Abstract
A scheme is proposed to describe the spin state of the Co3+ ions in the layered TbBaCo2O5.5 cobaltite near the metal–insulator transition. The spin state of the Co3+ ions in the metallic phase corresponds to a mixture of the HS(\(t_{{2g}}^{4}\)\(e_{g}^{2}\), S = 2) and LS(\(t_{{2g}}^{6}\)\(e_{g}^{0}\), S = 0) states taken at approximately the same proportion. The transition into a nonmetallic state occurs due to the transformation of the HS state into the LS state in octahedra and part of the LS state into the IS(\(t_{{2g}}^{5}\)\(e_{g}^{1}\), S = 1) state in pyramids (near TC ~ 280 K). The proposed scheme agrees with the well-known structural data obtained for the TbBaCo2O5.5 cobaltites. As follows from volumetric and linear expansion, the transition takes place over a wide temperature range T ≈ TMI ± 50 K. The study of thermal expansion shows that an LS/IS state is retained down to T = 80 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Interest in the ordered layered RBaCo2O5 + δ cobalt oxides is mainly caused by the detection of colossal magnetoresistance (MR) in hole lanthanum manganites [1, 2]. Although they do not exhibit the magnetoresistance properties comparable with those of hole manganites, they attract great attention due to their unusual magnetic and electrical properties and phase transitions [3–15]. The driving force of the cation ordering in the R1 –xBaxCoO3 – δ perovskites at x = 0.5 is the significant difference between the radii of the R3+ and Ba3+ rare-earth ions, which leads to cation ordering in the form of alternating layers with rare-earth (R) and alkali metal (Ba) ions. The layered RBaCo2O5 + δ cobaltites have a perovskite crystal structure, in which RO and BaO layers alternate with CoO2 layers located normal to axis c. They are strongly anisotropic because of their layered structure [3, 11]. Depending on the oxygen content 0 ≤ δ ≤ 1, the valence state of cobalt changes from Co2+ to Co4+, and Co ions have different oxygen environment (octahedra or pyramids with a square base). RBaCo2O5.5 only contains Co3+ ions, which are located in the crystal lattice of the same number of CoO6 octahedra and square CoO5 pyramids, and the oxygen pyramids and octahedra surrounding Co3+ ions are ordered [2].
The unusual electron, magnetic, and structural transitions in RBaCo2O5 + δ, δ ≈ 0.5, are of particular interest. The following sequential transitions were detected in them: metal–insulator (MI), paramagnetic (PM), ferromagnetic (FM), and antiferromagnetic (AFM) transitions [1–15]. In contrast to manganites, the MI transition in cobaltites is not related to magnetic ordering, which results from the magnetically active (antiferromagnetic) character of the RMnO3 matrix in the case of manganites and the weakly magnetic (paramagnetic) behavior of RCoO3 in the case of cobaltites (as the nature of magnetoresistance). Colossal magnetoresistance is promoted by the presence of FM clusters in an AFM matrix. The FM clusters in hole manganites are coupled to an AFM matrix by an exchange interaction, which causes an increase in the cluster size and a high MR [16, 17]. The increase in the magnetic cluster (polaron) size with decreasing temperature or in a magnetic field is explained by the unusual transport properties of layered manganites, namely, metal–nonmetal transition and higher MR [18]. A weakly magnetic cobaltite matrix exhibits cluster coalescence [19] and a low MR. The properties of the matrix cause the following new phenomena for layered cobalt oxides: a unidirectional electrical resistance anisotropy, exchange bias [20, 21], and the absence of exchange bias in MR hole manganites.
The physics of layered cobaltites is determined by the complex interaction between the charge, spin, orbital, and lattice degrees of freedom [1–5, 22]. The MI transition is accompanied by anomalous changes in the lattice parameters, the average cobalt–oxygen distance d(Co–O) in octahedra and pyramids [4, 6, 12, 13], and effective paramagnetic moment μeff [1, 2], which are determined by changes in the spin states of Co3+. Depending on the relation between the energies of intraatomic exchange and the crystal field, the Co3+ ions can be in a low-, intermediate-, or high-spin state. The differences between the spin-state energies in many cobaltites are small and can easily be overcome by temperature changes, which lead to transformation of the spin state of Co and unusual structural and phase transitions (including MI transition) [22].
At present, there is no agreement regarding the spin state of Co3+ and the origin of the MI transition in the layered RBaCo2O5.5 cobaltites. The spin state of the Co3+ ions in the relatively simple LaCoO3 compound is still unclear and has been the subject of controversy since the 1960s. The situation in the rare-earth layered RBaCo2O5 + δ cobaltites at δ ≈ 0.5 is more complex. First, unlike LaCoO3, the Co3+ ions in these compounds can be in two different oxygen environment positions (octahedral, pyramidal). Second, the Co3+ ions can also be in the following three different spin states: high-spin (HS, S = 2), intermediate-spin (IS, S = 1), and low-spin (LS, S = 0) states. In addition, the magnetic properties of the RBaCo2O5 + δ cobaltites depend on a paramagnetic R3+ ion [3, 4]. Depending on the type of rare-earth ion, this contribution can be significant, which substantially affects the spin moment of Co3+ determined from magnetic measurements.
