Abstract
According to the two-hit hypothesis of psychoneuropathology formation, infectious diseases and other pathological conditions occurring during the critical periods of early ontogenesis disrupt normal brain development and increase its susceptibility to stress experienced in adolescence and adulthood. It is believed that these disorders are associated with changes in the functional activity of the glutamatergic system in the hippocampus. Here, we studied expression of NMDA (GluN1, GluN2a, GluN2b) and AMPA (GluA1, GluA2) glutamate receptor subunits, as well as glutamate transporter EAAT2, in the ventral and dorsal regions of the hippocampus of rats injected with LPS during the third postnatal week and then subjected to predator stress (contact with a python) in adulthood. The tests were performed 25 days after the stress. It was found that stress altered protein expression in the ventral, but not in the dorsal hippocampus. Non-stressed LPS-treated rats displayed lower levels of the GluN2b protein in the ventral hippocampus vs. control animals. Stress significantly increased the content of GluN2b in the LPS-treated rats, but not in the control animals. Stress also affected differently the exploratory behavior of LPS-injected and control rats. Compared to the non-stressed animals, stressed control rats demonstrated a higher locomotor activity during the 1st min of the open field test, while the stressed LPS-injected rats displayed lower locomotor activity than the non-stressed rats. In addition, LPS-treated stressed and non-stressed rats spent more time in the open arms of the elevated plus maze and demonstrated reduced blood levels of corticosterone. To summarize the results of our study, exposure to bacterial LPS in the early postnatal ontogenesis affects the pattern of stress-induced changes in the behavior and hippocampal expression of genes coding for ionotropic glutamate receptor subunits after psychogenic trauma suffered in adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The role of early experiences in development of increased susceptibility to the stress-induced psychopathological states has been actively discussed during the recent years [1, 2]. In particular, this problem is considered within the framework of the two-hit hypothesis, which suggests that stress, infectious diseases, and other pathological states occurring during the critical periods of early ontogenesis disrupt early brain development and increase the organism susceptibility to stress experienced in adolescence and adulthood [3-5]. Bacterial infection, as one of the damaging factors, can be modeled experimentally by injecting lipopolysaccharide (LPS, endotoxin), a component of the cell wall of gram-negative bacteria. It was shown earlier that LPS injection in early ontogenesis can produce a long-lasting damaging effect on the CNS function and disrupt cognitive functions and emotional behavior [6-11]. Changes in the stress reactivity of animals injected with LPS in early ontogenesis have been demonstrated in several studies [12, 13].
The mechanisms of LPS-induced disorders of behavior and stress reactivity are poorly studied. It was suggested that disruptions in the functional activity of the glutamatergic system in the brain can be associated, in particular, with disorders in the formation of ionotropic NMDA and AMPA glutamate receptors [14], which have a complex subunit structure. NMDA receptors are heterotetramers consisting of the obligatory GluN1 subunit and variant GluN2a-d or GluN3 a, b subunits responsible for the functional and regional variability of NMDA receptors [15]. AMPA receptors consist of four GluA subunits (GluA1-4). AMPA receptors containing the GluA2 subunit are impermeable to calcium ions [16]. The subunit composition of NMDA and AMPA receptors in the brain of adult rats is formed during the first weeks of life [17-21]. LPS injection during this period leads to the short- and long-lasting alterations in the expression of genes encoding NMDA and AMPA receptor subunits in the hippocampus and cerebral cortex [10, 11, 14].
It is well known that NMDA and AMPA receptors are involved in cognitive functions [22-25] and psychoemotional responses [26]. Stress changes expression of these receptors in the hippocampus [27, 28]. It is assumed that ionotropic glutamate receptors mediate fear extinction, while disturbances in this process play a key role in the pathophysiology of post-traumatic stress disorder (PTSD) [29, 30]. Administration of NMDA receptor antagonists prevents stress-induced hormonal and behavioral disorders [31, 32], that evidencing the involvement of glutamate receptors in the regulation of stress responses.
EAAT2 glutamate transporter (GLT-1) is another protein involved in the glutamatergic system activity. EAAT2 is produced mainly by astrocytes; it is responsible for the recapture by these cells of up to 90% of glutamate from the synaptic gap, thus acting as the major regulator of extracellular glutamate levels [33]. Changes in the EAAT2 production in the hippocampus have been observed in the learned helplessness paradigm (model of depression) [34] and in adult rats subjected to social stress (maternal separation) in the neonatal period [35]. However, EAAT2 expression in the brain of stressed adult animals injected with LPS at the early age has not been studied before.
The purpose of the present work was to investigate the combined effects of the neonatal LPS injection and stress in adulthood on rat behavior, corticosterone levels, and expression of EAAT2 and subunits of NMDA and AMPA receptors in the dorsal and ventral regions of the hippocampus. The model of stress (predator stress caused by rat exposure to a python) and the testing protocol were chosen based on our earlier studies that have demonstrated that the predator stress affected expression of the studied genes [36]. Here, gene expression was analyzed separately in the ventral and dorsal regions of the hippocampus, because these regions differ in their functions and impact of stress on the expression of genes coding for the glutamate receptor subunits and EAAT2 [27, 37].
