culture medium. The graph shows a fast degradation of pure prometryn, which disappears in 9.0 h. The possible effect of the adjuvants of the herbicide formulation on prometryn degradation can be observed in the same graph. Although the culture was extended to more than 100 h, prometryn was not entirely removed. On the other hand, differences in the viable cell count along the batch cultures using Gesagard or pure prometryn are shown in Table 1. Notwithstanding that the initial cell count was greater when Gesagard was used as substrate, a clear delay in prometryn degradation took place.
Effect of adjuvants on prometryn removal in a submerged batch culture using free or immobilized cells. Removal of pure prometryn by free (filled circles) or immobilized cells (void circles). Prometryn removal in the Gesagard formulation containing adjuvants by free (filled diamonds) or immobilized cells (void triangles). Curves described by the logistic Eq. 1, using the parameters shown in Table 1. Immobilized cells (dotted lines). Free cells (solid lines)
After fitting the batch experimental data to the empirical logistic decay model (1), and then, by differentiation of the mathematical function, the behavior of the volumetric removal rates of prometryn R V,P can be estimated.
In this case, the model (1) is viewed as a purely empirical function, meaning that the equation parameters a, b, c, m, and z shown in Table 2, lack of biological or physical implications. Under these circumstances, the model was used only as a differentiable intermediate function (Marrón-Montiel et al. 2006; Arino et al. 2006). In this model, p i is the initial prometryn concentration, and p(t) is the prometryn concentration at the time t.
This equation was differentiated to obtain the transient behavior of the volumetric removal rate R V,P (t) of prometryn along the distinct batch cultures (Eq. 2)
Figure 3 shows the curves described by Eq. (2). In this figure, differences in the degradation kinetics of prometryn by free or attached cells can be observed. When the bacterial consortium grew on the MSP medium, the prometryn removal rates reached maximal values of 5.8 and 6.3 mg L−1 h−1, for free and attached cells, respectively. When the consortium was grown in the herbicide Gesagard, which contains prometryn plus adjuvants, the kinetic behavior of the consortium changed. In addition to the delay in prometryn degradation, a noteworthy decay in the top removal rates of free and attached cells, 0.78 and 1.22 mg L−1 h−1, respectively, was observed.
Effect of adjuvants on the instantaneous removal rates of prometryn (R V,P ) in submerged batch culture using free or immobilized cells. Curves described by Eq. 2, using the parameters shown in Table 1. Change in R V,P of pure prometryn by free (black solid line) or immobilized cells (black dotted line). Change in R V,P of prometryn in the Gesagard formulation containing adjuvants by free (gray solid line) or immobilized cells (gray dotted line)
Table 3 shows the final removal efficiencies of prometryn (η P ) determined by HPLC, COD (η COD), and the catabolic intermediary, cyanuric acid (η OOOT). These values were obtained in batch cultures of free or immobilized cells when the microbial consortium was grown on prometryn pure (t f = 9.0 h), or in the commercial formulation of the herbicide Gesagard, containing prometryn and adjuvants (t f = 104 h). Notwithstanding the larger time used for prometryn degradation contained in the Gesagard medium, the removal efficiencies of cyanuric acid were lower than those obtained when prometryn pure was totally degraded in a shorter time. Possibly, adjuvants were preferentially used as carbon sources; and mainly prometryn, not the refractory cyanuric acid, was used as nitrogen source for growth of the microbial consortium. In some cases, adjuvants could serve as co-substrates, facilitating the cometabolic degradation of recalcitrant compounds; also, non-ionic surfactants could augment the bioavailability of hydrophobic compounds increasing their biodegradation rates, and removal efficiencies. However, as occurred in this case, adjuvants negatively affect the biodegradation of recalcitrant compounds (Krogh et al. 2003; Ma et al. 2004; Ostroumov 2006).
Biodegradation of prometryn in the continuously operated biofilm reactor
When the concentration of presumptively inhibitory adjuvants was relatively high, at the beginning of the batch cultures, a delay in the degradation of prometryn was observed. In continuous regime, the substrate concentration, in steady-state operation, is certainly smaller than the concentration in the inflowing medium; therefore, it was considered that the operation of the packed bed reactor in continuous regime should lessen the negative effect that the adjuvants present in the medium MSG have on prometryn removal rates end efficiencies.
