Abstract
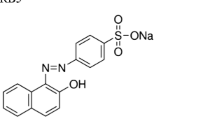
Water contamination by Remazol Brilliant Blue R (RBBR) can cause harmful effects on aquatic organisms due to its toxicity and recalcitrance in the environment. This study aimed to employ the oil palm fibers (OPFs)-immobilized white-rot fungus Trametes hirsuta AK04 to decolorize this dye and assess the alternative utilization of fungal-treated OPFs wastes. The fungus was able to utilize RBBR as a sole carbon and energy source. However, the decolorization efficiency was markedly enhanced by the supplementation of glucose as co-substrates. Veratyl alcohol (VA) was the best inducer to enhance the activities of laccase and manganese peroxidase associated with the decolorizing activity. The addition of 0.1 mM of VA along with glucose could accelerate the initial decolorization rate by the immobilized fungus, reaching 97% dye removal in 12 h. Fourier-transform infrared spectroscopy detected changes in the functional groups of dye and the formation of the degradation products, as well as changes within lignin and hemicellulose molecules in OPFs after decolorization. Sequentially, the fungal pretreatment of OPFs for 7–14 days resulted in increased lignin degradation and cellulose content, suggesting the possible use of treated OPFs as substrates for the further production of biofuels and other valuable products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Remazol Brilliant Blue R (RBBR) is an anthraquinone dye that is widely used in the textile industry. It represents the main class of toxic and recalcitrant organopollutants. Dye-containing wastewater can lead to a significant environmental problem if it discharges into water resources without any proper treatment. The wastewater is comprised of highly toxic chemical compounds such as several types of mixed dyes, metals, other contaminants with different concentrations, high organic content, suspended solids, and turbidity [1]. Dye pollution prevents sunlight penetration into water, inhibits the growth of aquatic biota, and decreases the concentration of dissolved oxygen in the water, all of which affect living organisms [2].
Numerous technologies have been employed for the treatment of dye-containing effluents, including physicochemical and biological methods. Fungal treatment is a promising biological method to treat dye wastewater since it is effective, easily applicable, low cost, and environmentally safe. White-rot fungi are used extensively for the decolorization of textile dyes due to their ability to produce extracellular ligninolytic enzymes such as lignin peroxidase, manganese peroxidase (MnP), and laccase (Lac) [3]. The supplementation of some nutrients and/or inducers can increase the production of these enzymes. Various groups of inducing compounds, such as metal ions, alcohol, aromatic and phenolic compounds related to lignin or its derivatives, have been reported to be able to induce the production of ligninolytic enzymes. For example, Ado et al., [4] reported enhanced Lac activities and complete degradation of RBBR by Trametes sp. B7 when supplemented with inducers at different concentrations such as Cu2+ (1–2 mM) and Ca2+ (3–4 mM) plus glucose as an additional carbon source. Galhaup et al., [5] found that an increase in the production of Lac (330 U/mL) in Trametes pubescens MB 89 was achieved by the addition of Cu2+ (2.0 mM) together with carbon (glucose) and nitrogen sources (peptone). Mani et al., [6] tested the effect of three inducers, namely syrinaldehyde, acetosyringone, and 2,2-azino-bis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) on the decolorization of Acid Orange 7 using Trametes versicolor. Of all inducers, the supplementation of acetosyringone could improve dye decolorization by 12–16%, reaching 94% removal of 100 mg/L dye within 24 h. Mostafa et al., [7] reported that the presence of different nutrients caused different effects on the decolorization efficiencies of Mordent Orange 1 by Cylindrocephalum aurelium RY06. They found that the addition of glucose (76%) gave the highest decolorization compared to fructose (71%), galactose (65%), and xylose (35%), while the different nitrogen sources such as yeast extract, ammonium nitrate, and ammonium tartrate led to 49–71% decolorization.
Fungal immobilization on lignocellulosic biomass is known as a technique to support fungal growth and increase ligninolytic enzyme production since it mimics the natural living conditions of the fungi. This technique, in particular, has been applied for the fungal degradation of lignin, which is considered an effective pretreatment method for lignocelluloses. It produces less fermentation inhibitors and requires low-energy consumption, as well as being a low-cost treatment compared to physical and chemical methods [8]. The pretreated biomass can be converted into fermentable sugars, further utilizing them for the fermentation of high-value products such as biofuels. The supplementation of certain mediators/inducers during the fungal pretreatment of lignocelluloses not only stimulated fungal growth and metabolite production but also enhanced the following enzymatic hydrolysis because of their physicochemical structure changes. Mishra et al., [9] studied the fungal pretretment of sweet sorghum bagasse using Coriolus versicolor with supplements of veratryl alcohol, syringic acid, catechol, gallic acid, vanillin, guaiacol, CuSO4, and MnSO4. The authors found that CuSO4, gallic acid, and syringic acid were the best supplements that enhanced lignin degradation and lowered cellulose consumption.
The immobilized fungus has also been employed for the degradation of several kinds of dye-containing wastewater, as it offered a high level of enzyme activity and more resistance to severe conditions than free cells of fungi. This method also has the advantages of reducing operation costs and increasing reusability. Boehmer et al., [10] have reported 80% decolorization efficiency of T. versicolor and Phanerochaete chrysosporium immobilized onto pine wood chip and oil palm fiber when tested with Levafix Blue, Remazol Brilliant Blue, and Red. Šušla et al., [11] have presented about the 94% decolorization of RBBR, Reactive Orange 16, Copper(II) Phthalocyanine using Dichomitus squalens immobilized onto polyurethane foam (PUF)–pine wood chip. Our previous work utilized Trametes hirsuta AK04 immobilized onto oil palm fibers (OPFs), one of the lignocellulosic wastes produced from palm oil mills, for the dephenolization and decolorization of palm oil mill effluent. The immobilized fungus was capable of removing phenolic compounds and color by 82.2 and 87.1%, respectively, with MnP and Lac involved in the treatment [12].