MATERIALS AND METHODS
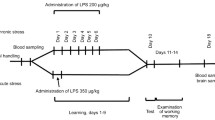
Treatment of rats. Experimental scheme. The study was performed in male Wistar rats in full compliance with the recommendations of the European Community Directive no. 86/609 EC and was approved by the Local Ethics Committee of the Institute of Evolutionary Medicine. The experimental scheme is presented in Fig. 1. Rat pups were kept with their mothers, one litter per cage (14 litters in total). The number of pups in a litter was limited to 7-8; if necessary, some female pups were left, but not used in the experiments. Bacterial LPS (serotype 055:B5 from Escherichia coli, 50 µg/kg; Sigma-Aldrich, USA) or pyrogen-free saline was injected intraperitoneally once a day on postnatal days 15, 18, and 21. The pups were taken from their mothers for no longer than 1 min. Each litter was divided into control (saline) and experimental (LPS) animals. At 1 month of age, the pups were separated from their mothers. The LPS dosage was chosen based on the earlier studies to possess a moderate pyrogenicity and to influence the expression of genes encoding NMDA and AMPA receptor subunits [10].
Exposure to stress. At the age of 3 months, a half of the experimental (n = 16) and control (n = 19) animals were subjected to stress by being threatened with death by a predator (python) and witnessing a death of other rat [38]. For this, a group consisting of the experimental and control rats (17-20 animals in total) was placed into a terrarium with a hungry python, where one of the rats was killed by the predator. The remaining rats were exposed to the stressful situation by being kept in the terrarium for the following 20-25 min. Then, the rats were taken from the terrarium and placed back into the cages, where they were kept until behavioral testis or collection of biological material for analysis. Non-stressed experimental and control animals were used for comparison.
Since we were interested in the long-lasting stress-induced changes, the experiments were performed 25 days after the stress in the stressed and non-stressed animals of the same age. Different groups of control and experimental rats were used for performing biochemical (saline, n = 17; LPS, n = 13) and behavioral (saline, n = 19; LPS, n = 16) studies.
Western blotting analysis of protein expression. The levels of NMDA (GluN2a, GluN2b) and AMPA (GluA1, GluA2) receptor subunits were determined by Western blotting. After the animals were sacrificed by decapitation, their entire brains were isolated, frozen immediately, and stored at –70°C. The ventral and dorsal regions of the hippocampus were isolated from the sections prepared with a Thermo Scientific MICROM HM microtome-cryostat (Thermo Scientific, USA) at –20°C according to the rat brain atlas [39] (Fig. S1 in the Supplement). Isolated brain structures were homogenized on ice in the optimized lysing buffer proposed by Kopec et al. [40] supplemented with 1× protease inhibitor cocktail (Pierce Protease Inhibitor Tablets, Thermo Fisher Scientific, USA) and incubated for 60 min at room temperature. The cell debris was removed by centrifugation (15 min, 14,000g, 20°C). Protein concentration in the samples was determined by the modified Lowry method [41]. The supernatant was diluted at a 1 : 1 ratio with 2× loading buffer (125 mM Tris-HCl, pH 6.8; 40% (v/v) glycerol; 4% sodium dodecyl sulfate; 2.5% β-mercaptoethanol; 0.02% Bromophenol Blue) and incubated in a thermostat (BioSan, Latvia) for 15 min at 70°C. Electrophoretic separation was performed under reducing denaturing conditions [42] in 7% polyacrylamide gel using molecular weight standards (Thermo Scientific PAGE Ruler Prestained Protein Ladder 10-170 kDa; Thermo Fisher Scientific) at 125 V. Each protein sample contained 6 µg of total protein per lane, because this amount of protein allowed us to work within the linear region of densitometric analysis for all used antibodies. Together with the analyzed specimens, each gel had a standard calibrator sample obtained by mixing several specimens from animals of different groups.
Fractionated proteins were transferred onto a nitrocellulose membrane (pore diameter, 45 µm) by the semi-dry transfer in 1× transfer buffer (Invitrogen Power Blotter 1-Step Transfer Buffer, Thermo Fisher Scientific) according to the manufacturer’s instructions. After the transfer, the membrane was stained with 0.1% Ponceau S solution in 5% acetic acid (Merck KGaA, Germany); the results were documented using a ChemiDoc MP gel visualization system (Bio-Rad, USA). Next, the membrane was blocked with 5% fat-free milk (Sigma-Aldrich, Switzerland) in PBS-T [0.01 M phosphate buffer, pH 7.4; 137 mM NaCl; 2.7 mM KCl containing 0.1% (v/v) Tween 20] for 1.5 h at room temperature, washed three times with PBS-T, and incubated overnight at 4°C with primary antibodies against GluN2a (ab169873, rabbit polyclonal antibodies), GluN2b (ab65783, rabbit polyclonal antibodies), GluA1 (ab109450, rabbit monoclonal antibodies), GluA2 (ab106515, mouse monoclonal antibodies), and EAAT2 (ab205248, rabbit monoclonal antibodies) in the same buffer. All primary antibodies (Abcam, Great Britain) were diluted 1/1000. Next, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulins G (31460, 1/60,000, Pierce Goat anti-rabbit IgG-HRP, Thermo Fisher Scientific) for GluN2a/2b, GluA1, and EAAT2 or anti-mouse immunoglobulins G (ab6808, 1/40,000, Sheep Anti-Mouse IgG H&L (HRP), Abcam) for GluA2. The membranes were developed with the chemiluminescent substrate Super-Signal™ West Pico PLUS (Thermo Fisher Scientific) and documented with a ChemiDoc MP system (Bio-Rad). Densitometric analysis was performed with the Image Lab 6.0.1 software (Bio-Rad); the optical density of the signal was normalized to the calibrator specimen based on the Ponceau S staining (total protein) of the corresponding lane.
