Abstract
Thiamethoxam is widely used to control pests, but it is harmful to nontargeted organisms and environments. In this study, the degradation of the insecticide was determined in liquid media, maize straw, and soil by fungal and bacterial strains, Phanerochaete sp. Th1 and Ensifer sp. Th2, isolated from soil. Both isolates utilized the compound used as a sole carbon, nitrogen, and sulfur source. The inoculation of both strains enhanced degradation rates compared to those of any single isolate. For example, the determination kinetics showed that the maximum degradation rates of Phanerochaete sp. Th1, Ensifer sp. Th2, and mixed culture of them were 0.53 ± 0.05, 0.74 ± 0.07, and 0.81 ± 0.08 mg/day, respectively. In addition, Phanerochaete sp. Th1 showed effective degradability towards hemicellulose, cellulose, and lignin in maize straw. Moreover, the inoculation of both strains increased thiamethoxam degradation in maize straw during solid-state fermentation. The inoculation of isolated strains also enhanced degradation in soil. The determination of metabolites and Cl– generated during thiamethoxam degradation showed that the fungus dechlorinated initially, whereas the bacterial strain removed Cl– after some transformation steps. This study demonstrates that isolated fungal and bacterial strains are suitable for thiamethoxam degradation in liquid media, rice straw, and soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neonicotinoids are the most widely used class of insecticides worldwide [1], with applications in over 120 countries [2]. Thiamethoxam having formula (E)-N-(3-(2-chlorothiazol-5-yl)methyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene)nitramide, is a neonicotinoid widely used in the agricultural sector to protect crops owing to its highly selective activity in controlling insects. Neonicotinoids cause reproductive and hormonal toxicity, genotoxicity, neurotoxicity, hepatotoxicity, and immunotoxicity in vertebrates [3]. Thiamethoxam harms many species, including birds, fish, and non-target insects [4], and invertebrates [5]. Moreover, thiamethoxam significantly affects microbial diversity and changes the bacterial community structure [6–8].
Thiamethoxam migrates through runoff and accumulates in water and soil owing to high water solubility and low soil adsorption. The compound has been detected up to 7.44 mg/kg in soil [9], 20.1–225 µg/L in freshwater [5], 67% of samples collected from the central Wisconsin groundwater [10]. Thiamethoxam exhibits environmental persistence, with the half-time varying from months to years [11]. Therefore, the elimination of thiamethoxam in contaminated environments is needed urgently.
Some studies on using physicochemical methods to eliminate thiamethoxam have been conducted. Examples include plasma discharge and TiO2 photocatalysis [12], heat-activated and ultrasound-activated persulfate [13], and sulfate-doped Ag3PO4 [14]. Biodegradation by microorganisms is a natural and effective method for eliminating organic pollutants. Several white-rot fungi and bacterial strains have been isolated and determined for thiamethoxam degradation, such as Pseudomonas sp. 1G [15], Ensifer adhaerens [16], Bacillus aeromonas, Pseudomonas putida [17], Enterobacter cloacae [18], Phanerochaete chrysosporium [19], P. aeruginosa, P. putida, P. fluorescens [20], and Labrys portucalensis [21]. The degradation pathways have also been analyzed in previous studies. However, only a few studies on the augmentation of thiamethoxam degradation in soil using inoculated microbial strains have been reported [22].
Composting has been applied using microorganisms to degrade maize straw components, that is, hemicellulose, cellulose, and lignin [23, 24]. Municipal solid waste composting was applied to degrade several pesticides, namely thiamethoxam, clothianidin, fludioxonil, and E-azoxystrobin [25]. He et al., [26] showed that applying a pesticide in a maize field caused the contamination of thiamethoxam in soil, maize straw, and maize cob. The contamination by thiamethoxam in the maize straw was 0.31–0.40 mg/kg and remained in the tissues for up to 30–40 days [26]. This study aimed to (1) isolate and characterize fungal and bacterial strains from soil using thiamethoxam as a sole carbon, nitrogen, and sulfur source, (2) apply them to degrade maize straw components (including both chopped corn straw and corn stalks) and thiamethoxam during solid-state fermentation, (3) determine the thiamethoxam degradation in soil by the isolated microbial strains, and (4) identify the intermediate products of thiamethoxam degradation.