The authors of the first works [1, 2] assumed that the Co3+ ions are in an LS/IS state at low temperatures and evolve to the HS state in both polyhedra at above transition temperature TMI [1, 2]. According to the soft X-ray absorption and the photoelectron spectroscopy of GdBaCo2O5.5, the HS state of the Co3+ ions is also retained at T < TMI [23]. The analysis [24] of the X-ray absorption spectra of TbBaCo2O5.5 did not detect a change in the spin state of the Co3+ ions at TMI. The Mössbauer spectroscopy of TbBaCo2O5.5 [25] suggests the IS → HS transition for the cobalt ions in octahedra and the retained HS state for cobalt in pyramids when temperature increases [25]. Thus, the data obtained are conflicting.
Magnetic methods are most widely used to determine the spin state of Co3+ [1, 2]. The complexity of the magnetic methods applied for this purpose is the problem of separating the contribution of the Co3+ ions from the PM contribution of rare-earth R3+ ions. Moreover, magnetization studies cannot determine the oxygen environment (octahedral or pyramidal) of the Co3+ ions. The authors of [4, 7] tried to determine the oxygen environment and the spin state of the Co3+ ions using magnetic and structural data. The structural data [4] obtained for GdBaCo2O5.5 revealed elongated octahedra and compressed pyramids in the metallic phase. Since the ionic radius of Co3+ increases with the spin state, these results could be interpreted as an increase in the spin state of the Co3+ ions in the octahedra and a decrease in the spin state in the pyramids. However, the spin state of the Co3+ ions in GdBaCo2O5.5 is LS/IS below TMI and HS/IS above TMI [7], which is in conflict with the structural data [7]. The authors of [4] suppose that the transition into a metallic state is due to a change in the LS state of the Co3+ ions to the HS state only in the octahedra without changing the IS state in the pyramids. The same conclusions about the spin state of the Co3+ ions in PrBaCo2O5.5 were made without regard for the PM contribution of the Pr3+ ions [13]. The authors think that the compression of pyramids can be attributed to either the steric effect or the formation of metallic bonds [4, 13]. This model has received wide acceptance, and many researchers use this model of the MI transition in RBaCo2O5.5.
However, the authors of [26] were doubtful of the reliability of the conclusions [4] regarding the spin state Co3+, since the method of determining the PM contribution of the Gd3+ ions that was used in [7] is incorrect (see below). Moreover, the magnetizations of samples [7] were anomalous high as compared to the data in [2, 8]. A method of determining the PM contribution of the Gd3+ ions was proposed in [26]. The studies [26] of the magnetization of GdBaCo2O5.5 as a function of temperature and magnetic field showed that the PM contribution of the Gd3+ ion almost coincided with the contribution of a free Gd3+ ion. As follows from the refined PM contribution of the Gd3+ ions, the Co3+ ions in GdBaCo2O5 + δ at δ ≈ 0.5 are still in the LS/IS state below TMI and transform into the HS/LS state above TMI [26]. The transition into the metallic state of GdBaCo2O5.5 takes place when the spin state of the Co3+ ions increases in the octahedra (LS–HS transition) and decreases in the pyramids (IS–LS transition). These conclusions agree with the structural data obtained by synchrotron X-ray diffraction of GdBaCo2O5.5 [4].
The purpose of this work is to determine the spin state of the Co3+ ions in the layered TbBaCo2O5 + δ cobaltite at δ ≈ 0.5. Although the magnetic properties of TbBaCo2O5.5 were extensively studied and the neutron and X-ray diffraction measurements were performed in many works, there are no unambiguous conclusions regarding the spin state of Co3+ in this compound in the MI transition range [2, 5, 6, 15, 23–25, 27]. This situation is mainly caused by the fact that the conclusions were drawn using the magnetic data that do not take into account the PM contribution of the Tb3+ ions [2, 5]. The spin state of the Co3+ ions in TbBaCo2O5.5 in this work is determined with allowance for the PM contribution of the Tb3+ ions. As follows from our data, the spin states of the Co3+ ions in TbBaCo2O5.5 and GdBaCo2O5.5 near the MI transition are identical. The Co3+ ions are in the LS/IS state below TMI and in the HS/LS state above TMI. The transition to the quasimetallic state occurs when the LS state changes to the HS state in octahedra and IS states to LS states in pyramids in the temperature range T ≈ TMI ± 50 K. This assumption explains the increase in the cobalt–oxygen distance d(Co–O) in the octahedra and its decrease in the pyramids in TbBaCo2O5.5 [6] because of the changes in the ionic radii of the Co3+ ions induced by changes in their spin state during transition into the metallic phase.