Determination of corticosterone levels in the blood. The corticosterone level was determined in the peripheral blood serum of the decapitated animals by competitive enzyme immunoassay using a Corticosterone (Human, Rat, Mouse) ELISA (RE52211) kit (TECAN Trading, Switzerland) according to the manufacturer’s recommendations. Spectrophotometric analysis was performed with an Immunochem-2100 Microplate Reader (HTI Diagnostics, USA). The concentration of corticosterone was calculated by the linear regression method. All experiments were performed in two independent repeats.
Behavioral tests. The orientation and exploratory behavior and the anxiety levels in rats were assessed in the open field test (OFT) and elevated plus maze (EPMT) tests. In the OFT, an open round platform 1 m in diameter was illuminated at 10 lx. A rat was placed in the center of the platform, and the test was conducted for 3 min. The following parameters were recorded: total distance covered (locomotor and exploratory activity), distance covered in the first minute (orientation and exploratory behavior), and time spent in the center of the platform and at its periphery (anxiety level). The general strategy of rat behavior (the shape of track) was analyzed as well (see Results for details).
EPMT was used to assess the anxiety level. The installation consisted of the central platform (10×10 cm) with two open and two closed arms (50×10 cm) elevated 40 cm above the floor. The closed arms had 30 cm-high walls and were illuminated at 5 lx. The open arms were illuminated at 10 lx. A rat was placed in a closed arm, and the test was conducted for 5 min. The following parameters were assessed: time spent in the closed and open arms; time spent for peeping out of the closed arms; the number of visits to the open and closed arms, the percentage of time spent in the open arms, and the grooming time in the closed arms. All behavioral experiments were recorded with web cameras placed above the installation; the video records were analyzed with a software (Field4W, Pole-7, Pole_Krest) developed at the Physiological Department of the Institute of Experimental Medicine.
The data were processed statistically with the SPSS Statistics 22 software (IBM Corp., USA) using the Kolmogorov–Smirnov test for the distribution normality assessment, the Levene’s test for verifying the equality of variances, two-factor analysis of variance (ANOVA), and the Student’s t-test with the Bonferroni correction as a post hoc test. The Pearson’s χ2 test was used to analyze the intergroup differences in the frequencies of different types of tracks in the OFT. The differences were considered significant at p < 0.05. The data in the graphs are presented as mean ± standard error. The graphs were plotted with the GraphPad Prism 8 software (GraphPad Software, USA).
RESULTS
The used LPS dose of 50 µg/kg did not affect animal development and body weight dynamics (see Fig. S2 and Table S1 in the Supplement).
Expression of genes for the ionotropic glutamate receptor subunits was studied at the protein level (see Fig. S3 in the Supplement for the results of membrane staining). The differences between the groups were more pronounced in the ventral vs. dorsal hippocampus. In the non-stressed rats, the levels of the NMDA receptor GluN2b subunit were lower in the animals injected with LPS at the early age were (n = 4) as compared to the control rats (n = 6) (Fig. 2, t = 4.75; p = 0.002, significant with consideration of the Bonferroni correction).
LPS injection affected the ratio between the GluN2a and GluN2b subunits, which was higher in the LPS-injected rats [F(1,17) = 6.97; p = 0.02], especially in the non-stressed animals (Fig. 2).
Stress also affected differently the expression of the NMDA receptor GluN2b subunit and AMPA receptor GluA1 subunit in the experimental and control rats [Figs. 2 and 3; interaction of the stress and group factors, respectively, F(1,20) = 4.71; p = 0.04 and F(1,19) = 4.87; p = 0.04]. The level of the GluN2b subunit in the experimental rats increased after the stress (t = 3.04; p = 0.025), whereas in the control rats, it displayed a trend for a decrease vs. the non-stressed animals.
Expression of genes encoding NMDA receptor subunits in the hippocampus of experimental and control rats (n = 4-8 in each group). Each point corresponds to a single animal. F, Fisher’s criterion, two-factor ANOVA; *, significant differences from the control group, #, significant differences for the non-stressed group; p < 0.05, Student’s t-test with the Bonferroni correction. Representative Western blots for each group of animals are shown left to the graphs.
No significant changes in the EAAT2 levels in the ventral and dorsal regions of the hippocampus were found (Fig. 4).
Therefore, neonatal injection of LPS affected expression of genes encoding ionotropic glutamate receptor subunits in the ventral, but not in the dorsal hippocampus. The most pronounced changes in the non-stressed and stressed -animals were observed for the NMDA receptors GluN2b subunit. To elucidate whether these changes influence animal behavior after psychogenic trauma, we studied rat behavior the OFT and EPMT using the same experimental paradigm, because animal behavior in these testes is known to depends on the activity of the GluN2b-containing NMDA receptors [43, 44].
