Abstract
This article reports the characteristics of thermoluminescence (TL) dosimeter of dysprosium doped strontium borate glass for NaO, Li2O, and CaO modifiers imposed by 10–90 Gy dose of 60Co gamma ray irradiation. The different elements are (70.0 – x) B2O3–20SrO–10NaO–xDy2O3, 10Li2O–20SrO–(70 – x)B2O3–(x)Dy2O3 and 10CaO–20SrO–(70.0 – x)B2O3–(x)Dy2O3 (x = 0, 0.10, 0.30, 0.50, 0.70 mol %). The glass was formed by melt quenching method and the ranges of Dy2O3 were 0.0 to 0.7 mol %. Calcium strontium borate doped with 0.3 mol % Dy2O3 shows very good linearity on dose response and gives an excellent sensitivity at 50 Gy dose of 60Co gamma ray irradiation. The samples N1, L1, C3 were completely amorphous. The optimums anneal temperature, time, concentration of Dy2O3 and heating rate were investigated for glass samples N1, L1, C3. The systematic characteristics of glow curve, TL intensity, reproducibility, sensitivity, kinetic parameters and effective atomic number were studied for three selected samples. All samples show the maximum peak from 154–200°C temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Thermoluminescence dosimetry (TLD) was developed for a long time as one of the most important technique for measuring radiation absorbed dose. Up to now, the increasing practice of radiation in personal, medical and environment has motivated the scientist to find a new thermoluminescence (TL) material. The borates glass as a TL material is under investigation due to its near tissue equivalent absorption coefficient, lower melting point, well solubility of rare earth ions and high thermal stability. Infact, real borate glass is sensitive and hygroscopic to its surrounding atmosphere. It’s hygroscopic exhibit a strong drawback on the performance of TL materials. A lot of careful examination has been guided to progress the achievement of current dosimeters for the purpose of discovery of TL materials. Strontium tetraborate is very pleasing materials because it is not hygroscopic as regular way present for inspection of borate compounds [1]. The sensitivity of polycrystalline dysprosium doped strontium tetraborate is 500% greater than LiF: Mg, Ti [2].
To find out a suitable TL material, we are proposing strontium borate glass with distinct modifiers. At first time the Alkali and alkali earth oxide (NaO, Li2O, and CaO) in glassy form have tested thermoluminescence dosimetry (TLD) properties. Alkaline and alkaline earth oxides are excellent for adjusting borate glasses as their realizable practical use to radiation absorbers [3, 4]. The Dy2O3 used as an activator in this report to find the excellent TL efficiency [5]. The objective is to choose one of the best modifier of strontium borate glass doped dysprosium that gives the best TL features. Recently a preliminary results for x-ray diffraction analysis, differential thermal analysis, thermal fading, TL dose response from 1 to 9 Gy doses of 60Co gamma ray irradiation of dysprosium doped strontium borate glass for different glass modifiers (Na, Li, Ca) were presented to EPJ [6]. The present report aims to extend the TL features of glow curves, reproducibility, kinetic parameters at 50 Gy dose and TL intensity, linearity and sensitivity of these materials exposed to high dose 10–90 Gy 60Co γ ray. It is important to verify the effective atomic number of these materials and their application to environmental radiation monitoring.
MATERIALS AND METHODS
Sample Treated
The glass sample of Dy2O3 doped with different modifiers (NaO, Li2O and CaO) of strontium borate at different Dy2O3 concentrations (from 0.0 to 0.70 mol %) was synthesized in Table 1 and they indicate the formation of various elements of the present samples. The glass was formed using melt quenching method. The different elements are (70.0 – x)B2O3– 20SrO–10NaO–xDy2O3, 10Li2O–20SrO–(70 – x)B2O3–(x)Dy2O3 and 10CaO–20SrO–(70.0 – x)B2O3–(x)Dy2O3 (x = 0, 0.10, 0.30, 0.50, 0.70 mol %). We used raw materials in powder form. The details preparations of glass were presented to reference [6]. The samples were annealed for four hour at four hundred degree (400°C) temperatures to take off any irrelevant stresses. Then it was cooled to 27°C. The composition of NSB: xDy, LSB: xDy and CSB: xDy are shown in Table 1–3.