Even though immobilized white-rot fungi on lignocelluloses have been used to improve dye decolorization, available information on the effect of certain inducers on ligninolytic enzyme activities during simultaneous decolorization and lignin biodegradation is limited. Han et al., [13] demonstrated that the presence of lignin and its metabolism enhanced the decolorization of azo dye by Echinodontium taxodii. Some low-molecular-weight phenol derivatives obtained during the long-term degradation of lignin may act as redox mediators in the Lac-catalyzed oxidation reactions of the dyes [13]. Hence, the metabolism of lignin in the presence of lignocellulose enhanced the enzyme activity of fungi, contributing to more efficient dye degradation. This phenomenon has been reported by Tychanowicz et al., [14], who showed an increase in Lac activity and the capability of dye decolorization by Pleurotus pulmonarius. These results were achieved when the fungus was cultivated on glucose/ammonium tartrate-corncob solid-state medium and supplemented with corncob-soluble phenolic extracts. The supplementation of these natural additives can stimulate the Lac production involved in the decolorization. Nevertheless, among the previous studies, Lac was the only ligninolytic enzyme secreted by the tested strains. This gap would impede the advancement of biological treatment of synthetic dye wastewater using different white-rot fungi with different enzyme secretion.
This study investigated the combined degradation of RBBR dyes and lignin using the white-rot fungus T. hirsuta AK04 in the presence of both natural and synthetic inducers. This fungus has been reported to produce Lac and MnP while dephenolizing and decolorizing palm oil mill effluent [12]. The effects of supplementation of different nutrients and inducers on decolorization and production of these two ligninolytic enzymes were determined when the strain was grown on lignocellulosic OPFs (with lignin) and in the free cell experiment (without lignin). The degradation of lignin was studied after dye removal and followed by fungal pretreatment for 7 and 14 days. This work reveals the efficient use of the fungal strain T. hirsuta AK04 to decolorize RBBR dye and pretreat lignocellulosic OPFs, which could serve as substrates for the further production of biofuels and other bioproducts. These findings lead to more economical and sustainable management of dye-containing wastewater treatment and the valorization of treated lignocellulosic wastes produced after dye removal.
MATERIALS AND METHODS
Microorganisms and dye. A white-rot fungus, namely T. hirsuta AK04, was tested in this study for its potential to decolorize RBBR dye in the free cell and immobilized cell systems. The strain T. hirsuta AK04 was isolated from a natural mushroom in southern Thailand [12]. This strain has been reported to produce extracellular ligninolytic enzymes during the dephenolization and decolorization of palm oil mill effluent [12]. The fungal starter cultures were grown on potato dextrose agar (PDA) plates and incubated at room temperature (28 ± 1°C) for 7 days. The fungal stock culture was kept at 4°C. Subcultures were carried out every 4 weeks.
The RBBR dye was obtained from Sigma-Aldrich (USA). The stock solution of the dye was prepared by dissolving it in distilled water and added to the medium to obtain the desired final concentration.
Fungal inoculum preparation. Inoculum preparation was performed as described by Kietkwanboot et al., [12]. Thirty agar plugs (8 mm in diameter) of actively growing fungal mycelium were inoculated into 250-mL Erlenmeyer flasks containing 100 mL of glucose yeast extract broth (GYEB) containing (g/L): glucose—100.0 and yeast extract—10.0. Flasks were incubated on an orbital shaker at room temperature (28 ± 1°C) and 120 rpm for 4 days. The fungal pellets were harvested by filtration and washed twice with 0.85% NaCl. The washed fungal pellets were used as inoculum for further decolorization experiments.
Decolorization under single and co-substrate conditions. Dye was added in a single substrate as the sole carbon source in a minimal salt medium (MS medium). The MS medium consisted of (g/L): Na2HPO4—12.8, NaCl—0.5, KH2PO4—3.0, and NH4Cl—1.0 [15]. To conduct the biodegradation experiment, 1 g of fungal pellet prepared as described above was inoculated into 10 mL of freshly prepared MS medium containing 300 mg/L RBBR in a 50-mL glass vial. The samples were incubated at room temperature (28 ± 1°C) and on an orbital shaker at 80 rpm for 2 days. For co-substrates, glucose (40 g/L) and/or peptone (10 g/L) were supplied as exogenous carbon and/or nitrogen sources in an N-free medium. These nutrients were selected as they had been reported to support the production of ligninolytic enzymes and dye decolorization in some fungal strains [16]. In addition, supplementation of these nutrients showed no additional color to the medium. The samples were prepared and incubated in the same manner with a single substrate. The triplicate samples were taken at time intervals of 0, 6, 12, 24, and 48 h to measure fungal growth, activity of ligninolytic enzymes (Lac and MnP), and dye decolorization. Heat-killed fungal pellets were used as a control to determine dye loss by sorption onto fungal biomass. Both samples and controls were prepared in the same manner and incubated under the same conditions. The supplement (glucose) with the highest degree of decolorization was selected for further experiment.
Effect of different inducers on dye decolorization. The effect of different inducers on dye decolorization by the fungal strain AK04 was investigated. Three different groups of compounds, including an aromatic compound, alcohol, and metal ions, were added as inducers for stimulating the production of ligninolytic enzymes. These inducers included syringic acid (SYR), veratryl alcohol (VA), CuSO4, and MnSO4. Initially, 1 g of fungal pellets prepared as described above was transferred to 10 mL of the medium containing 40 g/L glucose and 300 mg/L RBBR dye in a 50-mL vial. Each inducer was added individually with the following concentrations: SYR of 1 mM, VA of 0.4 mM, CuSO4 of 1 mM, and MnSO4 of 1 mM [9]. The samples were incubated under the same conditions as previously described. Triplicate samples were performed. Sampling was taken at time intervals (0, 6, 12, 24, and 48 h) to measure the fungal growth, ligninolytic enzyme production (Lac and MnP), and dye decolorization. The control was prepared in the same manner as the sample but in the absence of an inducer. A suitable inducer (VA) was used for the fungal immobilization experiment. Heat-killed fungal pellets were prepared to represent the dye loss by sorption onto fungal biomass.