The total covered distance in the OFT (Fig. 5) did not differ between the groups, however, the activity during the first minute (an indicator of orientation and exploratory behavior) increased in the stressed control rats and decreased in the experimental rats [interaction of stress and group factors: F(1,31) = 6.32; p = 0.017]. No statistically significant difference was revealed in the time spent in the field center and at the periphery, but the character of the track differed in the rat groups. Three types of the tracks were observed. The first type, that occupied mostly the field periphery (exploration of the entire field) was found in the majority of non-stressed control and experimental animals (66.7 and 70%, respectively) and in 70% of the control stressed rats. The second type, with frequent visits to the field center, was characteristic for the one-third of non-stressed control and experimental rats. The third track type, with the exploration only of a part of the field, was described in 44.4% of experimental stressed rats. Apparently, this indicated lower exploratory activity, and, possibly, increased anxiety level. The prevalence of different types of track was significantly different in the four groups (χ2 = 16.4; p = 0.012).
In the EPMT, stressed and non-stressed experimental rats stayed longer in the open arms of the maze (Fig. 6a; group factor F(1,27) = 4.70; p = 0.04); similar results were obtained for the percent of time spent by the animals in the open arms (group factor F(1,27) = 4.56; p = 0.04; Fig. S4 and Table S2 in the Supplement). These results corresponded to the trend for a lesser time spent at the field periphery displayed by the experimental rats in the OFT (F(1,31) = 2.98; p = 0.09). However, these results cannot be unambiguously interpreted as a decrease in anxiety, because the stressed experimental rats displayed longer grooming time in the closed arms: (Fig. 6b; interaction of factors stress and group: F(1,28) = 4.29; p = 0.048). No differences were found in the time spent in the closed arms, as well as in the time spent for peeping out of the closed arms and in the number of visits into the open and closed arms (Fig. 5 and Table S2 in the Supplement).
Therefore, the behavioral response to stress was different in the rats injected with LPS during the neonatal period and control animals.
To test the assumption that the observed differences can be associated with changes in the corticosterone levels, we analyzed blood levels of this hormone in the control and experimental rats subjected and not subjected to the psychogenic trauma. It was shown that the LPS injection at the early age decreased the blood level of corticosterone in both stressed and non-stressed rats [Fig. 7, LPS injection factor: F(1,23) = 6.22; p = 0.02].
Based on the results obtained results, injection of bacterial LPS in early postnatal ontogenesis during the period crucial for the formation of ionotropic glutamate receptors (group factor) influences the ratio between the GluN2a and GluN2b subunits in the ventral hippocampus, as well as rat behavior in the EPMT and blood levels of corticosterone. Different stress response of the control and experimental rats (combined influence of the group and stress factors) was manifested as differences in the expression of the NMDA receptor GluN2b and AMPA receptor GluA1 subunits and in the rat exploratory and emotional behavior.
DISCUSSION
In this work, expression of ionotropic glutamate receptor subunits in the hippocampus of rats after combined exposure to the neonatal injection of LPS and psychogenic trauma in adulthood was studied for the first time.
The group factor (LPS injection) had a pronounced effect on the GluN2a/GluN2b ratio in the ventral hippocampus and on rat behavior in the EPMT. We also showed that the non-stressed experimental rats displayed a decreased GluN2b levels in the ventral hippocampus. An increase in the GluN2a/GluN2b ratio was shown earlier in adult rats subjected to prenatal viral infection (via injection of pregnant females with a synthetic polyI:C molecule). However, in this case, the ratio between the subunits was increased due to the upregulated synthesis of the GluN2a subunit [45]. A similar increase in the synthesis of mRNA for the GluN2a subunit was observed in adult rats that had received a single injection of a high dose of LPS (100 µg/kg) on postnatal day 14 (the GluN2a/GluN2b ratio was not assessed) [14]. Our earlier studies in juvenile rats that were injected with LPS during the third postnatal week revealed a decrease in the GluN2b subunit level and increase in the GluN2a/GluN2b ratio in the dorsal and ventral regions of the hippocampus [11]. However, similar LPS injections upregulated GluN2b mRNA in the adult animals [10]. The activity of NMDA and AMPA receptors is directly associated with the long-term potentiation (LTP) in the hippocampal neurons. It was found that the LPS injections in the neonatal period led to the LTP disturbances in sexually immature animals [11, 46-48]. It should be noted that the most pronounced changes have been observed in the ventral hippocampus. Onufriev et al. [49] showed that LPS injections in adult rats led to different dynamics in the cell activity in the ventral and dorsal regions of the hippocampus (based on the LTP parameters and expression of genes encoding proinflammatory cytokines): the maximal response in the dorsal hippocampus was observed earlier than the maximum response in the ventral hippocampus.
LPS-induced rearrangements in the subunit composition of ionotropic glutamate receptors can influence behavior [43, 44]. Multiple studies have shown impaired exploratory behavior and changes in the anxiety levels in adult animals subjected to the LPS injections in the neonatal period [46, 50-52]. In particular, adult rats injected with LPS during the third postnatal week exhibited significantly reduced exploration of novel objects [53] and decreased number of rearings in the OFT [10]. The symptoms of anxiety and depression were recorded in adult rats and mice that had been injected with LPS on postnatal days 1 or 3 and 5 [46, 50, 54]. However, other authors [55] observed reduced anxiety (assessed by the time spent in the open arms in the EPMT) in the juvenile rats injected with LPS (100 µg/kg) on postnatal day 5. We found that adult rats injected with LPS during the third postnatal week spent less time in the open arms in the EPMT [10].