When the packed-bed biofilm reactor was continuously fed with MSG medium, eight flow rates were probed. Table 4 shows the changes in the operational characteristics of the continuously operated biofilm reactor along 6,000 h of operation.
The initial flow rate used was 0.06 L h−1, corresponding to a hydraulic retention time HRT = V L /F = 9.50 h and volumetric loading rates (B V ) of prometryn, COD and OOOT of 3.37, 14.79 and 1.80 g m−3 h−1, respectively. This flow rate was maintained until no appreciable change in concentrations was observed, meaning that a steady state was reached in the bioreactor. Then, different HRT values were probed. In Fig. 4 the chronological variations in the input and output concentrations of prometryn, COD and detached cells are shown for the different loading rates and HRTs used. The biofilm dynamics is relatively complex; it includes biomass attachment, growth, decay, lysis and detachment. Even when biofilm is weakened by lysis and biomass detachment, which is often caused by combined fluid shear and pressure forces acting on the biofilm, the biofilm growth remains balanced (Bottero et al. 2013).
The major variations in prometryn and COD were observed at the start-up and the end of the degradation process. At the highest loading rates, a decrease in the detached cells concentration was observed, possibly caused by the interstitial dilution rate in the packed bed.
Although at all the B V,P values tested the prometryn was totally removed from the liquid medium, part of the herbicide was biotransformed to cyanuric acid (OOOT). At low prometryn loading rates and high HRT values, about 44 % of the heterocyclic ring was degraded; this fraction gradually diminished to less than 10 % when B V,P values were increased, and HRT values were lowered. Evidently, an increase in the herbicide loading rate brings an increase in the supply of inhibitory adjuvants. This fact could help to explain the increase in OOOT accumulation and the lowering in COD removal at high loading rates in the PBR.
Although the PBR was continuously fed with the herbicide Gesagard, containing inhibitory adjuvants, the prometryn removal rates obtained were significantly higher than those reached in batch culture, even when the microbial consortium was batch cultivated in pure prometryn (Fig. 3)
To compare the behavior of the volumetric removal rates of prometryn, COD and OOOT (R V,P , R V,COD and R V,OOOT), at the different operational conditions, the corresponding volumetric removal rates were expressed in relative terms. The relative volumetric removal rates (R VR = R V /R VMAX ) were plotted as a function of the relative loading rates (B VR = B V /B VMAX ) (Fig. 5, upper graph). The highest loading rates probed were 20.2, 88.7 and 10.8 g m−3 h−1 for prometryn, COD and OOOT, respectively. The removal efficiencies of prometryn, COD, and the catabolic intermediary OOOT obtained in eight runs, are also shown in Fig. 5, lower graph. It is clearly appreciated that at all the HRTs probed prometryn was completely removed, though, at the lowest B V,P values, about 56 % of the prometryn was biotransformed to cyanuric acid. This fraction is increased to more than 90 % at the highest loading rates probed. The behavior of the COD removal rate observed in Fig. 5 denotes that besides prometryn, other compounds able to be chemically oxidized, presumptively adjuvants, were efficiently removed. In contrast, as a result of the recalcitrant nature of the triazinic ring species (Watanabe et al. 2005), the cyanuric acid that was not removed could not be determined by the COD technique.
Few processes regarding prometryn biodegradation can be found in the literature. Some of them were carried out in batch culture using free cells of Streptomyces sp. (Shelton et al. 1996), Arthrobacter aurescens (Strong et al. 2002), Rhodococcus sp. (Fujii et al. 2007) and Nocardioides sp. (Satsuma 2010). Generally, the best form to evaluate the microbial capacity to degrade a compound is through its specific removal rate (R V,X = R V,P /X), which is the decay rate expressed per unit of cell mass (X). Unfortunately, this rate could not be estimated with the kinetic data reported for prometryn degradation. Nevertheless, some works report removal efficiencies (η P ) or volumetric removal rates of prometryn (R V,P ), that can be compared with the present work. The highest prometryn removal rate reported, for a strain of Streptomyces sp., was 3.3 mg L−1 h−1, with a η P value of 100 % (Shelton et al. 1996). In the present work, the maximum R V,P value obtained in the continuously operated biofilm reactor containing an acclimated microbial consortium was 20.2 mg L−1 h−1, with a η P value of 100 %. On this basis, the use of a microbial community immobilized in a biofilm reactor operated in continuous regime offer better results than batch cultures of pure microbial strains.