Fungal immobilization. OPFs, solid residues from the palm oil industry, were used as immobilizing supports. The OPFs were collected from a palm oil mill in southern Thailand. The materials were washed thrice with tap water and autoclaved at 121°C for 20 min to eliminate the existing microorganisms. They were air-dried in an oven at 60°C. The average length of the OPFs pieces was about 2–3 cm. The fungal pellets were prepared as previously described. The fungal immobilization was performed according to the method of Kietkwanboot et al., [12] with slight modifications. The fungal pellets were mixed with the new GYEB and homogenized with stomacher blender (Seward Stomacher® 400 Circulator, England) to obtain a mycelial suspension. Ten-fifteen mL of cell suspensions were transferred to 500-mL Erlenmeyer flask containing 10 g of OPFs and 100 mL of GYEB. The flasks were incubated at room temperature (28 ± 1°C) under static conditions for 2 days. After incubation, the fungal mycelium was completely colonized on OPFs and ready to be used in decolorization experiments.
Effects of RBBR concentrations on decolorization efficiency. In the immobilized cell system, the effect of different concentrations of RBBR dye on fungal decolorization was examined. The fungal immobilization was performed as described above. The experiment was carried out in 500-mL Erlenmeyer flask containing 100 mL of samples with the following components (g/L): immobilized fungus—290 (wet weight), glucose—40, and dye—300, 500, or 1000. The culture was incubated under the same conditions, and sampling was performed in the same manner with a free cell system. The supernatant was used for analysis of the remaining color and the activities of ligninolytic enzymes, namely Lac and MnP.
The deactivated (autoclaved) immobilized fungus and OPFs alone were prepared as controls to represent the dye losses by sorption onto fungal biomass and immobilizing support. The control sets were incubated under the same conditions as those of the sample sets.
Effects of concentrations of VA on dye decolorization and lignin degradation. This experiment was conducted in the immobilized cell system. The fungal immobilization was performed as described above. The experiment was carried out in 500-mL Erlenmeyer flask containing 100 mL of samples with the following compositions: immobilized fungus—29 g (wet weight), glucose—40 g/L, dye—1000 mg/L, and VA of 0.1, 1, or 20 mM. The sample was incubated under the same conditions, and sampling was performed in the same manner as with the free cell system. The supernatant was used for analysis of the remaining color, the activities of ligninolytic enzymes, namely Lac and MnP, and the structural change of the dye by Fourier transform infrared (FTIR) spectroscopy. The solid part was used for analysis of the fungal growth by measuring N-acetylglucosamine content. The OPFs biomass loss after decolorization was determined. The composition of lignocellulosic OPFs was analyzed by the determination of the lignin, cellulose, and hemicellulose content. The change in the functional groups on OPFs surface was analyzed by FTIR. The heat-killed immobilized cells and OPFs alone were prepared to represent the sorption of the dye onto the fungal biomass and immobilizing support. The control sets were incubated under the same conditions as the sample sets.
Fungal pretreatment of lignocellulosic OPFs following dye decolorization. The effect of fungal pretreatment on the chemical composition of OPFs was determined to assess the efficient utilization or alternative use of biologically treated lignocellulosic wastes as substrate for the further production of biofuel/value-added products. After dye decolorization, the immobilized fungus was separated from the liquid medium. It was incubated for 7–14 days at room temperature under solid-state fermentation. During the incubation, the moisture content was periodically adjusted as needed by adding 15 mL of sterilized distilled water in each flask to obtain moisture at about 70%. After the incubation, the liquid residue was discarded. The solid parts, comprising the blends of fungal mycelium and pretreated OPFs, were separated from each other as previously stated. The following analyses were performed before and after fungal pretreatment: weight of OPFs biomass loss; chemical compositions of OPFs such as lignin, hemicellulose, cellulose, and sugar; functional groups on OPFs surfaces.
Determination of dye decolorization. The liquid portion was withdrawn from the culture vials and centrifuged at 4500 g for 15 min to remove suspended biomass. The concentration of RBBR was measured at OD595 [17]. The dye decolorization was calculated as follows:
where De is the efficiency of dye removal by the fungus (%), \({\text{Ci}}\) is the initial concentration of the dye, and \({\text{Cf}}\) is the final concentration of the dye.
Determination of fungal growth. The dry weight of fungal biomass was determined by filtering the fungal pellet through pre-weighed filter paper and washed twice with 0.85% NaCl. The liquid medium was removed, and the filtered biomass was dried at 60°C for 48 h until a constant weight was achieved.
Fungal growth in the form of immobilized cells was measured by using N-acetylglucosamine content [18]. Firstly, the blends of fungal mycelium and OPFs support were washed twice with 80% acetone. They were dried at 40°C and heated to 100°C for 15 min to separate the fungal mycelia from the OPFs material. The samples were washed and ground into small pieces. The fungal biomass was treated with 2 N HCl to turn fungal chitin into N-acetylglucosamine. One mL of glucosamine was added to 1 mL of acetylacetone reagent consisted of 1 mL acetylacetone and 50 mL 0.5 N Na-carbonate. The mixture was boiled for 20 min, cooled to room temperature, and 6 mL of ethanol was added. Then, 1 mL of Ehrlich’s reagent composed of 2.67 g of p-dimethyl aminobenzaldehyde in 100 mL of mixture of ethanol and concentrated hydrochloric acid (1 : 1, vol/vol) was added. The mixture was kept at 65°C for 10 min and measured spectrophotometrically at 530 nm.
Ligninolytic enzyme assays. Lac activity was determined by the method of Rodrígues et al., [19] with a slight modification using ABTS as a substrate. Lac from the genus Trametes exhibited the most specificity for ABTS over other substrates [20]. The reaction mixture included 500 μL 0.2 mM ABTS, 500 μL 20 mM Na-acetate buffer (pH 5.0), and 500 μL of culture broth. The measurements were carried out at room temperature at 436 nm (ε = 29 300 M–1 cm–1).
MnP activity was determined by the method of Heinfling et al., [21] with a slight modification using 6-dimethoxyphenol as a substrate. The reaction mixture included 500 μL 1 mM 2,6-dimethoxyphenol, 250 μL 0.1 M Na-acetate buffer (pH 4.5), 250 μL 0.1 mM H2O2, 250 μL 1 mM MnSO4, and 500 μL of culture broth. The measurements were carried out at room temperature at 469 nm (ε = 27 500 M–1 cm–1).