MATERIALS AND METHODS
Enrichment and isolation of thiamethoxam-degrading microorganisms. Cultivated soil samples were collected from a maize field in Dong Thap Province (10°36′43.1′′ N 105°22′02.2′′ E), Viet Nam, where farmers have extensively used pesticides. The soil was transferred to the laboratory within the day. The soil samples were mixed, pulverized, and sieved through 2-mm mesh before determining the physicochemical properties. The soil contained 45.5 ± 4.1% sand, 22.1 ± 2.0% silt, and 32.4 ± 2.8% clay. The pH of soil was 6.5 ± 0.4. Other chemical components included 2.5 ± 0.2% total C, 0.16 ± 0.0% total N, 33.7 ± 3.1 ppm P2O5, and 12.5 ± 1.0 ppm K2O.
Next, soil samples, each weighing 500 g, were transferred to plastic containers (length × width × depth = 15 × 25 × 20 cm). Dry soil was supplemented with 2 mg/kg thiamethoxam and incubated for 25 days, then with 5 mg/kg thiamethoxam for 25 days, and subsequently, 10 mg/kg thiamethoxam for 25 days. Sterile water was sprinkled at a soil moisture content of 50%. The container was capped with a plastic cover and placed in the dark at room temperature (~30°C). During the incubation process, sterile water was added to maintain the soil moisture, and the soil was mixed every five-day period.
After soil enrichment, 2 g of soil was dispensed in 500 mL flask containing 200 mL mineral medium (MM) supplemented with 10 mg/L thiamethoxam. After incubating for 10 days, 2 mL of mixture was transferred into new a portion of the MM supplemented with thiamethoxam at the same concentration. The process was conducted for 3 consecutive cycles, and the liquid medium was used to isolate thiamethoxam-degrading microorganisms.
For isolation, the enriched liquid media were diluted and spread onto agar plates of MM (2% agar, wt/vol) supplemented with 10 mg/L thiamethoxam as a sole carbon, nitrogen, and sulfur source. The plates were incubated for 10 days at room temperature. Emerged colonies of bacteria and fungi were purified and tested for their ability to degrade thiamethoxam. The bacterial strains were identified using the method described by Ha [27]. For the isolated fungal strain, the region of the internal transcribed spacers (ITS) of the ribosomal DNA (rDNA) was amplified with universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR products were sequenced by Bioneer Corporation (South Korea). The similarities in the sequences of the rDNA of bacterial strains and ITS fragments were identified by running the BLAST search in the EzBioCloud.
Thiamethoxam degradation in mineral medium (MM). Culture media. The MM was used to isolate bacteria and thiamethoxam degradation. The components of the MM included (g/L): Na2HPO4—2.79, KH2PO4—1.00, MgCl2·6H2O—0.20, and 1.0 mL of trace mineral solution. The trace mineral solution consisted of (g/L): H3BO3—0.30, FeCl2·6H2O—0.20, ZnCl2·7H2O—0.10, Na2MoO4·2H2O—0.03, MnCl2·4H2O—0.03), and CuCl2·2H2O—0.01. Ammonium sulfate was used as an additional nitrogen and sulfur source at 0.5 g/L. The pH was adjusted to 7.0 ± 0.1 using HCl and NaOH. The solid medium was obtained by adding 2.0% agar. All media were autoclaved at 121°C for 15 min.
Compatibility assay among isolated strains. An agar plate with MM supplemented with 10 mg/L thiamethoxam was inoculated in the middle with the hyphae of the fungal isolate. The plate was incubated at room temperature for 5 days when the fungal hyphae formed a colony with diameter of approximately 2 cm. The plate was inoculated with the bacterial strain approximately 1 cm from the fungal hyphae using a sterile toothpick and incubated for additional 5 days. Their antagonistic effects were exhibited based on the inhibition zone formation.
Thiamethoxam utilization by individual isolated strains and mixed culture. The bacterial strain was cultured in MM supplemented with 10 mg/L thiamethoxam for 15 days. The culture containing approximately 1.55 × 108 CFU/mL, was used for inoculation. A fresh MM was added with the spent medium to approximately 106 CFU/mL. The fungus was cultivated on potato dextrose agar slants at 30°C for 7 days. Formed spores were gently scraped from the agar surface and suspended in sterile distilled water, 106 spores/mL, before inoculating the MM. For the degradation experiments by a mixture, the bacterial strain and fungus had 0.5 × 106 CFU/mL and 0.5 × 106 spores/mL, respectively. The utilization of thiamethoxam by individual isolated strains and mixed culture of bacterial and fungal strains was conducted in the MM at thiamethoxam concentrations of 2, 10, 25, and 50 mg/L. The incubation was conducted at room temperature and a shaking speed of 150 rpm. The culture samples were collected to determine the remaining substrate and produced metabolites.