Identification of the bacterial isolates
Four cultivable bacterial strains were isolated from the biofilm formed in the support material at the end of acclimatization in batch culture. They were identified by sequencing and comparison with known 16S rDNA sequences at the NCBI GenBank. The identified bacteria were Enterobacter sp. (FJ472852.1, 98 % similarity), Acinetobacter sp. (HM246137.1, 98 % similarity), Microbacterium sp. (HM234007.1, 97 % similarity), and an uncultured Flavobacteriaceae bacterium (JQ328000 0.1, 96 % similarity).
Based on the results shown in Fig. 6, it was verified that the isolates KF619443, KF619444, KF619445 and KF619446 belong phylogenetically to the genera Enterobacter, Acinetobacter, Microbacterium and Flavobacterium. Since bacterial strains presenting 16S rDNA gene similarities between 97 and 99.5 % may belong to different species (Ramani et al. 2012), definitive identification of these isolates may require additional biochemical tests, or sequencing of additional gene loci.
Growth on prometryn of the bacterial isolates
The ability of the individual bacterial strains to grow on MSG medium, containing the herbicide Gesagard, on MSP medium, containing pure prometryn, and MSC medium, containing the metabolic intermediary cyanuric acid was evaluated.
Enterobacter and Acinetobacter had the ability to use all substrates for growth. Microbacterium grew well on MSG and MSP media, but not in the medium containing cyanuric acid, and the bacterium of the Flavobacteriaceae family could not grow in any of the tested substrates. However, it is known that microorganisms belonging to the family Flavobacteriaceae are able to form biofilms (Basson et al. 2008; Jacobs and Chenia 2011); thus, a possible role of this bacterium in the microbial consortium is biofilm formation.
Several xenobiotic compounds are degraded by members of the genera Microbacterium (Wang et al. 2009; Plotnikova et al. 2006), Enterobacter (French et al. 1998; Kryuchkova et al. 2013), and Acinetobacter (Lee et al. 2010; Singh et al. 2004; Sánchez-Sánchez et al. 2013), but, to our best knowledge, no strains of these genera have been reported as able to degrade prometryn.
Conclusions
In batch cultures, a considerable delay in the degradation of prometryn, presumptively caused by the elevated concentration of inhibitory adjuvants, was estimated by fitting raw experimental data to sigmoidal decay models, and differentiating them. This procedure could be a practical tool to analyze the kinetics of transient biodegradation processes.
In comparison with batch cultures, the substrate concentration, in steady-state continuous culture, is certainly smaller than the concentration in the inflowing medium; therefore, when the packed bed reactor was operated in continuous regime the negative effect of the adjuvants on the rates and removal efficiencies of prometryn was not observed.
Finally, by comparison of the prometryn removal rates reported in the literature, it can be concluded that the use of microbial communities immobilized in a biofilm reactor operated in continuous regime offer better results than batch cultures of pure microbial strains.
