Abstract
White-rot basidiomycetes such as Lentinus crinitus produce laccases with potential use in dye biodegradation. However, high productivity and enzymes with specific properties are required in order to make viable laccase production. We aimed to produce laccase from Lentinus crinitus grown in sugarcane bagasse for dye decolorization. Solid state cultivation medium had sugarcane bagasse added with a nutrient solution of 10 g/L glucose, 1 g/L KH2PO4, 0.5 g/L MgSO4, 0.001 g/L FeSO4, 0.01 g/L ZnSO4, and 0.01 g/L MnSO4. The addition of different nitrogen sources (peptone, urea, or peptone plus urea) and different nitrogen concentrations (0, 0.4, 0.6, 0.8, 1.0, and 1.2 g/L) were evaluated. Enzymatic extract was used in the decolorization of azo dyes, reactive blue 220 (RB220) and reactive black 5 (RB5), and anthraquinone dye, Remazol brilliant blue R (RBBR). The greatest laccase activity (4800 U/g dry mass) occurred when the peptone and urea mixture was added to the solid state cultivation medium. When the nitrogen concentration was 1 g/L, the laccase activity increased to 6555 U/g dry mass. The laccase activity peak occurred on the 10th day, and the maximum decolorization within 24 h was observed with enzymatic extracts obtained on different cultivation days, i.e., 6th day for RB220, 10th day for RB5, and 9th day for RBBR. Manganese and lignin peroxidases were not produced when nitrogen was added to the cultivation medium. The crude enzymatic extract was more effective in the decolorization of azo dyes (RB220 and RB5), more than 90% of decolorization, than anthraquinone dye with 77% decolorization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic dyes are utilized in different industrial sectors such as chemical, pharmaceutical, biomedical, food, leather, textile, plastic, and printing ones [1]. It is estimated that ten thousand types of dyes and pigments are yearly used by the industries, and their production can reach 7 × 105 tons per year [2]. The textile sector utilizes a great amount of dyes of different classes, and 10–15% of dyes used in fiber coloring are disposed in effluents due to losses that occur during dyeing process [3]. This feature combined to the high water consumption by the traditional textile finishing industry, results in the production of a great volume of colored effluents, with a variety of toxic compounds including dye degradation products [4].
The chromophore group is the main structural element responsible by the dye color and allows their classification in anthraquinone, azo, indigo, nitro, nitrous, phthalein, and triphenylmethane [5]. Azo dyes account for more than 50% of the annual worldwide production of dyes. This is the most utilized class of synthetic dyes in the textile industry and the most commonly found in industrial effluents [6]. Azo dyes are preferred due to their easy and affordable synthesis and stability, but the toxicity, mutagenicity, and carcinogenicity of synthetic azo dyes and/or their metabolites as aromatic amines are well described [7]. Anthraquinone dyes present a broad range of colors and shades, and are easily applicable, and, therefore, the second most important class of dyes extensively utilized in the textile industry [2]. Several chemical and physical methods have been developed in an attempt to treat and decolorize dye effluents. Approaches such as coagulation/flocculation, adsorption by organic or inorganic matrices, oxidation with ozone, decolorization by photocatalysis, irradiation, and filtration have been used [8]. Besides being costly, these methods have proven to be ineffective due to the stability of dyes and are able to generate highly toxic by-products [9]. This has increase the search for alternative and environmentally friendly methods such as biological processes.
White-rot fungi are among the most efficient organisms capable of degrading synthetic dyes due to their physiological characteristics [10]. White-rot basidiomycetes (WRB) are able to degrade all the components of the cell wall including the heterogeneous, complex, and stable polymer lignin. This capacity results in the production of an extracellular non-specific enzymatic complex consisting of oxidases such as laccase, manganese peroxidase, lignin peroxidase, and versatile peroxidases [11]. Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) catalyze the oxidation of phenolic substrates to the corresponding free radicals while reducing oxygen to water. Their typical substrates are 1,4-diphenols, but they are also able to oxidize a wide array of compounds as aromatic diamines and methoxy-substituted phenols [12]. Moreover, besides being utilized in the textile, food, paper, pharmaceutical, and chemical industries, laccases have shown to be efficient in the degradation of several xenobiotic compounds and synthetic dyes [13].