For the determination of utilization kinetics, thiamethoxam was supplemented at various concentrations (from 0.5 to 100 mg/L) in the MM with ammonium sulfate. The obtained results were used to calculate kinetic parameters.
Degradation of thiamethoxam and maize straw components during solid-state fermentation. The maize straw was obtained from the maize field at which soil was collected as described above after the autumn harvest and chopped to 0.5–1.0 cm sizes. The straw was dried at 45oC for 4 days. For the experiment with sterile control, the straw was autoclaved at 121°C for 15 min. The main components of the maize straw were as follows: 37.4 ± 1.5% cellulose, 28.7 ± 1.1% hemicellulose, 9.58 ± 1.0% lignin, 10.8 ± 0.9% moisture, 5.1 ± 0.3% ash, 40.5 ± 3.3% carbon, and 4.7 ± 0.6% nitrogen. Two hundred g of maize straw mixture in the dry state was transferred into a plastic box as described above. Thiamethoxam was spiked at 2 and 10 mg/kg in dry straw. The moisture content was set to approximately 50% mass, monitored, and adjusted during fermentation.
The isolated bacterial strain was cultured in the MM supplemented with 10 mg/kg thiamethoxam for 15 days, collected by centrifuging for 10 min at 6800 g, and resuspended in the fresh MM to 108 CFU/mL. For the fungus inoculation, the process was conducted as described above. Bacterial strain or fungus was inoculated to the maize straw at 106 CFU/g and 106 spores/g dry straw, respectively. For the inoculation of their mixed culture, each was 0.5 × 106 CFU/g and 0.5 × 106 spores/g dry straw, respectively. The incubation was conducted for the enrichment process for 25 days, as described above.
Thiamethoxam degradation in soil. Soil samples were collected from the maize field, transferred to the laboratory, and processed as described above. The soil treatment was conducted using free and immobilized cells. The treatment using free cells was performed as the fermentation method. For the immobilized cells, individual bacterial and fungal strains or mixture of them were suspended in 500 mL flasks containing 200 mL of MM to approximately 109 CFU/mL. A maize straw mixture weighing 100 g in the dry state was added to each flask. The flask was shaken at 50 rpm for 24 h at room temperature. The liquid medium was removed, and the straw was rinsed twice with fresh MM. The numbers of bacteria and fungi immobilized in the maize straw were determined based on the CFU/mL in the liquid medium.
The bacterial strain, fungus, or their mixed culture were inoculated at 106 CFU/g dry soil. Thiamethoxam was spiked at 2.0 mg/kg of dry soil (as the recommended dosage in crops [8]) and 10 mg/kg of dry soil. The moisture content was controlled at approximately 50% mass. Thiamethoxam concentrations in the soil were determined after 25 days of incubation.
Analytical methods. Thiamethoxam concentrations were determined by HPLC described by Ha [27], and the degradation products were analyzed using LC–MS described by Zhou et al., [16].
For thiamethoxam extraction, the soil, straw, and soil with straw samples of 10.0 g each were pulverized using a mortar and pestle, transferred into 50 mL centrifuge tube containing 10 mL acetonitrile and 5 mL deionized water. The tube was vortexed for 5 min and shaken at 500 rpm for 30 min. The liquid media were collected and filtered using a 0.22 µm syringe filter (Merck, Germany) to determine the substrate concentrations. The extraction efficiencies from the soil and straw were 94.7 and 91.6%, respectively.
Hemicellulose, cellulose, and lignin contents of maize straw were determined using the Van Soest’s method [28]. Hemicellulose was estimated based on the difference between the neutral-detergent and the acid-detergent fibers, whereas cellulose was determined as the difference between the acid-detergent fiber and the acid-detergent lignin. Lignin was estimated as the difference between the detergent lignin and the ash content.
Statistical analysis. The data obtained from at least three replicates were shown as the means ± standard deviation. The variance and the significant differences were calculated using Duncan’s test in SPSS software program version 22.0.