Irradiation
The samples were divided to tiny parts with equal mass and thickness. These pieces were weighted by analytical electronic device with a precision of 4 decimal. The samples were then labeled and placed in capsules. These capsules were sealed in black paper to safe from surrounding light. Each glass samples were irradiated by 60Co with 50 Gy dose at Universiti Kebangsaan Malaysia, UKM, Bangi. The samples were readout after one day of irradiation using a TL reader (Harshaw Model 4500). During the examinations of TL readouts the heating rates of NSB was 3°C s–1, LSB was 5°C s–1, and CSB was 7°C s–1 that provides the best behavior of traps in a luminescent material [6]. All TL data were normalized to unit mass.
Standard Error of the Measurements
Standard Error is taken for the data of each batch samples using a Poisson distribution. The calculation of the mean, \(\bar {x}\), the best estimation of the standard deviation, \({{\sigma }_{{{\text{est}}}}}\) and the standard error in the mean, \(\Delta \bar {x}\) for the data {\({{x}_{{{\text{obs}}}}}\)} are given as follow [7]:
Where N is the number of the data point.
RESULTS AND DISCUSSION
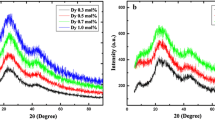
The diffraction of X-ray and differential thermal analysis with samples N1, L1, and C3 were presented to conference [6]. The intensity of XRD form of N1, L1, C3 samples were found no Bragg sharp peaks and they were completely amorphous. In DTA, we found the values of Trg from 0.63 to 0.64 and Hg from 0.53 to 0.58. Glass forming proficiency is excellent in the range 0.50 ≤ Trg ≤ 0.67. Kauzmann rule is good for the composition of samples. The stability of glass was very weak for Hg ≤ 0.1 and superior for Hg ≥ 0.5.
Pre-annealing Process
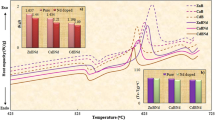
The samples were wormed at temperatures from 100 to 400°C for the time 10 to 60 minutes. Then the samples were irradiated with doses 50 Gy subjected to 60Co gamma irradiations. Figures 1 and 2 show the optimum anneal temperature of glasses up to 400°C. All samples were wormed at this temperature up to 60 min. Based on the result, the ideal annealing time and temperature for dysprosium doped with different modifiers strontium borate glass is summarized in Table 4.
Optimum Dy2O3 Concentration
The best optimum Dy2O3 doped with different modifiers of strontium borate glasses is important to determine the best TL performance. Figures 3, 4 and 5 show that the glow plots of Dy2O3 doped with different modifiers of strontium borate glasses at different kinds of Dy2O3 concentrations to 60Co irradiation at a delivery dose of 50.0 Gray at time temperature profile 5°C s–1. It is shown that single wide peak of TL glow curve existed in all glasses. In Figs. 3, 4 and 5 the highest peak temperature, Tm is located between 180 and 250°C. Usually, a perfect glow curve has a narrow peak existed from 180 to 250°C [8]. The best Optimum concentration Dy2O3 doped (mol %) of sample N1, L1 and C3 are given in Table 5.
Time Temperature Profile (TTP)
Actually, in the TL measurements the intensity of thermoluminescence dely on the heating rate. Figure 6 indicate the TL intensity with variation of the heating rate and its standard error bar for each glass samples irradiated at 50 Gy. The optimum heating rate of the glasses is given in Table 6. For the samples N1, L1 and C3 the time temperature profiles are 3, 5 and 7°C s–1 respectively. At heating rate measured from 1°C s–1 to 20°C s–1, the maximum TL response is observed with the lowest standard error. It reveals that the presece of different modifiers (NaO, Li2O, and CaO) on strontium borate glass that affects the TL response of the heating rate of time temperature profile (TTP).