The utilization of fungal laccases to biodegrade dyes demands the search for enzymes with specific characteristics and microorganisms that are able to produce a great quantity of enzymes. That requires constant research in cultivation and production strategies. Our research team has been investigating the cultivation of edible basidiomycetes to produce laccases capable of efficiently degrading dyes from different classes. Solid state cultivation (SSC) mimics the fungal natural environment, enables higher enzymatic productivity, allows the use of solid agro-industrial wastes and/or by-products as substrate or energy source, among other advantages [14]. Sugarcane bagasse is an affordable substrate for SSC of basidiomycetes and laccase production [15, 16]. Their composition, rich in cellulose (41–50%), hemicellulose (15–25%), and lignin (18–25%) favors its use in SSC of many fungi and the generation of value-added products [17]. In Brazil, 646 million tons of sugarcane were produced in the 2017–2018 crop, generating more than 180 million tons of sugarcane bagasse [18]. Therefore, the great availability and potential to aggregate value to the residue make sugarcane bagasse an appropriate substrate for laccase production.
The nature and concentration of nitrogen in the cultivation medium directly affect laccase production of WRB. The effect of nitrogen availability on laccase production in basidiomycetes is well-known and affects laccase production by changing laccase gene transcription rate [19]. However, different WRB species respond distinctly to nitrogen availability, and their response depends on other cultivation parameters as carbon-to-nitrogen (C/N) ratio of cultivation medium, carbon source, and fungal strain genetic diversity [20, 21].
Lentinus crinitus is a WRB with pantropical occurrence and broadly distributed in Brazil [22]. It is an edible mushroom consumed traditionally by Yanomami people in the Brazilian Amazon and natives of Colombian Amazon [23], and it is found locally in our area. This mushroom is capable to produce great amounts of mycelial biomass with antioxidant activity [24] besides being known as a robust species that produces enzymes such as laccase [25], cellulase, and xylanase [26] under different cultivation conditions [27]. Its capacity to degrade dyes of different classes has already been demonstrated [28, 29], but there are no data on the laccase production of this species by SSC. In this study, we evaluated the production of ligninolytic enzymes by L. crinitus in SSC with sugarcane bagasse and the enzymatic extract’s capacity to decolorize azo and anthraquinone dyes. New ways to produce laccase contribute to the knowledge on microorganisms to obtain enzymes that are capable to biodegrade dyes of different classes.
Material and methods
Microorganism
Lentinus crinitus (L.) Fr. (Polyporaceae) strain U9-1 from the culture collection of the Molecular Biology Laboratory of Paranaense University was used in the assays. The mycelium was grown on 2% (weight/volume) malt extract agar (MEA) medium at 28 ± 1 °C in the dark. Three MEA disks (6-mm diameter) covered with mycelia from a 7-day-old culture were used as inoculum.
Enzymatic production by solid state cultivation with sugarcane bagasse
Enzymatic production occurred in conical flasks (250 mL) with 4 g sugarcane bagasse moistened with 40 mL nutrient solution of 10 g/L glucose, 1 g/L KH2PO4, 0.5 g/L MgSO4, 0.001 g/L FeSO4, 0.01 g/L ZnSO4, 0.01 g/L MnSO4, and nitrogen solution. The nitrogen was supplied by peptone, urea, or peptone plus urea (6 g/L) added in appropriate volume to obtain 0.8 g/L nitrogen [25]. The nitrogen source that provided the highest laccase activity was used to evaluate the influence of nitrogen concentration during SSC and added to the nutrient solution to final nitrogen concentration of 0, 0.4, 0.6, 0.8, 1.0, and 1.2 g/L in the SSC. All nitrogen solutions were filtered (0.22 μm pore membrane) and added to the previously sterilized nutrient solution (autoclaved at 121 °C for 20 min) before being incorporated into the sugarcane bagasse. Sugarcane bagasse was provided by a sugar and alcohol industry from Southern Brazil. The by-product was washed five times with distilled water and dried at 65 °C. The assays were kept for 15 days at 28 ± 1 °C, in the dark, and the enzymatic activities were determined on the 5th, 10th, and 15th cultivation days. The best nitrogen concentration to produce high enzymatic activity was utilized to determine the laccase production every 24 h. Three flasks were withdrawn every 24 h, and each flask was analyzed in order to obtain an arithmetic average of the enzyme extract sample.
The enzymatic extracts were obtained by mixing 1 g of the bagasse colonized with mycelia and 10 mL sodium acetate buffer (0.1 M; pH 5.0). The mixture was homogenized with a glass rod until complete solid suspension. The suspension was kept at room temperature for 10 min; the enzymatic crude extract was recovered by centrifugation (8000g, at 4 °C for 10 min) and used for enzymatic determination.