EXPERIMENTAL
Polycrystalline TbBaCo2O5 + δ samples were synthesized from Tb4O7 (99.99% purity), Co3O4 (analytical grade), and BaCO3 (special purity grade) powders using solid-phase reactions. The powders taken in the necessary proportion were ground, pressed in pellets, and sintered at 1150°C for 24 h, and the pellets were then cooled to room temperature at a rate of 1 K/min. Oxygen content δ was determined by reducing a sample in a hydrogen atmosphere. The samples were single-phase with δ = 0.38 and had an orthorhombic structure (space group Pmmm, no. 47) with unit cell parameters a = 3.869(5) Å, b = 7.815(4) Å, and c = 7.515(5) Å. These results agree with the data in [28]. To change the oxygen content, we annealed the pellets in sealed ampules at a pressure of 5 atm. The Ag2O compound was as an oxygen source. The linear expansion coefficient was determined using an ULVAC-SINKU RIKO (Japan) quartz dilatometer in the temperature range 77–550 K in the dynamic mode at the rate of change of temperature of 2 K/min. The magnetization was determined on an MPMS-5XL (Quantum Design) device at T = 10–400 K and on a 7407 VSM (Lake Shore) vibrating-sample magnetometer at T = 280–500 K at the Center for Collective Use of the Institute of Metal Physics.
RESULTS AND DISCUSSION
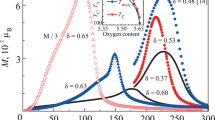
Figure 1 shows the temperature dependence of the magnetization M(T) of the TbBaCo2O5.47(2) polycrystal in a magnetic field H = 1 kOe. When the temperature decreases below the Curie temperature (TC = 279 ± 2 K), the magnetization increases sharply, reaches its maximum at Tmax ≈ 263 ± 1 K, and decreases sharply at T ~ 220 K. Based on neutron diffraction investigations, the authors of [27] explained this behavior of M(T) in the TbBaCo2O5.50 polycrystal by the formation of a canted FM structure below TC and its further transition into a noncollinear AFM state. Researchers usually suppose that the transition into the AFM state takes place at T = Tmax, although magnetization is retained within 30–40 K. A gradual transition from a canted FM state into a noncollinear AFM state is likely to occur in the temperature range from T = Tmax ~ 260 K to TN ~ 200 K (Fig. 1). The right inset to Fig. 1 shows the temperature dependences of saturation magnetization MS, which were obtained extrapolation of M(H) to 50 kOe, and the magnetizations at 50 kOe (PM contribution of the Tb3+ ions was subtracted) at T = 300–200 K. MS is seen to decrease below Tmax and the FM state exists in a narrow temperature range T ≈ 220–280 K. The increase in the magnetization below T = 200 K is explained the PM contribution of the Tb3+ ion (Fig. 1, solid line).
(Color online) Temperature dependences of the magnetization of TbBaCo2O5.47 (symbols 1) and the paramagnetic contribution of Tb3+ ions (solid line 2) at H = 1 kOe (see text). (left onset) Temperature dependence of the paramagnetic susceptibility at low temperatures. (right inset) temperature dependence of the saturation magnetization MS and the magnetization of a TbBaCo2O5.47 polycrystal at H = 50 kOe.
At low temperatures in the range T = 10–60 K, the paramagnetic susceptibility of the sample (left-side inset to Fig. 1) is described by the Curie–Weiss law with a paramagnetic temperature θPM ≈ –11 K and an effective magnetic moment μeff ≈ 8.3μB, which differs from the moment expected for a free Tb3+ ion (μeff = 9.72μB),
Rare-earth ion compounds are known to be Van Vleck paramagnets, and their paramagnetic properties are described by the Curie–Weiss law with μeff of a free ion [29, Chapter 9]. A negative value θPM < 0 characterizes the presence of AFM interactions and agrees with the AFM ordering of the Tb3+ ions in TbBaCo2O5.5 below θN = 3.44 K [15].
At present, the influence of the PM contribution of R3+ ions on the magnetic properties of RBaCo2O5 + δ is poorly understood. This contribution was not taken into account in [1, 2, 5, 8, 11, 13, 14, 30], and the authors of [3, 10, 15, 31] assumed that the contribution of R3+ ions coincides with the contribution of a free ion. In [4, 7], the contribution of the Gd3+ ion was determined using the results of studying the PM susceptibility of GdBaCo2O5.5 at low temperatures,
This method is inaccurate, since the effective moment depends on the presence of magnetic ions [29]. The value of μeff in Eq. (2), which differs from the moment of a free Gd3+ ion, is caused by the influence of Co ions, as in our experiment for the Tb3+ ion (inset to Fig. 1), since the PM susceptibility is determined by the contribution of Co and R3+ ions.
To determine the PM contribution of the Gd3+ ions, we [26] proposed to use the saturation of magnetization M(H) in a magnetic field at low temperatures. It was preliminarily shown that the contribution of the Co ions to the magnetization of GdBaCo2O5.5 at T = 10 K is low and the field dependence of magnetization at T = 10 K is described by a Brillouin function with the parameters characteristic of the free Gd3+ ion at θPM = –1.4 K,
where BS(x) is the Brillouin function, NA is Avogadro’s number, x = gμBJH/k(T – θPM), g is the Landé factor, μB is the Bohr magneton, J is the total magnetic moment, H is the magnetic field, and k is the Boltzmann constant.Footnote 1 At x ≪ 1, from Eq. (3) the PM contribution of the Gd3+ ions is [26]
Using the method proposed in [26], we determine the PM contribution of the Tb3+ ions using the results of studying the magnetization of TbBaCo2O5.47 in the range T = 10–150 K. The symbols in Fig. 2a show the experimental magnetization M(H) of the TbBaCo2O5.47 polycrystal at T = 10 K. Unlike GdBaCo2O5.52 [26], the M(H) dependence of the sample at T = 10 K is not described by Eq. (3) for the parameters of the Tb3+ ion at any values of θPM (Fig. 2a; solid lines 1, 2). The M(H) curves at T = 10 K are explained by the fact that TbBaCo2O5.47 at T = 10 K is not a pure paramagnet, since temperature T = 10 K is close to the AFM ordering temperature (θN = 3.44 K) of the Tb3+ ions [15], in contrast to GdBaCo2O5.5, in which ordering of the Gd3+ ions above T = 1.7 K was not detected [3, 4, 10].