FTIR analysis. FTIR spectroscopy was used to determine the change in the functional groups in the molecular structure of RBBR dye before and after treatments, as well as the change in the chemical bonds of untreated and pretreated OPFs. FTIR spectra were analyzed using Fourier transform infrared spectrometer (Vertex70 Bruker, Germany). The spectra were obtained with an average of 25 scans and a resolution of 4 cm–1, in the range of 4.000–400 cm–1.
Chemical compositions of OPFs. Analysis of the cellulose, hemicellulose, and lignin content in OPFs was performed using a detergent method. The neutral and acid detergents were used according to the procedure described by Van Soest et al., [22].
Sugar content in OPFs. Acid hydrolysis of OPFs was performed according to a modified method of Kasim and Kasim [23] to obtain sugar hydrolysate. OPFs were ground using a crusher (Sf 130, FnB Machinery and Solution Co., Ltd., Thailand) and sieved until the size approximately 0.1–0.5 mm. Twenty g of the samples were added to 200 mL of water and 1% H2SO4 (vol/vol). The samples were shaken at 100 rpm for 5 min and autoclaved at 121°C for 1 h. The amount of reducing sugar was determined by the dinitrosalicylic acid method.
RESULTS AND DISCUSSION
Dye decolorization in different media compositions. The decolorization activities of free cells of the fungal strain AK 04 under single and co-substrates conditions were investigated (Figs. 1a–1e). In the single substrate, dye was supplied as a sole carbon source in an original MS medium. For the co-substrtate, glucose and/or peptone were supplemented in an N-free medium to determine the effects of the exogenous carbon and/or nitrogen source on decolorization efficiency. More rapid initial decolorization (54%) was observed by autoclaved cells in a single substrate than in co-substrates (28%), after the first 12 h of incubation (Fig. 1b). While the decolorization was almost constant in the single substrate, it rose continuously in the co-substrates and reached the same level (58%) under both conditions after 48 h (Fig. 1b). The color of the medium still remained blue (Fig. S1). This indicates that dye removal was mainly due to its adsorption onto fungal biomass. In addition, the rate of the adsorption process was being interfered with the presence of other substrates in the medium. Similar results have been reported by Yesilada et al., [24], who found that 71% of Astrazone Blue was removed by adsorption after incubation with heat-killed pellets of Funalia trogii for 24 h.
For living pellets, the highest total decolorization (86%) was observed in the medium supplemented with glucose after 48 h of incubation (Fig. 1a). Lower decolorization (70 to 73%) was shown in the media supplemented with glucose plus peptone, peptone alone, or without supplementation (Fig. 1a). The results suggest that the addition of sugar (glucose) might generate more redox mediator, which played a major role in the transfer reducing equivalent from an electron donor to acceptor. This would accelerate the destruction process of the dye molecule [25]. Similar results were observed by Ambrósio and Campos-Takaki [25], who found that the presence of peptone interfered negatively with the removal of azo dye by the fungus Cunninghamella elegans. Jirasripongpun et al., [26] have reported the decreased decolorization of C.I. Reactive Red 195 by Enterobacter sp. in the presence of glucose plus peptone under anaerobic conditions. The metabolites generated during peptone consumption, such as nitrate or nitrite, acting as reducing power may compete with the dye molecule, leading to less decolorization. Additionally, in the present study, the results from the control sample showed that the fungus could metabolize the dye as a sole carbon and energy source (Fig. 1a). These were contributing to a slight fungal growth when grown on either single or co-substrates (Fig. 1e). Compared with the results of heat-killed cells, it proved that the color removal of the dye was also partly due to the biotic activity of the fungus.
The production of Lac was much lower (~2–10 U/L) than that of MnP in all treatments over the incubation period (Fig. 1c). The activities of MnP increased sharply (82–85 U/L) in the presence of either glucose or peptone during 48 h of incubation (Fig. 1d). Its production (71 U/L) was lower in the medium containing both peptone and glucose (Fig. 1d). The glucose assimilation resulted in a decrease in pH, favoring the optimal conditions for enzymatic activity in the fungus. The results of this study were in agreement with those reported by Bettin et al., [27] found that the cultivation of the Pleurotus sajor-caju strain PS-2001 in medium supplemented with glucose produced maximum enzyme activity. A simple carbon source can activate the primary metabolism of the fungus, thereby leading to increased enzyme production for the degradation of the compounds. These two enzymes played major roles in the decolorization of several reactive dyes by fungi. The enzyme production obtained in the present study corresponded with the decolorization efficiency described above (except for peptone supplementation). However, the activity of these enzymes in all treatments containing co-substrates was much higher than in the sample containing a single substrate. This finding suggests that co-substrates facilitate more metabolism (biodegradation) than single substrates, thus, resulting in lower dye adsorption (Fig. 1b). The color appearance of the treated medium changed from dark blue to light blue (single substrate) and purple and brown (co-substrates) (Fig. S1).
Effect of various inducers on RBBR decolorization. The effects of various inducers, including VA, SYR, CuSO4, and MnSO4 on the decolorizing activity of free cells of strain AK04 were examined in the medium containing co-substrates (dye and glucose). Higher decolorization efficiencies (96–98%) were observed in the medium supplemented with VA, MnSO4, and CuSO4 than in the medium supplemented with SYR and the control (Fig. 2a). However, compared to the control, the treatments with inducers (except for CuSO4) gave a more rapid decolorization rate. This suggests that the addition of some inducers such as VA, MnSO4, and SYR can accelerate the initial decolorization of the RBBR dye. The results of the decolorizing activity were consistent with those of enzyme production. The supplementation of all inducers showed a positive effect on Lac activity in the first 12 h of incubation, while faster effects on MnP activity were observed compared to the control (Figs. 2c, 2d). Of all inducers, the highest Lac and MnP activities were found in the media containing VA. This result shows that the addition of VA could enhance Lac and MnP production, which are associated with decolorization efficiency. The fungal growth in the medium with or without inducers showed a similar pattern, in which the growth slightly increased during the 48 h of incubation (Fig. 2e). A similar removal of dye (58%) was observed by adsorption with heat-killed cells in the media containing mixed inducers and control after 48 h (Fig. 2b).