Glow Curve
Figure 7 demonstrate TL intensity curves of samples N1, L1 and C3 exposed to high dose, 50 Gy. Only one wide peak of TL energetic level of traps was examined after 24 h of irradiation for all samples of the most favourable Dy-doped concentration of strontium borate glass. The glass sample of C3 gives the maximum peak temperature, Tm, response for high dose at range 200°C respectively that is accepable for an ideal glow curve.
TL Intensity
The TL intensity of samples (N1, L1, C3) was determined for doses ranging from 10 to 90 Gy. It is noted that all data points were presented using mean value of five individual readings. The TL response do not strongly depend on energy, and it tends to compass at higher energies from 10–90 Gy. From Fig. 8 it is shown that the TL response obtained for sample C3 is very high when compared with those of N1 and L1; the slope of the response curve of C3 is 35 890.48 nCg–1 Gy–1 where as those of L1 is 3318. 47nCg–1 Gy–1 and sample N1 is 7565.87 nCg–1 Gy–1. Therefore the TL response obtained for sample C3 is clearly stronger than those of samples L1 and N1. The discrimination of TL intensity for N1 sample is slightly higher than L1. The TL yield of all samples raises linearly with increase of dose from 10 to 90 Gy.
Sensitivity
Table 7 shows the relative sensitivity and sensitivity of N1, L1, and C3 glass samples. The TL sensitivity are found from the slope of the plot of Fig. 8. It illustrates that sample C3 has the highest relative sensitivity relative to samples N1 and L1. The TL material with high sensitivity using low dose irradiation is very important for their utilization in the assessment [9, 10]. The relative sensitivities are C3 : N1 : L1 = 2.4975 : 0.4202 : 0.0994.
Reproducibility
Study on reproducibility is very important TL nature to find the reusability of the materials after using many times. It is note that TL yields of a specific dose should be almost same after few experiments. Usually a TL sample is supposed to undergo any physiochemical change due to repeat of irradiation [11] (Manam and Sharma, 2004). We have investigated reproducibility of glass (N1, C3, L1) using γ irradiation with 5 Gy doses [6]. At present work the relative intensity of glass (N1, L1, C3) using γ irradiation with 50 Gy dose are given in Fig. 9. The data are taken after five times sequential cycles. The result of TL intensity verses number of exposure cycle confirms reusable of glass materials in radiation dosimeter.
Kinetic Trap Study
In this study, we applied Chen’s peak shape method to compute the parameters of kinetic trap for glow curve of 50 Gy dose by using Eq. (4), (5) and (6).
where T2 is temperature at IM/2 on the falling side and T1 is at IM/2 of rising side of glow curve. Tm indicate temperature at maximum, β the heating rate, Boltzmann’s constant k and \(\alpha \) is corresponding to \(\tau \), δ, and ω as shown in Fig. 10. The data is tabulated in Table 8. Here Eav and sav is average activation energy and frequency factor. These are appraise from the activation energy and frequency factor of \(\tau \), δ, and ω. It shows that all samples (N1, L1 and C3) possess second kinetic order as the value of μg is in the range of 0.6, which is more than 0.52 [12]. We can conclude that with increasing of heating rate, the activation energy decreases and the frequency factor becomes smaller.
Effective Atomic Number
The Zeff of the sample is calculated using Eq. (7).
With
Here a1, a2, are the fractional contents of electrons, Z1, Z2… atomic number in the composition, ni is the number of electrons per mole, Zi atomic number of element and NA is 6.02 × 1023 mol–1 (Avogadro’s number). The value of b is 2.9 to 3.5 [13]. The mean of the atomic mass of the optimum concentration Dy-doped different modifier (NaO, Li2O, and CaO) on strontium borate glass is in Table 9. The mean of Zeff of N1, L1, and C3 glass samples are in the range 22 to 24, which is more than an effective number of tissue. Jiang et al. has reported that the effective number of the LiSr4(BO3)3 is about 33.70 [14]. It is appripate investigation of borate material in TLD field which can be applied to environmental radiation monitoring.