The symbols in Fig. 2b show the experimental values of M(H) at T = 20–150 K, which are determined by the total contribution of the Tb3+, Co3+, and 2–3% Co2+ ions. The magnetization M(H) determined at T = 10–150 K demonstrates that the PM susceptibility of the Co ions tends to decrease with decreasing temperature (as in GdBaCo2O5 + δ [26]), which is characteristic of AFM at T ≪ TAFM [29]. Our estimates demonstrate that the contribution of the cobalt ions to the magnetization at T = 20 K is at most 0.2μB. At 20–50 K, the contribution of the Tb3+ ion is satisfactorily described by Eq. (3) with Tb3+ ion parameters J = 6, g = 1.5, and θPM = –(8 ± 2) K. At T = 75–150 K, the calculated values of M(H) bounded by the contribution of the Co3+ ions deviate slightly from the experimental values (Fig. 2b). The contribution of the Tb3+ ions to the paramagnetic susceptibility is determined by the following expression derived from Eq. (3) at x ≪ 1 (T > 300 K and H ~ 10 kOe):
where CM = NA\(\mu _{{\text{B}}}^{2}\mu _{{{\text{eff}}}}^{2}\)/3k = 11.82 is the Curie constant for the Tb3+ ion [32].
In a wide temperature range of 300–10 K, the PM susceptibility of plain GdCoO3 [33, 34] and TbCoO3 [35] is described by Eq. (5) with the parameters of free Gd3+ and Tb3+ ions, which indirectly supports our conclusions. Line 2 in Fig. 1 shows the PM contribution of the Tb3+ ions at H = 1 kOe. Our estimates demonstrate that the contribution of the Tb3+ ions to the magnetization of TbBaCo2O5.5 above TMI is predominant and the contribution of the Co3+ ions accounts for less than 14% of the total magnetization, which is almost half that in GdBaCo2O5.5 [26].
Figure 3 depicts the temperature dependence of the electrical resistivity of TbBaCo2O5.52 at T = 100–400 K, which is typical of layered cobaltites [1, 2]. It has a semiconductor character: ρ(T) decreases monotonically with increasing temperature and, in the temperature range 100–250 K, is described by the activation expression
with an activation energy ΔE ≈ 40 meV. An inflection point in the electrical resistivity is observed in the AFM–FM transition range (T ≈ 200–250 K). This inflection point shifts in a magnetic field toward low temperatures. An unusually high (for cobaltites) magnetoresistance
is observed in the same temperature range (Fig. 3, bottom insets). Analogous behavior of ρ(T) and MR0 was detected in GdBaCo2O5.5 [4, 26]. These results are explained by the fact that the carrier mobility in the AFM state is lower than that in the FM state and that a magnetic field widens the temperature range of the FM state toward low temperatures and narrows the range of the AFM state [4]. Below T ≈ TC ≈ 280 K, the linear dependence lnρ ~ 1/T changes and ρ(T) decreases sharply; at T = 330–338 K, an electrical resistivity jump takes place, which is thought to be related to a change in the spin state of Co3+ (Fig. 3, top inset). Above TMI ≈ 338 K, the electrical resistivity of the sample weakly depends on temperature, ρ ~ 2 × 10–3 Ω cm (Fig. 3, top inset). The sign of the electrical resistivity derivative dρ/dT remains negative, which indicates a semiconductor character of ρ(T) in the temperature range up to 400 K.
(Color online) Temperature dependence of the electrical resistivity ρ(T) of a TbBaCo2O5.52 polycrystal. (top inset) ρ(T) near TMI ~ 338 K. (bottom insets): (1) ρ(T) in the AFM–FM transition range at H = (solid line) 0 and (symbols) 15 kOe and (2) temperature dependence of the magnetoresistance at H = 15 kOe.