All the assays had a completely randomized design with three replicates. The results were evaluated using analysis of variance (ANOVA), and significant differences among arithmetic means were determined by the Scott-Knott test at 5% of probability.
Laccase assay
Laccase activity was determined using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) as substrate. The reaction mixture was 0.2 mL crude enzymatic extract, 0.7 mL water, 0.45 mL sodium acetate buffer (0.1 M; pH 5.0), and 0.15 mL ABTS (1 mM). ABTS oxidation rate was measured at 420 nm (ε = 36,000 M−1 cm−1) at 30 °C [30]. Laccase activity was expressed in international units (U) defined as the amount of enzyme required to oxidize 1 μmol ABTS per minute. The results were expressed as U/g of dry cultivation substrate.
Manganese peroxidase assay
The oxidation of phenol red in the presence of Mn2+ and H2O2 was used to determine manganese peroxidase (MnP) activity of the enzymatic extract [31]. The reaction mixture was enzymatic extract, 0.1 mL sodium lactate (0.25 M), 0.1 mL bovine serum albumin (0.5%; weight/volume), 0.1 mL sodium succinate buffer (0.2 M; pH 4.5), 0.05 mL MnSO4 (2 mM), 0.05 mL H2O2 (2 mM) in sodium succinate buffer (0.2 M; pH 4.5), and 0.1 mL phenol red (0.1%; weight/volume). The oxidation was monitored by absorbance increase at 610 nm (ε = 4460 M−1 cm−1). MnP activity was expressed in international units (U) defined as the amount of enzyme that oxidizes 1 μmol phenol red per minute.
Lignin peroxidase assay
The oxidation of methylene blue was used to determine lignin peroxidase (LiP) activity of the enzymatic extract [32]. The reaction mixture contained enzymatic extract, 0.1 mL methylene blue (1.2 mM), 0.6 mL sodium tartrate buffer (0.5 M; pH 4), and 0.1 mL H2O2 (2.7 mM). The methylene blue conversion to azure-C was monitored by increase in absorbance at 664 nm (ε = 52,400 M−1 cm−1). LiP activity was expressed in international units (U), which is defined as the amount of enzyme that oxidizes 1 μmol substrate per minute.
Dye decolorization assays
Fungal crude enzymatic extracts obtained every 24 h were used to decolorize the azo dyes reactive blue 220 (RB220) and reactive black 5 (RB5), and the anthraquinone dye Remazol brilliant blue R (RBBR). The two azo dyes, RB220 (monoazo) and RB5 (diazo), were chosen to represent different types of molecular arrangements. The RBBR dye was chosen because it has been widely used as a model in biodegradation studies. Decolorization was evaluated according to Marim et al. [21]. The dyes were diluted in sodium acetate buffer (0.1 M; pH 5.0), filtered (0.22 μm pore membrane), and used in sufficient volume to obtain 1 mg/mL (weight/volume) as final concentration in all assays. The decolorization reactions consisted of a mixture of 3.6 mL enzymatic extract and 0.4 mL dye solution kept at 28 ± 1 °C, in the dark, for 24 h. Aliquots of 300 μL were taken in aseptic conditions, and the decolorization was measured in the maximum absorbance of RB220 (592 nm), RBBR (595 nm), and MG (620 nm) after 24 h in a spectrophotometer (SpectraMax Plus 384 Molecular Devices, San Jose, USA). Decolorization reactions with ultrapure water, instead of enzymatic extract, was used as analytical control. The reduction of color (%) within 24 h was calculated in relation to the initial absorbance.
All the assays had a completely randomized design with three replicates. The results were evaluated using ANOVA, and significant differences among arithmetic means were determined by the Scott-Knott test at 5% of probability.
Results
The enzymatic extract produced after 15 days of L. crinitus cultivation on sugarcane bagasse with the nutrient solution and without nitrogen was approximately 2000 U/g dry mass laccase; 13 U/g dry mass LiP and MnP activity was not detected. Considering that the greatest detected ligninase activity was from laccase, and that this enzyme is the most important for dye decolorization, subsequent studies on enzymatic production regarding N concentration and dye decolorization considered only laccase production.