RESULTS AND DISCUSSION
Isolation and identification of fungal and bacterial strains degrading thiamethoxam. After enrichment and isolation, several fungal and bacterial strains degrading the thiamethoxam were isolated. A fungal strain and a bacterial strain used the herbicide as a sole carbon, nitrogen, and sulfur source to grow. Based on a BLAST search of the sequences, they are closely related to Phanerochaete sp. and Ensifer sp., respectively. The strains were Phanerochaete sp. Th1 and Ensifer sp. Th2, respectively. The isolates have been deposited at the Culture Collection in the Center for Biochemical Analysis (Dong Thap University, Viet Nam) with deposition numbers BTh1-2022 for Phanerochaete sp. Th1 and BTh2-2022 for Ensifer sp. Th2. In addition, the 16S rDNA sequences of them have been deposited to GenBank under accession numbers OQ592854 and OQ592855, respectively.
Thiamethoxam degradation by fungus and bacteria in liquid media. Before the experiment, the compatibility of the isolated strains was analyzed. The results showed that these strains had no antagonistic effects on each other. Therefore, they were suitable for conducting experiments with the mixed culture.
The thiamethoxam degradation rates of the fungus, bacteria, and their mixture differed at various substrate concentrations. The increase in the thiamethoxam concentrations decreased the degradation percentages (Fig. 1). Phanerochaete sp. Th1 showed higher degradation rates than Ensifer sp. Th2 at high concentrations (25 and 50 mg/L) but lower degradation performances at low concentrations (2 and 10 mg/L). For utilization as a sole carbon, nitrogen, and sulfur source, thiamethoxam at 2 mg/L was completely utilized within 15 days by Phanerochaete sp. Th1 (Fig. 1a) and 8 days by Ensifer sp. Th2 (Fig. 1b). At an initial concentration of 50 mg/L, the substrate was utilized at 37.0 ± 6.2 and 17.7 ± 4.4% by Phanerochaete sp. Th1 and Ensifer sp. Th2, respectively, for 25 days. Meanwhile, the mixed culture of these isolates completely utilized the compound taken at 2, 10, and 25 mg/L concentration within 5, 15, and 25 days, respectively (Fig. 1c). At 25 and 50 mg/L, thiamethoxam degradation by the mixed culture was similar to that by Phanerochaete sp. Th1 until 15 and 20 day, respectively, but the degradation rates were higher than those for any individual strain at the following time. Bacteria could effectively degrade the substrate when its concentration was reduced, probably resulting in increase of degradation.
Some previous studies described thiamethoxam degradation by isolated bacteria and fungi as a single carbon source, nitrogen, and sulfur source, namely Labrys portucalensis F11 [21] and Ensifer adhaerens TMX-23 [16]. Other microorganisms degrade the compound as a sole carbon source. Endophytic Enterobacter cloacae TMX-6 degrades 20% of the compound used as the sole carbon source at 10 mg/L after 15 days [18]. Twelve bacterial species isolated from agricultural soils degraded the substrate from 20.88 to 45.28% after 15 days in a liquid medium [17]. P. chrysosporium degraded 98, 74, and 27% of the compound for 25 days at initial thiamethoxam concentrations of 10, 20, and 50 mg/L, respectively, in Kirk’s liquid culture medium [19].
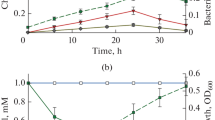
Thiamethoxam degradation kinetics. The utilization rates in this experiment were conducted in the MM medium supplemented with ammonium sulfate, during the exponential growth of isolated strains. The curves of utilization followed the saturation kinetics of the Edwards model given by the equation: V = Vmax[exp(–S/Ki) – exp(–S/Ki)]. The thiamethoxam utilization by Phanerochaete sp. Th1 and the mixed culture were the highest at 75 mg/L, while the highest rate of Ensifer sp. Th2 was found at 50 mg/L (Fig. 2).
The maximum utilization rates (Vmax) of Ensifer sp. Th2 and the mixed culture were not statistically different and significantly higher than that of Phanerochaete sp. Th1 (Table 1). Meanwhile, apparent half-saturation coefficient (Ks) of Ensifer sp. Th2 was significantly lower than that of Phanerochaete sp. Th1 and the mixed culture. The order of inhibition coefficient (Ki) values induced by thiamethoxam was as follows: Ensifer sp. Th2 < Phanerochaete sp. Th1 < mixed culture (Table 1). These results indicated that Phanerochaete sp. Th1 could tolerate at a higher thiamethoxam concentration compared to Ensifer sp. Th2, and both isolates cooperated during the degradation process.