Figure 4 (left axis) shows the temperature dependence of the experimental PM susceptibility \(\chi _{{\exp }}^{{ - 1}}\)(T) of the TbBaCo2O5.52 sample measured in a magnetic field H = 10 kOe. The \(\chi _{{\exp }}^{{ - 1}}\)(T) dependence is linear in the range T = 500–350 K, a small jump is observed below TMI ≈ 340 K, and the \(\chi _{{\exp }}^{{ - 1}}\)(T) is then obviously nonlinear. The value estimated according to the Curie–Weiss law (μeff/Co ≈ 7.55μB) is close to the data in [5] and is too high to be attributed to the spin state of Co3+. To separate the contribution of the Co3+ ions from the total magnetization of the sample, we used Eq. (5) to subtract the contribution of the Tb3+ ions and recalculated χ–1(T) for the cobalt ions (Fig. 4, right axis). The contribution of the Tb3+ ions increases the values of χ–1(T) in the metallic phase more than sixfold. In the temperature range 500–380 K, inverse susceptibility χ–1(T) linearly depends on temperature. The nonlinear part of χ–1(T) is observed below T ≈ 380 K: χ–1(T) changes slowly down to T ≈ 350 K, χ–1(T) jumps in the range TMI ≈ 345–335 K, and χ–1(T) decreases monotonically and nonlinearly when temperature decreases further. A similar change in the slope of χ–1(T) near TMI was detected in EuBaCo2O5 + δ crystals (see Fig. 2 in [1]). In the temperature range 500–380 K, the PM susceptibility is described by the Curie–Weiss law with a Néel temperature θN = ‒(155 ± 10 K) and μeff/Co = 3.28 ± 0.1μB; below TMI in the narrow range 325–280 K, μeff/Co = 1.40 ± 0.05μB and θC = 283 ± 2 K are used (Fig. 4b; solid lines μeff and θC(θN)).
(Color online) (a): (1) Temperature dependence of the paramagnetic susceptibility \(\chi _{{\exp }}^{{ - 1}}\)(T) of a TbBaCo2O5.52 polycrystal (left axis) and (2) χ–1(T) with subtracted contribution of the Tb3+ ion (right axis). (b) Temperature dependences of (solid line 1) effective moment μeff/Co and (solid line2) temperature θC(θN); (symbols and dashed line) differential values \(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\)/Co and θC(θN).
No linear segment is detected in the χ–1(T) dependence in the temperature range 380–280 K, which means that the transition is accompanied by changes in μeff(T) with temperature. To test this assumption, we separated linear segments in χ–1(T) in the temperature range 300–400 K and determined the differential values of μeff and θC(θN) by the Curie–Weiss law for each segment. The symbols in Fig. 4b show the temperature dependences of the differential values of \(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\) and θC(θN) thus determined. The MI transition is seen to occur over a wide temperature range (280–380 K): from the maximum (μeff/Co = 3.28 ± 0.10μB) at T = 500–380 K to the minimum (\(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\)/Co ≈ 0.5μB) at TMI ≈ 340 K upon an increase to \(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\)/Co ≈ 1.6μB at T ~ 280 K. The behavior of θC(T) is analogous: θC increases gradually from θN ≈ –150 K to θC ~ +320 K in the range T ≈ 400–340 K and then decreases weakly to θC ≈ +280 K. The values of μeff and \(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\) coincide in the range of the linear behavior of χ–1(T).
In the metallic state (T = 400–500 K), the value μeff/Co ≈ 3.28 ± 0.10μB corresponds best of all to a mixture of the HS(\(t_{{2g}}^{4}\)\(e_{g}^{2}\), S = 2) and LS(\(t_{{2g}}^{6}\)\(e_{g}^{0}\), S = 0) states with μeff/Co = 3.43μB taken at the proportion 1 : 1 among all possible states of the Co3+ ions (Fig. 4b). Below TMI near TC (T = 280–325 K), the value μeff/Co = 1.40 ± 0.05μB means that only up to one-fourth of the Co3+ ions are in the IS(\(t_{{2g}}^{5}\)\(e_{g}^{1}\), S = 1) state and the remaining ions are in the LS state. The predominance of the fraction of the LS state near TMI in TbBaCo2O5.5 was also detected in [15]. The X-ray and neutron diffraction studies [6, 27] also predicted the existence of an LS/IS state of the Co3+ ions below TMI.
Our magnetic data did not allow us to determine the type of oxygen environment of the Co3+ ions. The structural investigations [27] of TbBaCo2O5.5 revealed an increase in the oxygen–cobalt bond length d(Co–O) in the octahedra and a decrease in this length in the pyramids in the metallic phase, which can be interpreted as an increase in the ionic radius of Co3+ in the octahedra and its decrease in the pyramids. Based on our magnetic data and the structural data in [27], we conclude that the transition into a nonmetallic state is caused by the transition of the HS state into the LS state in the octahedra, as in GdBaCo2O5.5 [26]. In the pyramids, only part (about one-fourth) of the Co3+ ions transform from the LS to the IS state, and the remaining ions are still in the LS state (at T ≈ TC).
A mixture of Co3+ ions with an approximately identical proportion of the IS and LS states is assumed to exist in GdBaCo2O5.5 below TMI [1, 2, 4, 7, 13]. This is partly true: this assumption is likely to be correct for temperatures T ≪ TMI [3, 10]. The spontaneous magnetization of GdBaCo2O5.5 without a twinned structure that was determined by extrapolation of the magnetization from high fields increases gradually upon cooling from MS ~ 0.3μB/Co at T = TC ≈ 280 K to MS ~ 0.6μB/Co at T ≈ 200 K (see Figs. 22 and 24 in [3]). At T = 1.8 K, it reaches MS ~ 1μB/Co in a magnetic field higher than 300 kOe, which corresponds to the ratio 1 : 1 of the LS/IS states of Co3+ at T → 0 [10]. Upon cooling below TC ≈ 270 K, the spontaneous magnetization of GdBaCo2O5.5 with a twinned structure increases gradually to MS ~ 0.5μB/Co at T = 78 K (see Fig. 3 in [10]). Half of the IS (S = 1) spins is parallel to an applied field due to the twinned structure of the crystal and the other half is opposite the field, which leads to the saturation moment MS ~ 0.5μB/Co [3, 10].