References
Arino J, Wang L, Wolkowicz GSK (2006) An alternative formulation for a delayed logistic equation. J Theor Biol 241(1):109–119. doi:10.1016/j.jtbi.2005.11.007
Basson A, Flemming LA, Chenia HY (2008) Evaluation of adherence, hydrophobicity, aggregation, and biofilm development of Flavobacterium johnsoniae-like isolates. Microb Ecol 55:1–14. doi:10.1007/s00248-007-9245-y
Bottero S, Storck T, Heimovaara, TJ, van Loosdrecht MCM, Enzien MV, Picioreanu C (2013) Biofilm development and the dynamics of preferential flow paths in porous media. Biofouling (in press)
French CE, Nicklin S, Bruce NC (1998) Aerobic degradation of 2,4,6-trinitrotoluene by Enterobacter cloacae PB2 and by pentaerythritol tetranitrate reductase. Appl Environ Microbiol 64:2864–2868
Fujii K, Takagi K, Hiradate S, Iwasaki A, Harada N (2007) Biodegradation of methylthio-s-triazines by Rhodococcus sp. strain FJ1117YT, and production of the corresponding methylsulfinyl, methylsulfonyl and hydroxy analogues. Pest Manag Sci 63:254–260. doi:10.1002/ps.1331
Galíndez-Nájera SP, Llamas-Martínez MA, Ruiz-Ordaz N, Juárez-Ramírez C, Mondragón-Parada ME, Ahuatzi-Chacón D, Galíndez-Mayer J (2009) Cyanuric acid biodegradation by a mixed bacterial culture of Agrobacterium tumefaciens and Acinetobacter sp. in a packed bed biofilm reactor. J Ind Microbiol Biotechnol 36:275–284. doi:10.1007/s10295-008-0496-5
Geyer HJ, Rimkus GG, Scheunert I, Kaune A, Schramm K-W, Kettrup A, Zeeman M, Muir DCG, Hansen LG, Mackay D (2000) Bioaccumulation and occurrence of endocrine-disrupting chemicals (EDCs), persistent organic pollutants (POPs), and other organic compounds in fish and other organisms including humans. In: Beek B (ed) The handbook of environmental chemistry, Vol. 2 Part J. Bioaccumulation, vol 2. Springer, Berlin, pp 1–166
Hach (2012) Hach Water Analysis Handbook. Hach Company, Loveland
Jacobs A, Chenia HY (2011) Biofilm formation and adherence characteristics of an Elizabethkingia meningoseptica isolate from Oreochromis mossambicus. Ann Clin Microbiol Antimicrob 10:16. doi:10.1186/1476-0711-10-16
Jin ZP, Luo K, Zhang S, Zheng Q, Yang H (2012) Bioaccumulation and catabolism of prometryne in green algae. Chemosphere 87:278–284. doi:10.1016/j.chemosphere.2011.12.071
Krogh KA, Halling-Sørensen B, Mogensen BB, Vejrup KV (2003) Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review. Chemosphere 50:871–901. doi:10.1016/S0045-6535(02)00648-3
Kryuchkova YV, Burygin GL, Gogoleva NE, Gogolev YV, Chernyshova MP, Makarov OE, Fedorov EE, Turkovskaya OV (2013) Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol Res. doi:10.1016/j.micres.2013.03.002
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Lee JK, Lee WJ, Cho Y-J, Park DH, Lee Y-W, Chung J (2010) Variation of bacterial community immobilized in polyethylene glycol carrier during mineralization of xenobiotics analyzed by TGGE technique. Korean J Chem Eng 27:1816–1821. doi:10.1007/s11814-010-0291-7
Ma J, Lin F, Zhang R, Yu W, Lu N (2004) Differential sensitivity of two green algae, Scenedesmus quadricauda and Chlorella vulgaris, to 14 pesticide adjuvants. Ecotoxicol Environ Safe 58:61–67. doi:10.1016/j.ecoenv.2003.08.023
Macías-Flores A, Tafoya-Garnica A, Ruiz-Ordaz N, Salmerón-Alcocer A, Juárez-Ramírez C, Ahuatzi-Chacón D, Mondragón-Parada ME, Galíndez-Mayer J (2009) Atrazine biodegradation by a bacterial community immobilized in two types of packed-bed biofilm reactors. World J Microbiol Biotechnol 25:2195–2204. doi:10.1007/s11274-009-0125-0
Marja TK, Kaoukonen S, Kilpi-Koski J, Malin I, Kairesalo T, Romantschuk M, Tuominen J, Kontro MH (2008) Atrazine and terbutryne degradation in deposits from groundwater environment within the boreal region in Lahti, Finland. J Agric Food Chem 56:11962–11968. doi:10.1021/jf802528a