The degradation rates in the medium with the addition of ammonium sulfate were higher than those of thiamethoxam utilization when the compound was used as the sole carbon, nitrogen, and sulfur source. For instance in the medium with ammonium sulfate, the degradation at 10 mg/L (3.43 µM) by Phanerochaete sp. Th1 and Ensifer sp. Th2 was 74.6 ± 9.7 and 98.0 ± 8.1% on the 10 day, respectively. The degradation by the mixture was almost complete after 8 days (Fig. 3).
The addition of co-substrates increased the cell density, facilitating the degradation rate. Boufercha et al., [21] showed that the degradation by Labrys portucalensis F11 depended on thiamethoxam concentrations and increased with the addition of nitrogen and sulfur sources in media. The addition of glucose caused Pseudomonas sp. 1G to degrade by 70% of both thiamethoxam and imidacloprid (50 mg/L) after 14 days [15].
Metabolites and Cl– produced during thiamethoxam degradation. During thiamethoxam degradation by individual isolates, metabolites were produced. For the degradation by Phanerochaete sp. Th1, an intermediate product with m/z 274 was proposed to have the elemental composition of C8H11N5O4S [19]. Other transient products having m/z of 161 and 101, corresponded to the elemental compositions of C4H8N4O3 and C3H7N3O, respectively [19]. For the degradation by Ensifer sp. Th2, metabolites with m/z 248 and 192 were identified to be C8H10ClN3O2S and C5H6ClN3OS, respectively [21]. These metabolites were also detected during the degradation by the mixed culture.
Cl– was released during the substrate degradation (Fig. 2). On the third day, the released Cl– concentrations were 82.8 ± 10.9, 32.2 ± 14.1, and 70.0 ± 9.0% of thiamethoxam transformed by Phanerochaete sp. Th1, Ensifer sp. Th2, and the mixed culture, respectively. The corresponding data for the 10 day were 80.4 ± 9.7, 80.6 ± 8.1, and 84.0 ± 8.7%. These results indicate that Phanerochaete sp. Th1 initially dechlorinated the substrate, and Ensifer sp. Th2 dechlorinated the substrate after some transformation steps.
Degradation of straw components and thiamethoxam during solid-state fermentation. In the control without inoculation, the low amounts of hemicellulose, cellulose, and lignin were reduced. In addition, thiamethoxam was dissipated in the control treatments. The dissipation of these components occurred probably owing to the chemical and physical processes. The inoculation of Phanerochaete sp. Th1 and Ensifer sp. Th2 enhanced the degradation of straw components and thiamethoxam in all treatments. Ensifer sp. Th2 exhibited higher thiamethoxam degradation than Phanerochaete sp. Th1 at 2 mg thiamethoxam/kg treatment of dry straw, whereas the degradation by the fungus was higher than by bacterial strain at 10 mg/kg (Table 2). The degradation by any strain at this concentration was higher than that at 10 mg/kg. However, the specific degradation rates at 10 mg/kg were higher than those at 2 mg/kg. For example, the degradation of thiamethoxam at 2 mg/kg in nonsterilized straw by the mixed culture was 1.94 ± 0.04 mg after 25 days, whereas that at 10 mg/kg was 5.59 ± 0.66 mg.
The degradation rates of the sterile and nonsterile substrates were not statistically different, indicating that the degradation of straw components and the insecticide was mostly performed by Phanerochaete sp. Th1 and Ensifer sp. Th2. The inoculation of the isolates in the straw resulted in faster thiamethoxam removal than the natural dissipation in a previous study [26]. The degradation of hemicellulose, cellulose, lignin, and thiamethoxam of the mixed culture was higher than that by individual isolates in several treatments. Moreover, the degradations of hemicellulose, cellulose, and lignin were not statistically different at 2 and 10 mg thiamethoxam/kg dry straw. These results indicated that thiamethoxam at these concentrations did not affect the degradation of the straw components.