The main objective of this study was to investigate the specific features of the response to psychogenic trauma in adult rats injected with LPS at the early age. Modeling of the life-threatening situations, in particular, exposure of laboratory rodents to a predator (or its odor), is frequently used for PTSD modeling [56]. This disorder is characterized by a delayed development of psychoneurological impairments [57]. The model used by us in this has been used before for analyzing the course of post-stress changes in the expression of genes encoding NMDA and AMPA receptor subunits in the rat hippocampus, medial prefrontal cortex, and amygdala. The most pronounced changes were found in the hippocampus 25 days after the stress [36]. For this reason, we used the same the time interval to assess the specific features of animals injected with LPS in the neonatal period. In contrast to previous research, we did not find any statistically significant effect of the stress factor on the studied parameters, which might be associated with individual features of animal used in the study. Similar phenomenon has been described in clinical and experimental works, in which PTSD developed only in some humans and animals subjected to stress [56, 58]. Nevertheless, we found that the LPS-injected rats displayed higher stress reactivity as judged from changes in the GluN2b levels. No changes in the biosynthesis of EAAT2 protein in the hippocampus of rats 25 days after the psychogenic trauma were found. Earlier, downregulated expression of this protein was shown in the hippocampus of mice subjected to chronic stress [59]. It is possible that stress used in our study was insufficient for affecting EAAT2 expression.
The main result of our study is identification of the combined effect of LPS injection in the neonatal period and psychogenic trauma on the expression of genes coding for the NMDA receptor GluN2b subunit and AMPA receptor GluA1 subunit, as well as on the exploratory and emotional behavior of rats. To the best of our knowledge, this is the first study on the expression of genes encoding ionotropic glutamate receptors subunits in the used experimental paradigm. The studies on specific features of stress reactivity in animals injected with LPS in the neonatal period assessed by the behavioral and hormonal parameters have been performed before. Thus, Shanks et al. [60] found an increased secretion of adrenocorticotropic hormone (ACTH) in response to the restraint stress in adult rats injected with endotoxin on postnatal days 3 and 5. Moreover, the animals displayed a decreased sensitivity to glucocorticoids, indicating dysregulation of the negative feedback regulatory mechanisms of stress response. Similar results, such as stronger or more prolonged response to stress (assessed by the corticosterone levels) in animals injected with LPS at the early age were also obtained in other works [13, 46]. Walker et al. [12] reported that a 3-day combined stress induced weaker hormonal response in rats injected with LPS at the same early period, although the behavioral stress reactivity of these animals was higher than in the control rats.
In the present study, rats injected with LPS during the third postnatal week demonstrated lower corticosterone levels both in the absence of psychogenic trauma and 25 days after the trauma. The lowest concentrations of corticosterone were recorded in the LPS-injected rats subjected to stress. In another independent study performed by us earlier [9], low corticosterone levels were found in the non-stressed rats that had received three LPS injections (25 or 50 µg/kg) during the third postnatal week. It is possible that the discrepancies in the obtained results are due to different endotoxin of and different times of injections. It should be also noted that the decrease in the blood level of glucocorticoids and disturbances in the negative feedback mechanisms are typical for PTSD [61, 62], which was presumably modeled in our work by the exposure of rats to stress.
In conclusion, our study has shown that the injection of bacterial LPS in the early postnatal ontogenesis affects the stress-induced changes in animal behavior, as well as expression of genes coding for ionotropic glutamate receptor subunits in the hippocampus of adult animals subjected to the psychogenic trauma. A possible relationship between impaired behavioral responses and altered gene expression was indicated, in particular, by Lei et al. [43], who demonstrated that Rislenemdaz (GluN2b-selective NMDA receptor antagonist) caused changes in the animal activity in the OFT. The final conclusion about the association between the neurochemical and behavioral changes found by us can be made only after additional neuropharmacological studies.
Abbreviations
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- EAAT2:
-
excitatory amino acid transporter 2
- EPMT:
-
elevated plus maze test
- LPS:
-
lipopolysaccharide, endotoxin
- NMDA:
-
N-methyl-D-aspartate
- OFT:
-
open field test
- PTSD:
-
post-traumatic stress disorder
References
Lee, R. S., Oswald, L. M., and Wand, G. S. (2018) Early life stress as a predictor of co-occurring alcohol use disorder and post-traumatic stress disorder, Alcohol. Res., 39, 147-159.
Fisher, P. A., Beauchamp, K. G., Roos, L. E., Noll, L. K., Flannery, J., and Delker, B. C. (2016) The neurobiology of intervention and prevention in early adversity, Annu. Rev. Clin. Psychol., 12, 331-357, https://doi.org/10.1146/annurev-clinpsy-032814-112855.
Van Camp, G., Cigalotti, J., Bouwalerh, H., Mairesse, J., Gatta, E., et al. (2018) Consequences of a double hit of stress during the perinatal period and midlife in female rats: mismatch or cumulative effect? Psychoneuroendocrinology, 93, 45-55, https://doi.org/10.1016/j.psyneuen.2018.04.004.
Jaric, I., Rocks, D., Cham, H., Herchek, A., and Kundakovic, M. (2019) Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model, Front. Mol. Neurosci., 12, 74, https://doi.org/10.3389/fnmol.2019.00074.
Koss, K. J., and Gunnar, M. R. (2018) Annual research review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology, J. Child Psychol. Psychiatry, 59, 327-346, https://doi.org/10.1111/jcpp.12784.
Dinel, A.-L., Joffre, C., Trifilieff, P., Aubert, A., Foury, A., Le Ruyet, P., and Layé, S. (2014) Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood, J. Neuroinflammation, 11, 155, https://doi.org/10.1186/s12974-014-0155-x.