Inducers are commonly used for the induction of the synthesis of inducible enzymes and are also utilized as the substrate of enzymes. Aromatic compounds have been widely used to induce and enhance ligninolytic enzyme activities because these compounds are related to lignin or lignin derivatives. Thakkar et al., [28] have reported that the supplementation of glucose and VA as a carbon source and an enzyme inducer, respectively, can markedly enhance Lac production. Arora and Gill [29] reported that VA was a suitable inducer for the enhancement of Lac production in different fungi. It was reported that VA played a role as a feedback control mechanism that directly regulated the synthesis of Lac. This inducer also influenced the membrane structure to enable the secretion of intracellularly localized Lac into the medium [30]. On the other hand, Mishra et al., [9] have demonstrated that CuSO4 and SYR improved Lac production during the pretreatment of sweet sorghum bagasse by Coriorus versicolor. SYR has a similar structure to the syringyl unit of the lignin-related compounds, while copper is a cofactor in the catalytic center of Lac production. Nevertheless, the induction and production of ligninolytic enzymes vary greatly depending on the microbial species and environmental conditions used in the experiments.
The color of the medium supplemented with VA, CuSO4, and MnSO4 changed from dark blue to light yellow (Fig. S2). These results were in agreement with those reported by Lee et al., [31]. They found that the decolorization of RBBR by Phlebia brevispora produced a yellowish medium. In the present study, the color of the medium supplemented with SYR turned red (Fig. S2). This phenomenon was most likely due to the oxidation of SYR by Lac and the production of intermediate compounds such as 2,6-dimethoxy-1,4-benzoquinone. This degrading product can be further polymerized to form polyhydroquinones, which might produce colored compounds [6].
Effects of dye concentrations on fungal decolorization. The effects of dye concentrations on the decolorization activity of the immobilized fungal strain AK04 were investigated. Three different concentrations of dye, varying from 300, 500, and 1000 mg/L, were tested in a glucose medium. The immobilized fungus showed higher total decolorization rates (> 80%) at initial concentrations of 500 and 1000 mg/L than that of 300 mg/L in the first 6 h of incubation (Fig. 3a). However, almost similar decolorization (96–98%) was found at all dye concentrations after 12 h of incubation. The decolorization of dye ranging from 42 to 82% was shown for heat-killed immobilized fungus (Fig. 3b), suggesting that dye adsorption onto the lignocellulosic immobilizing materials and the fungal mycelial biomass was one of the main mechanisms of dye removal. The adsorption decreased with an increase in dye concentration (Fig. 3b) since the total number of sorption sites available for reaction was constant while the dye ions in the medium increased with increasing dye concentration. Zhu et al., [32] have reported that at a low concentration of dye (<300 mg/L), the removal of the dye was mainly attributed to the surface adsorption mechanism. On the other hand, at a high concentration of dye, the removal of the dye decreased, and a precipitation of the excess dye ions tended to occur.
The higher decolorization rates at 500 and 1000 mg/L RBBR were in accord with the activities of enzyme production (Figs. 3c–3d). A higher production of Lac was observed in the experiments at these concentrations during the first 6 h of incubation (Fig. 3c). In contrast, MnP activity was the same at all dye concentrations during the same period. However, the increase in dye concentration led to an increase in enzyme production at the end of the experiment (Figs. 3c–3d). The activities of Lac and MnP in the immobilized cell system were higher than those in the free cell system when compared at the same dye concentration (Figs. 2c–2d vs. 3c–3d). This was attributed to the fact that lignocellulosic OPFs used as supports are composed of cellulose, hemicellulose, and lignin. These carbohydrate-rich materials provide additional nutrients and/or natural inducers that support fungal growth and improve ligninolytic enzyme production in white-rot fungi [12]. The result of the present study was in line with those Kietkwanboot et al., [12] reported. The authors reported more Lac and MnP activities in the OPFs-immobilized T. hirsuta AK04 than in the free fungal pellets during the dephenolization and decolorization of palm oil mill effluent. Han et al., [13] revealed that lignin metabolism played an important role in enhanced Lac activity and azo dye removal by Echinodontium taxodii cultured in lignin-containing medium. The metabolites of lignin may function as redox mediators in the oxidation of dyes. The amount and type of lignin present in lignocellulose are the factors that contribute to the activity of the Lac enzyme produced.
The results of this study indicated that the high initial concentrations of RBBR up to 1000 mg/L had no negative effects on decolorization efficiency (Fig. 3) or immobilized fungal growth (Table 2). This dye concentration can enhance ligninolytic enzyme production, which further increases decolorization. Similarly, Chang et al., [33] have investigated the effects of initial azo dye concentrations on the decolorization efficiency of Pseudomonas luteola immobilized in polyacrylamide. They found that by increasing the initial concentration of C.I. Reactive Red 22 up to 3000 mg/L, the decolorization rate and azo dye reductase activity increased. Erkurt et al., [34] have shown that the concentration of RBBR ranging from 20 to 100 mg/L did not cause any inhibitory effect on the growth of Funalia trogii and Coriolus versicolor. By contrast, Sumandono et al., [35] have reported an increasing concentration of RBBR from 100 mg/L to 500, 1000, and 1500 mg/L led to a reduction in fungal growth. It might be possible that the concentration of dye used in the present study is lower than fungal toxicity threshold.
Effects of concentrations of VA on dye decolorization. Based on the results of the free-cell experiment, VA was a suitable inducer for enhancing RBBR removal by the strain AK04 compared to others (Fig. 2). The effects of variations in the concentrations of VA on dye removal and ligninolytic enzyme activity in the immobilized cell system were investigated. Three concentrations of VA of 0.1, 1, and 20 mM were tested in glucose medium containing RBBR at 1000 mg/L. The increase in VA concentrations from 0.1 to 20 mM led to a decrease in the decolorization rate in the first 6 h of incubation (Fig. 4a). The most rapid decolorization rate was found in the presence of 0.1 mM VA, reaching 97% dye removal after 12 h of incubation. However, after 48 h, similar decolorization (94–96%) was observed in all treatments (Fig. 4a). These results suggest that the concentrations of VA had an effect on the initial dye removal rate. The control showed a lower degree of decolorization than that after the treatments with 0.1 and 1 mM VA. Moreover, about half of the dye (54%) was adsorbed by heat-killed immobilized cells (Fig. 4b), showing that the dye was partly removed through biosorption onto OPFs materials and fungal mycelial biomass.