The fungus exhibited higher degradation of straw components than Ensifer sp. Th2. The degradation rates of hemicellulose and cellulose by Phanerochaete sp. Th1 were not statistically different, but they were significantly higher than those of lignin. Meanwhile, the degradation percentages of hemicellulose, cellulose, and lignin by Ensifer sp. Th2 were not statistically different in all the treatments. Lignin is a highly branched, irregular three-dimensional organic polymer. Its degradation was slower than that of hemicellulose and cellulose in some previous studies [29, 30]. The enhancement of lignin degradation and the conversion of intermediate metabolites by the synergistic mechanism between microorganisms has been reported [31].
Some species of Phanerochaete degrade maize straw components during composting. P. chrysosporium degrades 40.0% cellulose and 64.3% lignin of mixed maize straw and canola residue after 30 days [23]. The mixed culture of P. chrysosporium, Trametes versicolor, and Pleurotus ostreatus degraded 43.36, 31.29, and 48.36% of lignin, cellulose, and hemicellulose, respectively, under optimal conditions [24].
Composting has been applied to degrade pesticides and other toxic compounds. Thiamethoxam elimination using municipal solid waste composting was determined [25]. Some Phanerochaete species degrade organic toxic compounds during composting, such as herbicides [32], and 4-nonylphenol [33]. This study showed that Phanerochaete sp. Th1 could be used to effectively compost and degrade thiamethoxam.
Thiamethoxam dissipation in soil. The thiamethoxam degradation rates in the inoculated soil samples were typically higher than those in the noninoculated soil (Table 3). The treatment with free and immobilized forms was significantly higher than that of any individual isolate at 10 mg/kg. These results indicated that both Phanerochaete sp. Th1 and Ensifer sp. Th2 adapted to the new environment. More than 90% thiamethoxam at 2 mg/kg of dry soil was dissipated in the augmented soil without sterilization, whereas the degradation at 10 mg/kg of dry soil was less than 90% (Table 3).
The degradation in soil inoculated with Ensifer sp. Th2 was significantly higher than that with Phanerochaete sp. Th1 at 2 mg thiamethoxam/kg dry soil; the rates of each isolate at 10 mg/kg were not statistically different. The results prove that Ensifer sp. Th2 exhibits better degradation of thiamethoxam at low concentrations. The degradation in soil inoculated with immobilized Phanerochaete sp. Th1 was significantly higher than that with the free counterpart in most treatments. Phanerochaete sp. Th1 might degrade maize straw, which provided nutrients for the fungus to grow and degrade the insecticide. However, the degradation performances of the free and immobilized cells of bacteria and the mixed culture were not statistically different in other treatments (Table 3).
The reduction in thiamethoxam concentration in the nonsterile soil was significantly higher than that in the sterile soil, indicating that indigenous microorganisms could degrade the compound. Thiamethoxam dissipation was also observed in the sterile soil; probably, the substrate was degraded by physical and chemical processes, or it was absorbed into soil components and could not be extracted.
Some previous studies described thiamethoxam dissipation in soil [7, 34]. Yu et al., [6] observed that thiamethoxam biodegradations in soil for 112 days were 44.68, 37.35, and 34.74% at 0.02, 0.2, and 2.0 mg/kg, respectively. Thiamethoxam degradation in soil based on the substrate concentrations and soil types has been investigated [7]. The half-lives of thiamethoxam at 1.8, 18.0, and 180.0 mg/kg silty loam soil were 6.2, 9.5, and 76.2 days, respectively [7]. At the recommended dosage (2 mg thiamethoxam/kg soil), the average degradation percentages were 30.28 and 91.20% in the sterilized and nonsterilized soils for 60 days, respectively [8]. The amendment of Bacillus aerophilus IMBL 4.1 in clay loam soil increased thiamethoxam degradation [22]. Recently, Li et al., [35] found that the supplementation with P. chrysosporium improved thiamethoxam degradation in both sterilized and nonsterilized wetland soils.
In this study, Phanerochaete sp. Th1 and Ensifer sp. Th2 isolated from soil utilized thiamethoxam used as a single carbon, nitrogen, and sulfur source. The degradation rates of their mixed culture were higher than those of the individual cultures. Phanerochaete sp. Th1 also effectively degraded hemicellulose, cellulose, and lignin in maize straw. The inoculation of the mixed culture enhanced the degradation of not only thiamethoxam but also maize straw components, namely hemicellulose, cellulose, and lignin, during the solid-state fermentation. Moreover, the treatment with the isolates enhanced thiamethoxam degradation in the soil. This study showed that Phanerochaete sp. Th1 and Ensifer sp. Th2 effectively degraded the insecticide in liquid media, maize straw, and soil.