Doenni, V. M., Song, C. M., Hill, M. N., and Pittman, Q. J. (2017) Early-life inflammation with LPS delays fear extinction in adult rodents, Brain. Behav. Immun., 63, 176-185, https://doi.org/10.1016/j.bbi.2016.11.022.
Lei, Y., Chen, C.-J., Yan, X.-X., Li, Z., and Deng, X.-H. (2017) Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats, Brain Res., 1671, 43-54, https://doi.org/10.1016/j.brainres.2017.06.014.
Trofimov, A., Strekalova, T., Mortimer, N., Zubareva, O., Schwarz, A., et al. (2017) Postnatal LPS challenge impacts escape learning and expression of plasticity factors Mmp9 and Timp1 in rats: effects of repeated training, Neurotox. Res., 32, 175-186, https://doi.org/10.1007/s12640-017-9720-2.
Trofimov, A. N., Rotov, A. Y., Veniaminova, E. A., Fomalont, K., Schwarz, A. P., and Zubareva, O. E. (2020) Changes in behavior and the expression of ionotropic glutamate receptor genes in the brains of adult rats after neonatal administration of bacterial lipopolysaccharide, Neurosci. Behav. Physiol., 50, 1239-1248, https://doi.org/10.1007/s11055-020-01025-7.
Zubareva, O. E., Postnikova, T. Y., Grifluk, A. V., Schwarz, A. P., Smolensky, I. V., et al. (2020) Exposure to bacterial lipopolysaccharide in early life affects the expression of ionotropic glutamate receptor genes and is accompanied by disturbances in long-term potentiation and cognitive functions in young rats, Brain. Behav. Immun., 90, 3-15, https://doi.org/10.1016/j.bbi.2020.07.034.
Walker, A. K., Nakamura, T., Byrne, R. J., Naicker, S., Tynan, R. J., et al. (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis, Psychoneuroendocrinology, 34, 1515-1525, https://doi.org/10.1016/j.psyneuen.2009.05.010.
Walker, A. K., Nakamura, T., and Hodgson, D. M. (2010) Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats, Stress, 13, 506-515, https://doi.org/10.3109/10253890.2010.489977.
Harré, E.-M., Galic, M. A., Mouihate, A., Noorbakhsh, F., and Pittman, Q. J. (2008) Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain, Eur. J. Neurosci., 27, 644-653, https://doi.org/10.1111/j.1460-9568.2008.06031.x.
Hansen, K. B., Yi, F., Perszyk, R. E., Furukawa, H., Wollmuth, L. P., et al. (2018) Structure, function, and allosteric modulation of NMDA receptors, J. Gen. Physiol., 150, 1081-1105, https://doi.org/10.1085/jgp.201812032.
Liu, S., Lau, L., Wei, J., Zhu, D., Zou, S., et al. (2004) Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia, Neuron, 43, 43-55, https://doi.org/10.1016/j.neuron.2004.06.017.
Wenzel, A., Fritschy, J. M., Mohler, H., and Benke, D. (1997) NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins, J. Neurochem., 68, 469-478, https://doi.org/10.1046/j.1471-4159.1997.68020469.x.
Babb, T. L., Mikuni, N., Najm, I., Wylie, C., Olive, M., et al. (2005) Pre- and postnatal expressions of NMDA receptors 1 and 2B subunit proteins in the normal rat cortex, Epilepsy Res., 64, 23-30, https://doi.org/10.1016/j.eplepsyres.2005.02.008.
Du Bois, T. M., and Huang, X.-F. (2007) Early brain development disruption from NMDA receptor hypofunction: relevance to schizophrenia, Brain Res. Rev., 53, 260-270, https://doi.org/10.1016/j.brainresrev.2006.09.001.
Lippman-Bell, J. J., Zhou, C., Sun, H., Feske, J. S., and Jensen, F. E. (2016) Early-life seizures alter synaptic calcium-permeable AMPA receptor function and plasticity, Mol. Cell. Neurosci., 76, 11-20, https://doi.org/10.1016/j.mcn.2016.08.002.
Yuan, T., and Bellone, C. (2013) Glutamatergic receptors at developing synapses: the role of GluN3A-containing NMDA receptors and GluA2-lacking AMPA receptors, Eur. J. Pharmacol., 719, 107-111, https://doi.org/10.1016/j.ejphar.2013.04.056.
Sweatt, J. D. (2016) Neural plasticity and behavior – sixty years of conceptual advances, J. Neurochem., 139, 179-199, https://doi.org/10.1111/jnc.13580.
Diering, G. H., and Huganir, R. L. (2018) The AMPA receptor code of synaptic plasticity, Neuron, 100, 314-329, https://doi.org/10.1016/j.neuron.2018.10.018.
Lisman, J. (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling, Philos. Trans. R. Soc. Lond. B Biol. Sci., 372, https://doi.org/10.1098/rstb.2016.0260.
Sarantis, K., Antoniou, K., Matsokis, N., and Angelatou, F. (2012) Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation of the NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus, Neurochem. Int., 60, 55-67, https://doi.org/10.1016/j.neuint.2011.10.018.
Barkus, C., McHugh, S. B., Sprengel, R., Seeburg, P. H., Rawlins, J. N. P., and Bannerman, D. M. (2010) Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion, Eur. J. Pharmacol., 626, 49-56, https://doi.org/10.1016/j.ejphar.2009.10.014.