Total decolorization (a), adsorption with heat-killed cells (b), Lac (c) and MnP (d) activities of OPFs-immobilized T. hirsuta AK04 in the media containing 1000 mg/L RBBR supplemented with glucose and VA, compared to the control. All treatments were incubated at 80 rpm and room temperature. 1—0.1, 2—1, 3—20 mM VA, 4—control.
The results revealed the most rapid production and the highest activity of Lac in the presence of 0.1 mM VA after 12 h of incubation (Fig. 4c). The increase in VA concentrations resulted in a decrease in Lac activity. Meanwhile, the MnP activities were comparable in all treatments during the entire incubation (Fig. 4d). However, the control showed the lowest production of both enzymes (Figs. 2c–2d), suggesting that the production of enzymes was enhanced by the addition of VA. These were probably because VA is an aromatic inducer that acts as a redox mediator for dye transformation. Thus, it is necessary to add an inducer to enhance the enzymatic activity. This method can reduce time and cost during the treatment of dye-containing wastewater. The results of the present study were consistent with those of Andriani et al., [36], who found that the addition of 0.1 mM VA could enhance the production of Lac and MnP by T. hirsuta AA-017 grown on Indonesian sorghum biomass. Tychanowicz et al., [14] found an increase in Lac production (100–430 U/mL) and dye decolorization when P. pulmonarius was cultivated on glucose/ammonium tartrate-corncob solid-state medium supplemented with corncob-soluble phenolic extracts of 0.55–1.34 μmol/mL. In contrast, neither decolorization nor Lac production detected when the phenolics added were lower than 0.1 μmol/mL. Although the addition of some redox mediators could result in increased decolorization by some ligninolytic fungi, these compounds, at a certain concentration, showed inhibitory effects on Lac production [37]. In addition, the ratio of mediatior to dye strongly influenced the degradation of the recalcitrant dyes by the fungal Lac [38].
FTIR analysis of fungal-treated dye. Compared to untreated RBBR dye, the presence of an absorption band at 1632 cm–1 in the treated RBBR showed C–O, C=C, and C=O stretching vibrations (Table 1). Conversely, the disappearance of absorption bands at 1018, 1053, 1617, and 1643 cm–1 was associated with the absence of a C–N group in the treated RBBR sample [39]. This demonstrates the complete destruction between the anthraquinone and the monobenzene rings in the RBBR molecule. The biodegradation of RBBR by T. hirsuta D7 immobilized in light expanded clay aggregate was suggested to begin with the hydrolysis of C–N bonds [40]. The bands at 2922 and 3267 cm–1 suggested the stretching vibration of the N–H group (Table 1). These results indicate the change in the RBBR structure after fungal decolorization. The degradation and deamination of the RBBR chromophore by Lac enzymes may lead to dye fragmentation made up of its derivative molecules [39]. The peaks at 1413 and 1256 cm-1 were assigned to an asymmetric C–H deformation of alkane [41]. Moreover, the presence of new absorption bands appearing at around 1012–1256 cm–1 and 450 cm–1 and 770–897 cm–1 regions can be attributed to the functional groups such as S=O, C–O, asymmetric P–O–C and asymmetric P–O–S stretching vibrations, as well as the bond formation of C–CO–C and Si–O–Si of the formed derivatives of the decolorized RBBR dye [39, 41]. The results were in line with the color change from dark blue to light yellow as observed in the treated dye sample supplemented with VA (Fig. S2). This occurrence proved that the chromophore was damaged. Similarly, the orange color of the C14 byproduct was formed after RBBR treatment with T. versicolor Lac [42].
The degradation of RBBR by the fungal genus Trametes and its Lac, including T. hirsuta D7 and T. versicolor, produced metabolites such as sodium 2-((3-aminophenyl)sulfonyl)ethyl sulfate (C8) and 1-amino-4-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (C14) and its dihydroxyl form [40, 42]. The degradation products, namely phthalic acid and hydroxylphthalic acid, were detected following ring cleavage. Moreover, Alam et al., [40] demonstrated a reduction in the cytotoxicity of RBBR on human dermal fibroblast cells after decolorization with the fungus T. hirsuta D7. Osma et al., [43] also showed that the treatment of RBBR with immobilized Lac from T. pubescens can effectively reduce phytotoxicity on seed germination and root elongation of ryegrass (Lolium perenne).
Fungal growth and chemical compositions of OPFs. Fungal growth and the changes in chemical composition of OPFs before and after the decolorization process are presented in Table 2. The OPFs biomass loss was observed to be between 11.0 and 13.3% after 24 h of dye decolorization, suggesting that some parts of lignocellulosic OPFs were degraded during the fungal growth (Table 2). The growth of the immobilized fungal strain AK04 was determined by its glucosamine content. It increased (from 231 to 399 mg/g) after 24 h of decolorization in all treatments with VA induction (Table 2). When the VA concentrations increased from 0.1 to 1.0 mM, glucosamine contents were 397 and 399 mg/g, respectively. However, the increase in VA concentration to 20 mM led to a decreased glucosamine content (368 mg/g). Thus, the high concentration of VA had an inhibitory effect on fungal growth (Table 2) as well as on the decolorization activity (Fig. 4). Lower glucosamine content (342 mg/g) was found in the control (Table 2). These results indicate that fungal growth was supported by the supplementation of an inducer.