CONCLUSION
The TL properties of various Dy-doped of different modifier (NaO, Li2O, and CaO) on strontium borate glass system have been investigated subjected to Co-60 gamma irradiation at range 10 to 90 Gy. The optimum Dy-doped has identified for NaSr(BO3)3 is 0.1 mol % of Dy2O3, LiSr(BO3)3 is 0.1 mol % of Dy2O3 and CaSr(BO3)3 is 0.30 mol % Dy2O3. The analysis of XRD and DTA of the present sample confirms the amorphous stable state [6]. The analysis of glow curve, reproducibility, TL intensity, sensitivity, and effective atomic number, Zeff has been investigated for all set of samples. It is concluded that calcium strontium borate glass doped with 0.3 mol % of dysprosium oxide are the best dosimeter among three modifiers.
REFERENCES
Santiago, M., Grasseli, C., Caselli, E., Lester, M., Lavat, A., and Spano, F., Phys. Status Solidi A, 2001, vol. 185. no. 2, p. 285.
Lavat, A., Graseli, C., Santiago, M., Pomarico, J., and Caselli, E., Cryst. Res. Technol., 2004, vol. 39, no. 10, p. 840.
Gautam, C., Yadav, A.K., and Singh, A.K., ISRN Ceram., 2012, vol. 2012, p. 17.
Aboud, H., Wagiran, H., Hossain, I., and Hussain, R., Int. J. Phys. Sci., 2012, vol. 7, no. 6, p. 922.
Prokic, M. and Yukihara, E., Radiat. Meas., 2008, vol. 43, no. 2, p. 463.
Hamzah, S.A., Saeed, M.A., Wagiran, H., and Hashim, I.H., EPJ Web of Conferences, 2017, 156 00007. https://doi.org/10.1051/epjconf/201715600007
Supian, S. and Evans, C.J., Statistics and Nuclear Counting: Theory, Problems and Solutions, Serdang: Universiti Pertanian Malaysia, 1992.
Lim, T.Y., Wagiran, H., Husin, R., and Hashim, S., Appl. Radiat. Isot., 2015, vol. 102, p. 10.
Alajerami, Y., Thermoluminescence and optical characteristics of lithium potassium borate glass for radiation therapy dose measurement, PhD Thesis, Universiti Teknologi Malaysia, 2014.
Marcazzo, J., Santiago, M., D’Angelo, C., Furetta, C., and Caselli, E., Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, vol. 286, no. 2, p. 183.
Manam, J. and Sharma, S., K, Nucl. Instrum. Methods Phys. Res., Sect. B, 2004, vol. 217, no. 2, p. 314.
McKeever, S.W.S., Moscovitch, M., and Townsend, P.D., Thermoluminescence Dosimetry Materials: Properties and Uses., Ashford: Nuclear Technology Publishing. 1995.
Furetta, C., Prokic, M., Salamon, R., Prokic, V., and Kitis, G., Nucl. Instrum. Methods Phys. Res., Sect. A, 2001, vol. 456, no. 3, p. 411.
Jiang, L.H., Zhang, Y.L., Li, C.Y., Hao, J.Q., and Su, Q., J. Mater., 2007, vol. 61, p. 5107.
ACKNOWLEDGMENTS
The authors would like to thank the Universiti Kebangsaan Malaysia (UKM), Bangi for providing irradiation facilities. The authors would like to thank the Ministry of Higher Education (MOHE) of Malaysia and Universiti Teknologi Malaysia (UTM) for supporting the research and providing research grant (R.J130000.7826.4F736).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hamzah, S.A., Saeed, M.A., Hossain, I. et al. Thermoluminescence of Dysprosium Doped Strontium Borate Glass for (NA, LI, CA) Modifiers Irradiated to High Dose 60CO. High Energy Chem 53, 238–245 (2019). https://doi.org/10.1134/S0018143919030068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143919030068