As is seen in the inset to Fig. 1, the spontaneous magnetization increases gradually from MS ~ 0.11μB/Co at T = TC ≈ 280 K to MS ~ 0.23μB/Co at T ≈ 260 K. Actually, MS ~ 0.11μB/Co at T = 280 K corresponds to the LS/IS state of the Co3+ ions at the ratio 0.7 : 0.3 for T ≈ 280 K, which was obtained above from fitting χ–1(T) by the Curie–Weiss law. However, the maximum value (MS ~ 0.45μB/Co) is significantly lower than the expected value (about 1μB/Co) for the LS/IS state at the ratio 1 : 1 because of the twinned and noncollinear FM structure of the TbBaCo2O5.52 crystal.
Therefore, the spin state of Co3+ as a function of the type of rare-earth ion R3+ is of interest. Using the well-known results on the PM susceptibility of the RBaCo2O5.5 (R = Pr, Nd, Sm, Gd, Tb, Dy, Ho) cobaltites [1, 2, 7, 11–14], we estimated possible spin states of the Co3+ ions near the MI transition with allowance for the PM contribution of the R3+ ions. For simplicity, the PM contribution was assumed to coincide with the contribution of a free R3+ ion [3]. Using these estimates, we can assume that the Co3+ ions in all these cobaltites below TMI are in an IS/LS state. The Co3+ ions in the metallic phase in all cobaltites except for those with R = Ho and Pr are in an HS/LS state.Footnote 2
Anomalous lattice expansion is detected along with the spin transition (Fig. 5). The thermal expansion was measured on a TbBaCo1.96O5 + δ single crystal along three directions in a 3 × 3 × 2.5 mm3 parallelepiped. One parallelepiped axis is perpendicular to axis c and coincides with the [120] growth direction, and two other axes are directed along axis c.
The symbols in Fig. 5 show the temperature dependence of the volumetric expansion ΔV/V near T ≈ TMI ± 100 K. The volumetric expansion coefficient αV(T) = 1/V ⋅ dV/dT in the metallic phase (400–500 K) is higher than that in the dielectric phase (T < TC), 3.9 × 10–5 and 3.2 × 10–5 K–1, respectively. The deviations of ΔV/V(T) from the linear dependence of the lattice (solid lines) take place at T ≈ TMI ± 40 K and are indicated by arrows. The nonmonotonic (S-like) change in the lattice volume ΔV/V(T) at the transition range is noteworthy: the lattice volume decreases upon heating above T ≈ 300 K, increases sharply at T ≈ TMI ± 10 K, and then again decreases. The linear expansion ΔL/L(T) near TMI also exhibits nonmonotonic (S-like) behavior (Fig. 5, top inset). An analogy between ΔV/V(T) and \(\mu _{{{\text{eff}}}}^{{{\text{diff}}}}\)(T) in Fig. 4b is visible. The results of thermal expansion are likely to reflect the fact of different temperature changes in the spin states of the Co3+ ions in different polyhedra near the MI transition. The linear expansion ΔL/L(T) has an anisotropic character (Fig. 5, left inset). During the transition into the metallic state, the lattice expands along axis c and is compressed along [120] normal to axis c. The lattice expansion ΔL/L(T) along axis c, the lattice compression normal to axis c, and the increase in the volume ΔV/V during the transition into the metallic phase agree qualitatively with the neutron diffraction data in [5, 6]. The linear and volumetric expansion curves plotted upon heating and cooling exhibit hysteretic behavior, supporting the fact that the MI transformation is a first-order phase transition.
The temperature dependence of the linear thermal expansion coefficient α(T) = 1/L ⋅ dL/dT of TbBaCo1.96O5 + δ has a well-pronounced peak at T = TMI ≈ 338–340 K and a weak anomaly at TN ≈ 190 K (Fig. 5, bottom inset). A good correlation between the linear thermal expansion coefficient α(T) and the effective magnetic moment μeff(T) is clearly visible (Fig. 4b). Coefficient α(T) deviates from the linear temperature dependence (solid lines) induced by thermal lattice expansion in the range T ≈ 380–290 K, i.e., the temperature range of changing the spin state of Co3+.
The α(T) anomaly at TN ≈ 190 K is smaller than α(T) at TMI by almost two orders of magnitude, which excludes the possibility of its explanation by changing the spin state of the Co3+ ions near this temperature. Allowing for the magnetic data (Fig. 1), we assume that this anomaly can be caused by magnetostriction phenomena during the transition from a canted FM state to a noncollinear AFM state. The thermal expansion of TbBaCo1.96O5 + δ confirms the retention of the LS/IS state of the Co3+ ions at low temperatures down to at least T = 80 K.