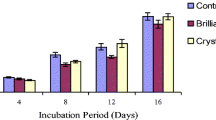
The addition of all nitrogen sources favored the laccase activity of L. crinitus (Fig. 1). The laccase activity peak occurred on the 15th cultivation day when the nitrogen source was urea. However, when peptone or the combination of peptone and urea were used, the laccase activity peak anticipated to the 10th cultivation day. The peptone and urea combination also favored greater (p = 0.0014) laccase activity (4800 U/g dry mass) at the activity peak (Fig. 1). The combination of the two nitrogen compounds promoted an increase in activity of 40 and 139% in relation to the culture media only with peptone or urea, respectively. Therefore, the peptone and urea combination was chosen for the follow-up of laccase production with different N concentrations.
The increase in N concentration from the mixture of peptone and urea in the bagasse increased the laccase activity (Fig. 2). The laccase activity peak occurred on the 10th cultivation day for all nitrogen concentrations, except for 0.6 g/L nitrogen which happened on the 15th day. The greatest laccase activity (p ≤ 0.05) of 6555 U/g dry mass occurred in the presence of 1.0 g/L N, an activity which was 44% greater than the average maximum activity observed for the other nitrogen concentrations and 240% greater than the maximum activity in the absence of nitrogen (15th cultivation day). Concentrations of 1.2 or ≤ 0.8 g/L N in SSC implied in the reduction of the laccase enzymatic activity (Fig. 2). The activity of LiP and MnP was zero for the treatments, in which there was nitrogen addition to the cultivation medium. It suggests that the utilized combined nitrogen source do not favor LiP and MnP production.
The follow-up of the laccase production in the best cultivation condition (sugarcane bagasse with nutrient solution of 1.0 g/L N from peptone plus urea) confirmed the laccase activity peak of 6004 U/g dry mass and 25 U g−1 h−1 on the 10th cultivation day (Fig. 3).
Laccase activity (●) and 24 h decolorization (average ± standard deviation) of reactive blue 220 (RB220), Remazol brilliant blue R (RBBR), and reactive black 5 (RB5) by the enzymatic extract of Lentinus crinitus grown on sugarcane bagasse added with nutrient solution and 1.0 g/L nitrogen (peptone and urea) along 12 cultivation days. Averages indicated by the same letter do not differ statistically by the Scott-Knott test (p ≤ 0.05)
The decolorization of RB220 and RBBR started with the enzymatic extracts obtained after 24 h of cultivation (Fig. 3). RB220 was decolorized more efficiently than RBBR from the beginning; however, RB5 was only decolorized from the 6th cultivation day (Fig. 3). The maximum decolorization of RB220 was 90 ± 2.7%, when the laccase production was from 3000 to 6004 U/g dry mass from the 6th to the 10th cultivation day. The maximum RBBR decolorization was 77 ± 1.8% on the 9th cultivation day, when the laccase production had already surpassed 5000 U/g dry mass. RB5 had maximum decolorization of 95 ± 3.0% in the laccase production peak in the 10th cultivation day, but presented low decolorization efficiency from the 6th to the 9th cultivation days and later from the 11th to the 12th cultivation days.
Discussion
We found that L. crinitus did not produce MnP in SSC with sugarcane bagasse. These results are in agreement with Almeida et al. [28] that cultivated the same strain in liquid medium added with coffee husks or citric pulp and reported laccase but not LiP and MnP production. When L. crinitus was cultivated in synthetic liquid medium with glucose or fructose added with various nitrogen sources [29], MnP and LiP activities were not detected also. In an opposite way, Conceição et al. [33] reported that when L. crinitus was cultivated on SSC in a mixture of barley and cassava residues, MnP but not laccase was produced. Cambri et al. [26] cultivated L. crinitus in liquid and solid synthetic media with different combinations of maltose and urea and reported no LiP production, MnP production in few growing conditions, and laccase production mostly in solid medium. Ligninase production by WRB depends on several factors such as C/N ratio, presence of phenolic or aromatic inducers, and type of lignocellulosic waste used as substrate [34]. Moreover, the expression of different ligninases depends on the evaluated fungal species and strain [35]. Therefore, laccase or peroxidase production by fungal species is intrinsically linked to the cultivation medium and fungal strains, which demands an investigation of the conditions that favor production of enzymes of biotechnological interest. However, the ideal conditions for the production of a ligninase do not always favor the production of the other [36].