REFERENCES
Douglas, M.R. and Tooker, J.F., Environ. Sci. Technol., 2015, vol. 49, no. 8, pp. 5088–5097. https://doi.org/10.1021/es506141g
Craddock, H.A., Huang, D., Turner, P.C., Quirós-Alcalá, L., and Payne-Sturges, D.C., Environ. Heal. Global Access Sci. Source, 2019, vol. 18, pp. 1–16. https://doi.org/10.1186/s12940-018-0441-7
Zhao, G.P., Yang, F.W., Li, J.W., Xing, H.Z., Ren, F.Z., Pang, G.F., et al., Environ. Toxicol. Chem., 2020, vol. 39, no. 10, pp. 1884–1893. https://doi.org/10.1002/etc.4842
Finnegan, M.C., Baxter, L.R., Maul, J.D., Hanson, M.L., and Hoekstra, P.F., Environ. Toxicol. Chem., 2017, vol. 36, no. 10, pp. 2838–2848. https://doi.org/10.1002/etc.3846
Saraiva, A.S., Sarmento, R.A., Rodrigues, A.C., Campos, D., Fedorova, G., Zlabek, V., Gravato, C., et al., Ecotoxicol. Environ. Saf., 2017, vol. 137, pp. 240–246. https://doi.org/10.1016/j.ecoenv.2016.12.009
Yu, B., Chen, Z., Lu, X., Huang, Y., Zhou, Y., Zhang, Q., et al., Sci. Total. Environ., 2020, vol. 725, p. 138328. https://doi.org/10.1016/j.scitotenv.2020.138328
Wu, C., Wang, Z., Ma, Y., Luo, J., Gao, X., Ning, J., et al., J. Hazard. Mater., 2021, vol. 405, p. 124275. https://doi.org/10.1016/j.jhazmat.2020.124275
Zhang, H., Zhang, Z., Song, J., Mei, J., Fang, H., and Gui, W., Environ. Pollut., 2021, vol. 274, p. 116540. https://doi.org/10.1016/j.envpol.2021.116540
Zhang, P., He, M., Wei, Y., Zhao, Y., Mu, W., and Liu, F., Crop Prot., 2016, vol. 90, pp. 1–8. https://doi.org/10.1016/j.cropro.2016.07.028
Bradford, B.Z., Huseth, A.S., and Groves, R.L., PLoS One, 2018, vol. 13, p. e0201753. https://doi.org/10.1371/journal.pone.0201753
Goulson, D., and Kleijn, D. J. Appl. Ecol. 2013, vol. 50, pp. 977–987. https://doi.org/10.1111/1365-2664.12111
Li, S., Cao, X., Liu, L., and Ma, X., Desalin. Water Treat., 2015, vol. 53, no. 11, pp. 3018–3025. https://doi.org/10.1080/19443994.2014.884478
Lebik-Elhadi, H., Frontistis, Z., Ait-Amar, H., Madjene, F., and Mantzavinos, D., Process Saf. Environ. Prot., 2020, vol. 134, pp. 197–207. https://doi.org/10.1016/j.psep.2019.11.041
Lee, Y.J., Kang, J.K., Park, S.J., Lee, C.G., Moon, J.K., and Alvarez, P.J.J., Chem. Eng. J., 2020, vol. 402, p. 126183. https://doi.org/10.1016/j.cej.2020.126183
Pandey, G., Dorrian, S.J., Russell, R.J., and Oakeshott, J.G., Biochem. Biophys. Res. Commun., 2009, vol. 380, no. 3, pp. 710–714. https://doi.org/10.1016/j.bbrc.2009.01.156
Zhou, G.C., Wang, Y., Zhai, S., Ge, F., Liu, Z.H., Dai, Y.J., et al., Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 9, pp. 4065–4074. https://doi.org/10.1007/s00253-012-4638-3
Rana, S., Jindal, V., Mandal, K., Kaur, G., and Gupta, V.K., Environ. Monit. Assess., 2015, vol. 187, no. 5, p. 300. https://doi.org/10.1007/s10661-015
Zhan, H., Wan, Q., Wang, Y., Cheng, J., Yu, X., and Ge, J., Chemosphere, 2021, vol. 269, p. 128751. https://doi.org/10.1016/j.chemosphere.2020.128751
Chen, A., Li, W., Zhang, X., Shang, C., Luo, S., Cao, R., et al., J. Hazard. Mater., 2021, vol. 417, p. 126017. https://doi.org/10.1016/j.jhazmat.2021.126017
Zamule, S.M., Dupre, C.E., Mendola, M.L., Widmer, J., Shebert, J.A., Roote, C.E., et al., Ecotoxicol. Environ. Saf., 2021, vol. 209, p. 111814. https://doi.org/10.1016/j.ecoenv.2020.111814
Boufercha, O., Monforte, A.R., Boudemagh, A., Ferreira, A.C., Castro, P.M.L, and Moreira, I.S., Int. J. Mol. Sci., 2022, vol. 23, no. 22, p. 14326. https://doi.org/10.3390/ijms232214326
Rana, S., and Gupta, V.K. J. Pharmacogn. Phytochem. 2019, vol. 8, no. 1, pp. 294–298.