Extrapolating the thermal expansion of nickel below and above the transition temperature, we estimated the increase in the unit cell volume during the MI transition at ΔV/V ≈ 2 × 10–4 at TMI. Approximately the same value of ΔV/V was obtained for a GdBaCo2O5.5 polycrystal [36]. Volume increment ΔV/V near TMI demonstrates that the transition into a metallic state is accompanied by an increase in the spin state of the Co3+ ions. The results of studying the thermal expansion of a TbBaCo1.96O5 + δ single crystal support the fact that the spin state of Co3+ changes over a wide temperature range T ≈ TMI ± (40–50) K. The physical properties, such as ρ(T) (Fig. 3), χ–1(T) (Fig. 4), α(T), ΔL/L, and ΔV/V (Fig. 5), change sharply only in a narrow temperature range T = TMI ± 10 K, where χ–1(T) and μeff change jumpwise.
CONCLUSIONS
Magnetic and structural investigations are the main methods for determining the spin state of the Co3+ ions in the layered RBaCo2O5.5 cobaltites near the MI transition. However, unambiguous conclusions regarding the spin state of the Co3+ ions cannot always be drawn using the results of these investigations, which is mainly caused by the fact that conclusions were usually based on the magnetic data that incorrectly took into account the PM contribution of the R3+ ions. In this work, we studied the magnetization of TbBaCo2O5.5 over a wide temperature range and found that the PM contribution of the Tb3+ ions was determined by the Curie–Weiss law with the parameters of a free Tb3+ ion at θPM = –8 ± 2 K. The spin states of the Co3+ ions in TbBaCo2O5.5 and GdBaCo2O5.5 were found to be identical with allowance for the PM contribution of the Tb3+ ions. The Co3+ ions are in an HS/LS state above TMI and in an LS/IS state below TMI. The transition into a metallic state takes place when the Co3+ ions in the octahedra transform from the LS to the HS state and the Co3+ ions in the pyramids transform from the IS to the LS state, according to the structural data indicating expansion of the octahedra and compression of the pyramids. As follows form the data obtained for volumetric and linear expansion, the MI transition occurs over a wide temperature range T ≈ TMI ± (40–50) K when the spins state of the Co3+ changes. The hysteretic behavior of the volumetric and linear expansion demonstrates that the MI transition in the compound under study is a first-order phase transition. Thermal expansion data indicate that the LS/IS state of the Co3+ ions is retained down to T = 80 K.
NOTE ADDED IN PROOF (FEBRUARY 13, 2020)
In conclusion, we note that the authors of [5, 12, 37, 38] considered the role of orbital ordering in the metal–insulator transition in layered cobaltites. Neutron diffraction of TbBaCo2O5 + δ at δ = 0.5 [37] and X‑ray diffraction of GdBaCo2O5.5 [38] demonstrate that the spin states of the Co3+ ions differ in the low-temperature phase and they are located in two different octahedra and two different pyramids because of orbital ordering. It is assumed that, in the low-temperature TbBaCo2O5 + δ at δ = 0.5 and T = 260 K, the Co3+ ions in octahedra are in the LS state and half the ions in pyramids is in the HS state and the other half is in the LS state [37]. The increase in μeff/Co approximately from 0.5μB to 1.5μB when temperature decreases from TMI to TC in both TbBaCo2O5 + δ at δ ≈ 0.5 (see Fig. 4) and GdBaCo2O5 + δ at δ ≈ 0.5 (see Fig. 5 in [26]) is thought to be caused by an increase in the fraction of the IS state from a few percent to 20–25% in the IS/LS state of the pyramids. The same μeff/Co(T) results could also be interpreted as an increase in the fraction of HS in the HS/LS state of the pyramids; the only difference is that the fraction of HS states would be approximately half as much. In the orbital ordering model approximation, the transition into the low-temperature insulator state occurs from the HS into the LS state of the Co3+ ions in octahedra. In pyramids, half the Co3+ ions is retained in the LS state and the other half should transform into the HS state. This model is in conflict with our data and the well-known data [15] on measuring the paramagnetic susceptibility, since the effective moment in the low-temperature phase should be twice as large, about μeff/Co ≈ 2.5μB. On the other hand, the authors of [15] state that the magnetic moment per Co3+ ion increases from 1.22μB to 2.8μB when the magnetic field increases from 1 to 50 kOe. The substantiation of the proposed model and, in particular, the presence of the HS state in the low-temperature phase need additional investigations.
Notes
As follows from [13], the Co3+ ions in the metallic phase of PrBaCo2O5.50 are in an HS/IS state at the ratio 1 : 1 if the PM contribution is taken into account.
REFERENCES
C. Martin, A. Maignan, D. Pelloquin, et al., Appl. Phys. Lett. 71, 1421 (1997).
A. Maignan, C. Martin, D. Pelloquin, et al., J. Solid State Chem. 142, 247 (1999).
A. A. Taskin, A. N. Lavrov, and Yoichi Ando, Phys. Rev. B 71, 134414 (2005).
C. Frontera, J. L. García-Muñoz, A. Llobet, et al., Phys. Rev. B 65, 180405(R) (2002).
Y. Moritomo, T. Akimoto, M. Takeo, et al., Phys. Rev. B 61, 13325(R) (2000).
H. Kusuya, A. Machida, Y. Moritomo, et al., J. Phys. Soc. Jpn. 70, 3577 (2001).
M. Respaud, C. Frontera, J. L. García-Muñoz, M. A. Aranda, B. Raquet, J. M. Broto, H. Rakoto, M. Goiran, A. Llobet, and J. Rodriguez-Carvajal, Phys. Rev. B 64, 214401 (2001).