Pacheco, A., Aguayo, F. I., Aliaga, E., Muñoz, M., García-Rojo, G., et al. (2017) Chronic stress triggers expression of immediate early genes and differentially affects the expression of AMPA and NMDA subunits in dorsal and ventral hippocampus of rats, Front. Mol. Neurosci., 10, 244, https://doi.org/10.3389/fnmol.2017.00244.
Costa-Nunes, J., Zubareva, O., Araújo-Correia, M., Valença, A., Schroeter, C. A., et al. (2014) Altered emotionality, hippocampus-dependent performance and expression of NMDA receptor subunit mRNAs in chronically stressed mice, Stress, 17, 108-116, https://doi.org/10.3109/10253890.2013.872619.
Furini, C., Myskiw, J., and Izquierdo, I. (2014) The learning of fear extinction, Neurosci. Biobehav. Rev., 47, 670-683, https://doi.org/10.1016/j.neubiorev.2014.10.016.
Matsumoto, Y., Morinobu, S., Yamamoto, S., Matsumoto, T., Takei, S., et al. (2013) Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder, Psychopharmacology (Berlin), 229, 51-62, https://doi.org/10.1007/s00213-013-3078-9.
Réus, G. Z., Abelaira, H. M., Stringari, R. B., Fries, G. R., Kapczinski, F., and Quevedo, J. (2012) Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats, Metab. Brain Dis., 27, 175-182, https://doi.org/10.1007/s11011-012-9281-2.
Padovan, C. M., and Guimarães, F. S. (2004) Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats, Pharmacol. Biochem. Behav., 77, 15-19, https://doi.org/10.1016/j.pbb.2003.09.015.
Blacker, C. J., Millischer, V., Webb, L. M., Ho, A. M. C., Schalling, M., et al. (2020) EAAT2 as a research target in bipolar disorder and unipolar depression: a systematic review, Mol. Neuropsychiatry, 5, 44-59, https://doi.org/10.1159/000501885.
Zink, M., Vollmayr, B., Gebicke-Haerter, P. J., and Henn, F. A. (2010) Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression, Neuropharmacology, 58, 465-473, https://doi.org/10.1016/j.neuropharm.2009.09.005.
Zhang, X. H., Jia, N., Zhao, X. Y., Tang, G. K., Guan, L. X., et al. (2013) Involvement of pGluR1, EAAT2 and EAAT3 in offspring depression induced by prenatal stress, Neuroscience, 250, 333-341, https://doi.org/10.1016/j.neuroscience.2013.04.031.
Kovalenko, A. A., Zakharova, M. V., Nikitina, V. A., Schwarz, A. P., Karyakin, V. B., et al. (2018) Alterations in the expression of genes that encode subunits of ionotropic glutamate receptors and the glutamate transporter in brain structures of rats after psychogenic stress, Neurochem. J., 12, 135-141, https://doi.org/10.1134/S181971241802006X.
Nasca, C., Bigio, B., Zelli, D., de Angelis, P., Lau, T., et al. (2017) Role of the Astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress, Neuron, 96, 402-413.e5, https://doi.org/10.1016/j.neuron.2017.09.020.
Beznin, G. V., Pshenichnaya, A. G., Kusov, A. G., and Tsikunov, S. G. (2012) Morphological and functional bases of behavioral deviations in the model of acute vital stress in rats, Med. Akadem. Zhurn., 12, 37-39.
Paxinos, G., and Watson, C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th Edition, Academic Press.
Kopec, A. M., Rivera, P. D., Lacagnina, M. J., Hanamsagar, R., and Bilbo, S. D. (2017) Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue, J. Neurosci. Methods, 280, 64-76, https://doi.org/10.1016/j.jneumeth.2017.02.002.
Harrington, C. R. (1990) Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determinations on fractions from brain tissue, Anal. Biochem., 186, 285-7, https://doi.org/10.1016/0003-2697(90)90081-j.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 227, 680-685, https://doi.org/10.1038/227680a0.
Lei, T., Dong, D., Song, M., Sun, Y., Liu, X., and Zhao, H. (2020) Rislenemdaz treatment in the lateral habenula improves despair-like behavior in mice, Neuropsychopharmacology, 45, 1717-1724, https://doi.org/10.1038/s41386-020-0652-9.
Delawary, M., Tezuka, T., Kiyama, Y., Yokoyama, K., Inoue, T., et al. (2010) NMDAR2B tyrosine phosphorylation regulates anxiety-like behavior and CRF expression in the amygdala, Mol. Brain, 3, 37, https://doi.org/10.1186/1756-6606-3-37.
Rahman, T., Zavitsanou, K., Purves-Tyson, T., Harms, L. R., Meehan, C., et al. (2017) Effects of immune activation during early or late gestation on N-methyl-d-aspartate receptor measures in adult rat offspring, Front. Psychiatry, 8, 77, https://doi.org/10.3389/fpsyt.2017.00077.
Tishkina, A., Stepanichev, M., Kudryashova, I., Freiman, S., Onufriev, M., et al. (2016) Neonatal proinflammatory challenge in male Wistar rats: effects on behavior, synaptic plasticity, and adrenocortical stress response, Behav. Brain Res., 304, 1-10, https://doi.org/10.1016/j.bbr.2016.02.001.