For the best decolorization treatment (0.1 mM VA supplementation), a small amount of cellulose (16.7%) was degraded after decolorization. Meanwhile, no lignin or hemicellulose degradation was observed. The small amount of cellulose loss might be in the easily accessible parts utilized for fungal growth. While decolorization efficiency was similar among all VA concentrations after 12 h of incubation, a higher degradation of lignin (32.3%) and hemicellulose (20.9%) was shown with 1 mM VA supplementation (Table 2). These findings show that the capabilities of VA as mediators for dye decolorization and lignin degradation required different concentrations of this compound.
FTIR analysis of OPFs before and after dye decolorization. The FTIR spectra of OPFs biomass were analyzed to determine the changes in the functional groups on their surface before and after dye decolorization (Fig. 5). Prior to decolorization, the raw biomass showed a peak at 1028 cm–1 belonging to the C–O stretching of primary alcohol present in lignin and hemicellulose [44]. The absorption bands at 1383 and 1628 cm–1 were attributed to C–O stretching and C=C stretching of the aromatic ring found in lignin. The band at 1740 cm–1 represented the presence of C=O stretching vibrations of the ester carbonyl group in hemicellulose [45]. The peak at 2921 cm–1 was associated with C–H stretching of lignin polymer. The bands at 3305 and 3660 cm–1 corresponded to O–H stretching of the lignin and hemicellulose polymer [44]. The changes in the intensities of the peaks before and after decolorization suggested reduced functional groups such as C–H and C–O. Conversely, the increased functional groups like alcohols (O–H), unjugated C–O, C=O and C=C might be attributed to the changes within the lignin and hemicellulose molecules as well as the residual lignin contained in the isolated cellulose [45].
Fungal pretreatment of lignocellulosic OPFs following dye decolorization. After dye removal, the effect of fungal pretreatment on the chemical composition of OPFs was determined in order to assess the efficient utilization or alternative use of treated lignocellulosic wastes as substrate for the further production of biofuel and value-added products. The fungus induced with 0.1 mM VA and grown on OPFs was investigated for lignin degradation in solid-state fermentation for 7 and 14 days. About 22.0–26.5% loss of OPFs biomass was shown, suggesting partial consumption of lignocellulosic biomass by the fungus (Table 3). This corresponded to a continued growth of the immobilized fungus over the pretreatment periods. The increase in cellulose content agreed with an increased reducing sugar in OPFs since the amount of cellulose conserved by the fungal pretreatment can be converted to fermentable sugars. These compounds can be used as substrates for subsequent biofuel production. In contrast, the contents of lignin and hemicellulose tended to decrease during the pretreatment period, indicating that this strain is a selective degrader for both hemicellulose and lignin. These implied that ligninolytic and hemicellulolytic enzymes produced by the fungus played key roles in the fungal pretreatment of OPFs biomass. Nevertheless, type of substrate, fungal–substrate interaction, and culture conditions are contributors to the efficiency of fungal pretreatment [9]. The decrease in the intensity of some significant bands such as O–H, C–H, C=O, C=C, and C–O functional groups indicates the changes caused by lignin and hemicellulose degradation on OPFs observed after 7- and 14-days fungal pretreatment compared to that of 24-h decolorization (Fig. S3 vs. Fig. 5).
The fungus T. hirsuta AK04 was able to utilize RBBR dye as a sole carbon and energy source. The decolorization activity was enhanced by the supplementation of glucose as co-substrate. Of all tested compounds, VA was the best inducer to enhance the activities of Lac and MnP associated with decolorizing activity. There is more Lac and MnP activity in the immobilized cell system than in the free cell system, suggesting the influence of lignin in inducing and producing these enzymes. The addition of 0.1 mM VA along with glucose could accelerate the initial decolorization rate by the immobilized fungus, reaching 97% dye removal in 12 h. FTIR spectra presented changes in the functional groups of dye and the formation of the degradation products, as well as changes within lignin and hemicellulose of OPFs after decolorization. The extended lignin degradation and cellulose content of OPFs following fungal pretreatment suggest that the fungal-treated OPFs wastes can be utilized as substrates for further production of bioproducts.
REFERENCES
Al-Tohamy, R, Ali, S.S., Li, F., Okasha, K.M., Mahmoud, Y.A.G., Elsamahy, T., et al., Ecotoxicol. Environ. Saf., 2022, vol. 231, p. 113160.
Bonugli-Santos, R.C., Durrant, L.R., and Sette, L.D., Wat. Air Soil Poll., 2012, vol. 223, pp. 2333–2345.
Chatterjeea, S., Deya, S., Sarmab, M., Chaudhuric, P., and Dasa, S., Appl. Biochem. Microbiol., 2020, vol. 56, no. 6, pp. 708–717.
Ado, B.V., Onilude, A.A., and Amande, T., J. Adv. Microbiol., 2018, vol. 13, pp. 1−14.
Galhaup, C., Wagner, H., Hinterstoisser, B., and Haltrich, D., Enzyme Microb. Technol., 2002, vol. 30, pp. 529−536.
Mani, P., Kumar, V.T.F., Keshavarz, T., Chandra, T.S., and Kyazze, G., Energies, 2018, vol. 11, p. 3455.
Mostafa, A.A-F., Elshikh, M.S., Al-Askar, A.A., Hadibarata, T., Yuniarto, A., and Syafiuddin, A., Bioproc. Biosyst. Eng., 2019, vol. 42, pp. 1483−1494.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M.J., Bioresour. Technol., 2010, vol. 101, pp. 4851–4861.
Mishra, V., Jana, A.K., Jana, M.M., and Gupta, A., 3 Biotech., 2017, vol. 7, p. 110.
Boehmer, U., Suhardi, S.H., and Bley, T., Eng. Life Sci., 2006, vol. 6, pp. 417−420.
Šušla, M., Novotny, Č., and Svobodova, K., Bioresour. Technol., 2007, vol. 98, pp. 2109−2115.
Kietkwanboot, A., Tran, H.T.M, and Suttinun, O., Water Air Soil Pollut., 2015 vol. 226, p. 345.
Han, Y., Shi, L., Meng, J., Yu, H., and Zhang, X., PLoS One, 2014, vol. 9, no. 10, p. e109786.
Tychanowicz, G.K., Zilly, A., de Souza, C.G.M., and Peralta, R.M., Process Biochem., 2004, vol. 39, pp. 855–859.