Marrón-Montiel E, Ruiz-Ordaz N, Rubio-Granados C, Juárez-Ramírez C, Galíndez-Mayer CJ (2006) 2,4-d-degrading bacterial consortium isolation, kinetic characterization in batch and continuous culture and application for bioaugmenting an activated sludge microbial community. Process Biochem 41:1521–1528. doi:10.1016/j.procbio.2006.02.012
Ostroumov SA (2006) Biological effects of surfactants. CRC Press, Boca Raton, pp 127–145
PAN Pesticides Database (2013) http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC34259. Accessed 07 July 2013
Pedersen JA, Yeager MA, Suffet IH (2003) Xenobiotic organic compounds in runoff from fields irrigated with treated wastewater. J Agric Food Chem 51:1360–1372. doi:10.1021/jf025953q
Plotnikova EG, Rybkina DO, Anan’ina LN, Yastrebova OV, Demakov VA (2006) Characteristics of microorganisms isolated from technogenic soils of the Kama region. Russ J Ecol 37:233–240. doi:10.1134/S1067413606040035
Ramani A, Rein K, Shetty KG, Jayachandran K (2012) Microbial degradation of microcystin in Florida’s freshwaters. Biodegradation 23(1):35–45. doi:10.1007/s10532-011-9484-y
Relman DA (1993) Universal bacterial 16S rDNA amplification and sequencing. In: Persing HD, Smith TF, Tenover CF, White ST (eds) Diagnostic molecular microbiology. Principles and applications. ASM Press, Washington, pp 489–495
Sánchez-Sánchez R, Ahuatzi-Chacón D, Galíndez-Mayer J, Ruiz-Ordaz N, Salmerón-Alcocer A (2013) Removal of triazine herbicides from aqueous systems by a biofilm reactor continuously or intermittently operated. J Environ Manag 128:421–426. doi:10.1016/j.jenvman.2013.05.050
Satsuma K (2010) Mineralization of s-triazine herbicides by a newly isolated Nocardioides species strain DN36. Appl Microbiol Biotechnol 86:1585–1592. doi:10.1007/s00253-010-2460-3
Schuler LJ, Rand GM (2008) Aquatic risk assessment of herbicides in freshwater ecosystems of South Florida. Arch Environ Contam Toxicol 54:571–583. doi:10.1007/s00244-007-9085-2
Shelton D, Khader J, Pogel B (1996) Metabolism of twelve herbicides by Streptomyces. Biodegradation 7(2):129–136. doi:10.1007/BF00114625
Singh P, Suri CR, Cameotra S (2004) Isolation of a member of Acinetobacter species involved in atrazine degradation. Biochem Biophys Res Commun 317:697–702. doi:10.1016/j.bbrc.2004.03.112
Strong LC, Rosendahl C, Johnson G, Sadowsky MJ, Wackett LP (2002) Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl Environ Microbiol 68:5973–5980. doi:10.1128/AEM.68.12.5973- 5980.2002
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Fumar S (2011) MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Villeneuve A, Larroudé S, Humbert JF (2011) Herbicide contamination of freshwater ecosystems: impact on microbial communities. In: Stoytcheva M (ed) Pesticides: formulations, effects, fate. InTech. Rijeka, Croatia, pp 285–312
Wang C, Li D, Wang C (2009) Naphthalene, phenanthrene, anthracene and pyrene by Microbacterium sp. Chin J Appl Environ Biol 15:361–366. doi:10.3724/SP.J.1145.2009.00361
Watanabe N, Horikoshi S, Kawasaki A, Hidaka H, Serpone N (2005) Formation of refractory ring-expanded triazine intermediates during the photocatalyzed mineralization of the endocrine disruptor amitrole and related triazole derivatives at UV-irradiated TiO2/H2O interfaces. Environ Sci Technol 39:2320–2326. doi:10.1021/es049791l
Acknowledgments
This work was supported by a grant obtained from SIP, IPN. The authors wish to thank COFAA-IPN and SNI-Conacyt for fellowships to D. Ahuatzi-Chacón, N. Ruiz-Ordaz, C. Juárez-Ramírez and J. Galíndez-Mayer; and to SIP-IPN for the financial support of J. F. Pérez-Bárcena and K. L. Castillo-Martínez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Bárcena, J.F., Ahuatzi-Chacón, D., Castillo-Martínez, K.L. et al. Effect of herbicide adjuvants on the biodegradation rate of the methylthiotriazine herbicide prometryn. Biodegradation 25, 405–415 (2014). https://doi.org/10.1007/s10532-013-9669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-013-9669-7