Onufriev, M. V., Freiman, S. V., Peregud, D. I., Kudryashova, I. V., Tishkina, A. O., et al. (2017) Neonatal proinflammatory stress induces accumulation of corticosterone and interleukin-6 in the hippocampus of juvenile rats: potential mechanism of synaptic plasticity impairments, Biochemistry (Moscow), 82, 275-281, https://doi.org/10.1134/S0006297917030051.
Postnikova, T. Y., Griflyuk, A. V., Ergina, J. L., Zubareva, O. E., and Zaitsev, A. V. (2020) Administration of bacterial lipopolysaccharide during early postnatal ontogenesis induces transient impairment of long-term synaptic plasticity associated with behavioral abnormalities in young rats, Pharmaceuticals (Basel), 13, https://doi.org/10.3390/ph13030048.
Onufriev, M. V., Uzakov, S. S., Freiman, S. V., Stepanichev, M. Y., Moiseeva, Y. V., et al. (2018) The Dorsal and ventral hippocampus have different reactivities to proinflammatory stress: corticosterone levels, cytokine expression, and synaptic plasticity, Neurosci. Behav. Physiol., 48, 1024-1031, https://doi.org/10.1007/s11055-018-0665-6.
Benmhammed, H., El Hayek, S., Nassiri, A., Bousalham, R., Mesfioui, A., et al. (2019) Effects of lipopolysaccharide administration and maternal deprivation on anxiety and depressive symptoms in male and female Wistar rats: neurobehavioral and biochemical assessments, Behav. Brain Res., 362, 46-55, https://doi.org/10.1016/j.bbr.2019.01.005.
Sulakhiya, K., Keshavlal, G. P., Bezbaruah, B. B., Dwivedi, S., Gurjar, S. S., et al. (2016) Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin, Neurosci. Lett., 611, 106-111, https://doi.org/10.1016/j.neulet.2015.11.031.
Walker, F. R., March, J., and Hodgson, D. M. (2004) Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat, Behav. Brain Res., 154, 63-69, https://doi.org/10.1016/j.bbr.2004.01.019.
Spencer, S. J., Heida, J. G., and Pittman, Q. J. (2005) Early life immune challenge – effects on behavioural indices of adult rat fear and anxiety, Behav. Brain Res., 164, 231-238, https://doi.org/10.1016/j.bbr.2005.06.032.
Custódio, C. S., Mello, B. S. F., Filho, A. J. M. C., de Carvalho Lima, C. N., Cordeiro, R. C., et al. (2018) Neonatal immune challenge with lipopolysaccharide triggers long-lasting sex- and age-related behavioral and immune/neurotrophic alterations in mice: relevance to autism spectrum disorders, Mol. Neurobiol., 55, 3775-3788, https://doi.org/10.1007/s12035-017-0616-1.
Fan, L.-W., Tien, L.-T., Mitchell, H. J., Rhodes, P. G., and Cai, Z. (2008) Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide, Eur. J. Neurosci., 27, 1475-84, https://doi.org/10.1111/j.1460-9568.2008.06121.x.
Zoladz, P. R., and Diamond, D. M. (2016) Predator-based psychosocial stress animal model of PTSD: preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis, Exp. Neurol., 284, 211-219, https://doi.org/10.1016/j.expneurol.2016.06.003.
Zoladz, P. R., Park, C. R., Fleshner, M., and Diamond, D. M. (2015) Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset, Physiol. Behav., 147, 183-192, https://doi.org/10.1016/j.physbeh.2015.04.032.
Auxéméry, Y. (2012) Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context, Encephale, 38, 373-380, https://doi.org/10.1016/j.encep.2011.12.003.
Ding, X.-F., Li, Y.-H., Chen, J.-X., Sun, L.-J., Jiao, H.-Y., et al. (2017) Involvement of the glutamate/glutamine cycle and glutamate transporter GLT-1 in antidepressant-like effects of Xiao Yao san on chronically stressed mice, BMC Complement. Altern. Med., 17, 326, https://doi.org/10.1186/s12906-017-1830-0.
Shanks, N., Larocque, S., and Meaney, M. J. (1995) Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress, J. Neurosci., 15, 376-384.
Zoladz, P. R., Fleshner, M., and Diamond, D. M. (2012) Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities, Psychoneuroendocrinology, 37, 1531-1545, https://doi.org/10.1016/j.psyneuen.2012.02.007.
Olff, M., Güzelcan, Y., de Vries, G.-J., Assies, J., and Gersons, B. P. R. (2006) HPA- and HPT-axis alterations in chronic posttraumatic stress disorder, Psychoneuroendocrinology, 31, 1220-1230, https://doi.org/10.1016/j.psyneuen.2006.09.003.
Acknowledgments
Spectrophotometric studies and visualization of membranes were carried out at the Center for the Shared Use of Scientific Equipment for Physiological, Biochemical, and Molecular Biology Research at the Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences.
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 17-04-02116).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals have been followed. This article does not describe any research involving human subjects.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nikitina, V.A., Zakharova, M.V., Trofimov, A.N. et al. Neonatal Exposure to Bacterial Lipopolysaccharide Affects Behavior and Expression of Ionotropic Glutamate Receptors in the Hippocampus of Adult Rats after Psychogenic Trauma. Biochemistry Moscow 86, 761–772 (2021). https://doi.org/10.1134/S0006297921060134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921060134