Chen, Y., Wang, Y., Xu, Z., Liu, Y., and Duan, H., Bioresour. Technol., 2019, vol. 293, p. 122075. https://doi.org/10.1016/j.biortech.2019.122075
Chu, X., Awasthi, M.K., Liu, Y., Cheng, Q., Qu, J., and Sun, Y., Bioresour. Technol., 2021, vol. 320 (pt. A), p. 124174. https://doi.org/10.1016/j.biortech.2020.124174
Kozlov, G., Alekseev, E., and Chermenskaya, T., Agric. Biotechnol., 2022, vol. 42, p. 102378. https://doi.org/10.1016/j.bcab.2022.102378
He, M., Song, D., Jia, H.C., and Zheng, Y., J. Environ. Sci. Health B, 2016, vol. 51, no. 9, pp. 594–601. https://doi.org/10.1080/03601234.2016.1181903
Ha, D.D. Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1, Environ. Eng. Res., 2022, vol. 27, no. 4, p. 210158. https://doi.org/10.4491/eer.2021.158
Van Soest, P.J., Rovertson, J.B., and Lewis, B.A., J. Dairy Sci., 1991, vol. 74, pp. 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Tuomela, M., Vikman, M., Hatakka, A., and Itävaara, M., Bioresour. Technol., 2000, vol. 72, pp. 169–183. https://doi.org/10.1016/S0960-8524(99)00104-2
Yu, M., Zeng, G., Chen, Y., Yu, H., Huang, D., and Tang, L., Process Biochem., 2009, vol. 44, no. 1, pp. 17–22. https://doi.org/10.1016/j.procbio.2008.09.005
Xu, C., Su, X., Wang, J., Zhang, F., Shen, G., Yuan, Y., Yan, L., Tang, H., Song, F., and Wang, W., Bioresour. Technol., 2021, vol. 331, p. 125066. https://doi.org/10.1016/j.biortech.2021.125066
Castillo, M, Andersson, A, Ander, P, Stenström, J, and Torstensson, L., World J. Microbiol. Biotechnol., 2001, vol. 17 pp. 627–633. https://doi.org/10.1023/A:1012420422111
Huang, D., Qin, X., Xu, P., Zeng, G., Peng, Z., Wang, R., et al., Bioresour. Technol., 2016, vol. 221, pp. 47–54. https://doi.org/10.1016/j.biortech.2016.08.104
Kumar, N., Srivastava, A., Chauhan, S.S, and Srivastava, P., Plant Soil Environ., 2014, vol. 60, pp. 332–335. https://doi.org/10.17221/106/2014-PSE
Li, W., Chen, A., Shang, C. Zhang, X., Chai, Y., Luo, S., et al., J. Environ. Chem. Eng., 2022, vol. 10, no. 5, p. 108333. https://doi.org/10.1016/j.jece.2022.108333
ACKNOWLEDGMENTS
Authors thank all who have provided supports. We are also thankful an anonymous reviewer whose suggestions helped improve and clarify this manuscript.
Funding
This study was supported by Dong Thap University (Viet Nam) for research groups.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The manuscript does not have potential conflicts of interest. The research does not involve human participants or animals.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Oanh, N.T., Duc, H.D. Degradation of Thiamethoxam by Mixed Culture of Phanerochaete sp. Th1 and Ensifer sp. Th2. Appl Biochem Microbiol 59, 858–866 (2023). https://doi.org/10.1134/S0003683823060091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823060091