S. Roy, M. Khan, Y. Q. Guo, et al., Phys. Rev. B 65, 064437 (2002).
F. Fauth, E. Suard, V. Caignaert, et al., Phys. Rev. B 66, 184421 (2002).
Z. X. Zhou, S. McCal., C. S. Alexander, et al., Phys. Rev. B 70, 024425 (2004).
H. D. Zhou and J. B. Goodenough, J. Solid State Chem. 177, 3339 (2004).
E. Pomjakushina, K. Conder, and V. Pomjakushin, Phys. Rev. B 73, 113105 (2006).
C. Frontera, J. L. García-Muñoz, A. E. Carillo, et al., Phys. Rev. B 74, 054406 (2006).
Y. Diaz-Fernandez, L. Malavasi, and M. C. Mozzati, Phys. Rev. B 78, 144405 (2008).
M. Baran, V. I. Gatalskaya, R. Szymczak, et al., J. Phys.: Condens. Matter 15, 8853 (2003).
E. L. Nagaev, Phys. Usp. 39, 781 (1996).
E. Dagotto, New J. Phys. 7, 67 (2005).
N. I. Solin, J. Magn. Magn. Mater. 401, 677 (2016).
V. A. Ryzhov, A. V. Lazuta, V. P. Khavronin, P. L. Molkanov, Ya. M. Mukovskii, and A. E. Pestun, Phys. Solid State 56, 68 (2014).
N. I. Solin, S. V. Naumov, S. V. Telegin, et al., JETP Lett. 104, 49 (2016).
N. I. Solin, S. V. Naumov, and S. V. Telegin, J. Exp. Theor. Phys. 128, 281 (2019).
N. B. Ivanova, S. G. Ovchinnikov, M. M. Korshunov, I. M. Eremin, and N. V. Kazak, Phys. Usp. 52, 789 (2009).
Z. Hu, Hua Wu, T. C. Koethe, et al., New J. Phys. 14, 123025 (2012).
M. Hidaka, M. Soejima, R. P. Wijesundera, et al., Phys. Status Solidi B 243, 1813 (2006).
M. Kopcewicz, D. D. Khalyavin, I. O. Troyanchuk etal., J. Phys.: Condens. Matter 14, 9007 (2002).
N. I. Solin, S. V. Naumov, and S. V. Telegin, JETP Lett. 107, 203 (2018).
M. Soda, Y. Yasui, T. Fujita, et al., J. Phys. Soc. Jpn. 72, 1729 (2003).
E. Rautama and M. Karppinen, J. Solid State Chem. 83, 1102 (2010).
S. V. Vonsovskii, Magnetism (Wiley, New York, 1971).
T. I. Arbuzova, S. V. Telegin, S. V. Naumov, et al., Solid State Phenom. 215, 83 (2014).
S. Kolesnik, B. Dabrowski, O. Chmaissem, et al., Phys. Rev. B 86, 1064434 (2012).
J. S. Smart, Effective Field Theories of Magnetism (Saunders, London, 1966).
V. A. Dudnikov, D. A. Velikanov, N. V. Kazak, C. R. Michel, J. Bartolome, A. Arauzo, S. G. Ovchinnikov, and G. S. Patrin, Phys. Solid State 54, 79 (2012).
N. B. Ivanova, N. V. Kazak, C. R. Michel, A. D. Balaev, and S. G. Ovchinnikov, Phys. Solid State 49, 2126 (2007).
A. Muñoz, M. Martínez-Lope, J. A. Alonso, et al., Eur. J. Inorg. Chem. 2012, 5825 (2012).
K. R. Zhdanov, M. Yu. Kameneva, L. P. Kozeeva, and A. N. Lavrov, Phys. Solid State 52, 1688 (2010).
V. P. Plakhty, Y. P. Chernenkov, S. N. Barilo, et al., Phys. Rev. B 71, 214407 (2005).
M. García-Fernandes, V. Scarnoli, U. Staub, et al., Phys. Rev. B 78, 054424 (2008).
ACKNOWLEDGMENTS
We thank A.V. Korolev and D.A. Shishkin for the magnetic measurements, as well as A.V. Telegin for fruitful discussion.
Funding
This work was performed in terms of a state assignment of the Federal Agency of Scientific Organizations, project Spin no. AAAA-A18-118020290104-2. This work was supported in part by the Russian Foundation for Basic Research (project 20-02-00461).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Solin, N.I., Naumov, S.V. & Kazantsev, V.A. Spin State of the Co3+ Ions in the Layered TbBaCo2O5.5 Cobaltite in the Metal–Insulator Transition Range. J. Exp. Theor. Phys. 130, 690–698 (2020). https://doi.org/10.1134/S1063776120050106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063776120050106