Tian, J.H., Pourcher, A.M., and Peu, P., Lett. Appl. Microbiol., 2016, vol. 63, pp. 30−37.
Stajić, M., Persky, L., Friesem, D., Hadar, Y., Wasser, S.P., Nevo, E., et al., Enzyme. Microb. Technol., 2006, vol. 38, pp. 65–73.
Tavares, M.F., Avelino, K.V., Araújo, N.L., Marim, R.A., Linde, G.A., Colauto, N.B., et al., Braz. J. Microbiol., 2020, vol. 51, pp. 99−106.
Aidoo, K.E., Hendry, R., and Wood, B.J.B., Eur. J. Appl. Microbiol. Biotechnol., 1981, vol. 12, pp. 6−9.
Rodriguez, E., Pickard, M.A., and Vazquez-Duhalt, R., Curr. Microbiol., 1999, vol. 38, pp. 27−32.
Han, M.J., Choi, H.T., and Song, H.G., Int. J. Microbiol., 2005, vol. 43, pp. 555–560.
Heinfling, A., Martinez, M.J., Martinez, A.T., Bergbauer, M., and Szewzyk, U., Appl. Environ. Microbiol., 1998, vol. 64, pp. 2788−2793.
Van Soest, P.J., Robertson, J.B., and Lewis, B.A., J. Dairy. Sci., 1991, vol. 74, pp. 3583–3597.
Kasim, F. and Kasim, A., Int. J. Adv. Sci., 2013, vol. 3, no. 3, pp. 24−27.
Yesilada, O., Asma, D., and Cing, S., Process Biochem., 2003, vol. 38, pp. 933−938.
Ambrosio, S.T.,and Campos-Takaki, G.M., Bioresour. Technol., 2004, vol. 91, pp. 69−75.
Jirasripongpun, K., Nasanit, R., Niruntasook, J., and Chotikasatian, B., Sci. Technol. Asia, 2007, vol. 12, pp. 6−11.
Bettin, F., Montanari, Q., Calloni, R., Gaio, T.A., Silveira, M.M., and Dillon, A.J.P., J. Ind. Microbiol. Biotechnol., 2009, vol. 36, pp. 1−9.
Thakkar, A.T., Pandya, D.C., and Bhatt, S.A., Biosci. Biotechnol. Res. Asia., 2020, vol.17, pp. 65−72.
Arora, D.S., and Gill, P.K., Bioresour. Technol., 2000, vol. 73, pp. 283−285.
Dekker, R.F., Vasconcelos, A.F.D., Barbosa, A.M., Giese, E.C., and Paccola-Meirelles, L., Biotechnol. Lett., 2001, vol. 23, pp. 1987−1993.
Lee, A.H., Jang, Y., Kim, G-H., Kim, J-J., Lee, S-S., and Ahn, B-J., Eng. Life. Sci., 2017, vol. 17, pp. 125−131.
Zhu, M-X., Lee, L., Wang, H-H., and Wang, Z., J. Hazard. Mater., 2007, vol. 149, pp. 735−741.
Chang, V.S., Nagwani, M., Kim, C-H., and Holtzapple, M.T. Appl. Biochem. Biotechnol., 2001, vol. 94, pp. 1−28.
Erkurt, E.A., Ünyayar, A., and Kumbur, H., Process Biochem., 2007, vol. 42, pp. 1429−1435.
Sumandono, T., Saragih, H., Migirin, Watanabe, T., and Amirta, R., Proc. Environ. Sci., 2015, vol. 28, pp. 45−51.
Andriani, A., Maharani, A., Yanto, D.H.Y., Pratiwi, H., Astuti, D., Nuryana, I., et al., Bioresour. Technol. Rep., 2020, vol. 12, p. 100562.
Soares, G.M.B., de Amorim, M.T.P., and Costa-Ferreira, M., J. Biotechnol., 2001, vol. 89, pp. 123–129.
Camarero, S., Ibarra, D., Martínez, M.J., and Martínez, Á.T., Appl. Environ. Microbiol., 2005, vol. 71, pp. 1775−1784.
Syafiuddin, A. and Fulazzaky, M.A., Biotechnol. Rep., 2021, vol. 29, p. e00573.
Alam, R., Ardiati, F.C., Solihat, N.N., Alam, M.B., Lee, S.H., and Yanto, D.H.Y., et al., J. Hazard. Mater., 2021, vol. 405, p. 124176.
Velayutham, K., Madhava, A.K., Pushparaj, M., Thanarasu, A., Devaraj, T., Periyasamy, K., et al., Environ. Technol., 2018, vol. 39, pp. 2900–2907.
Pype, R., Flahaut, S., and Debaste, F., Environ. Technol. Innov., 2019, vol. 14, p. 100324.
Osma, J.F., Toca-Herrera, J.L., and Rodríguez-Couto, S., Bioresour. Technol., 2010, vol. 101, pp. 8509−8514.
Sharma, S., Sharma, V., and Kuila, A., 3 Biotech., 2016, vol. 6, p. 139.
Kubovský, I., Kačíková, D., and Kačík, F., Polymers, 2020, vol. 12, p. 485.
Sahoo, S., Chakraborti, C.K., Behera, P.K., and Mishra, S.C., J. Young Pharm., 2012, vol. 4, pp. 138−145.
ACKNOWLEDGMENTS
We would like to thank Dr. A. Naknaen for her technical assistance during laboratory work. Technical guidance by Dr. A. Kietkwanboot in fungal cultivation and enzyme analysis is thankfully acknowledged. The authors would like to express their sincere thanks to the Center of Excellence on Hazardous Substance Management (Thailand) for their invaluable support in terms of facilities and scientific equipment.
Funding
This work was supported by the Thailand International Cooperation Agency (TICA) through the Thailand International Postgraduate Program (TIPP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Mahdy, S., Suttinun, O. Decolorization of Remazol Brilliant Blue R by White-rot Fungus Trametes hirsuta AK04 Immobilized on Lignocellulosic Oil Palm Fibers. Appl Biochem Microbiol 59, 867–880 (2023). https://doi.org/10.1134/S0003683823060078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823060078