The solid state cultivation using sugarcane bagasse as a substrate favored L. crinitus laccase production. Valle et al. [25] who worked on L. crinitus submerged cultivation in synthetic culture medium (10 g/L glucose, 2.8 g/L nitrogen from urea with ethanol, and guaiacol as inducers) reported maximum laccase production of 15,000 U/L on the 12th cultivation day with 52 U L−1 h−1. In our study, the maximum volumetric laccase production of 65,550 U/L and 273 U L−1 h−1 was 4.4 times greater when obtained in SSC with sugarcane bagasse. Agro-industrial residues have been utilized in SSC of WRB for ligninase production, because they present excellent cost benefit and provide support and/or carbon source for fungal growth [14]. Sugarcane bagasse, besides its great abundance, is rich in lignin, cellulose, hemicellulose, presents low ash content, and allows good aeration of the culture medium, advantageous aspects in bioconversion processes [37]. Additionally, sugarcane bagasse contains polyphenols, such as flavonoids and tannins, soluble in water [38]. Polyphenols can induce the production of ligninolytic enzymes [39], and these compounds, solubilized during the cultivation period, could have induced the laccase production by L. crinitus. Almeida et al. [28] cultivated the same strain of L. crinitus used in this study in liquid medium containing coffee husks or citric pulp, as the only carbon sources, added with urea (0.7 g/L or 11.2 g/L nitrogen, respectively). Both agro-industrial by-products contain polyphenols, and the authors suggested that the greater polyphenol proportion in coffee husks could have contributed for a greater laccase production with this by-product of approximately 41,250 U/L and 156 U L−1 h−1. However, in our study, the volumetric laccase production with SSC in sugarcane bagasse was 59% greater, and the productivity was 75% greater than the one observed in submerged cultivation with coffee husks. We can suppose that the smaller amount of water available in the SSC may have favored the increase in the concentration of soluble polyphenols, and this could have contributed for a greater laccase production and productivity. Thus, SSC with sugarcane bagasse to produce L. crinitus laccase reduces the use of expensive nutrients and chemical inducers.
The supplementation of the cultivation medium with the combination of a protein (peptone) and non-protein (urea) nitrogen source favored L. crinitus laccase production. This species, and specially the strain evaluated in this study, has demonstrated greater laccase production in liquid medium, containing non-protein nitrogen source (urea). Valle et al. [25] reported that the urea increased L. crinitus U9-1 laccase production in synthetic culture medium. Almeida et al. [28] showed that only urea, in detriment to other protein and non-protein sources, induced laccase production by L. crinitus U9-1 in liquid medium with agro-industrial by-products. However, the effect of nitrogen source on the ligninase production in WRB is complex and depends on the chemical nitrogen nature and its concentration and the relation of both to the chemical nature and concentration of the carbon source, especially in the presence of lignocellulosic material [36].
In our study, the nitrogen concentration strongly interferes with laccase. The fungal response to the nitrogen availability depends on the fungus species and strain, the cultivation mode, and the carbon source. When L. crinitus was cultivated in liquid culture media with maltose or glycerol (20 g/L) and different urea concentrations (4, 20, or 100 mM), the combination of maltose and 100 mM urea induced the expression of more complex protein profiles than the combination of glycerol and 100 mM urea [26]. Previously, our group observed that the combination between the carbon source and the nitrogen concentration strongly affects laccase production of L. crinitus as well as Almeida et al. [28]. When cultivated in liquid medium with coffee husks, addition of 0.7 g/L nitrogen raised laccase production, but in citric pulp–based liquid medium, 11.2 g/L nitrogen stimulated the greatest laccase production [28]. These results demonstrate the strength of this species and its capacity to adapt to various cultivation conditions.
The follow-up of laccase production throughout the cultivation showed increase in the production already in the fifth cultivation day. Compared with the performance of this strain in different growth conditions, the observed results in our study with SSC in sugarcane bagasse are the ones that presented greater laccase productivity. When L. crinitus U9-1 was grown in submerged cultivation in synthetic medium (10 g/L glucose and 2.8 g/L nitrogen from urea) and using copper as inducer (150 μM), the laccase production started to increase on the 10th cultivation day reaching the maximum productivity only on the 22nd day (~ 26 U L−1 h−1) [25]. Similarly, L. crinitus U9-1 cultivated in a liquid medium containing coffee husks or citric pulp, presented increase in laccase productivity from the 6th day (~ 140 U L−1 h−1) and 10th day (~ 125 U L−1 h−1), respectively [28]. Our results indicate a better performance of L. crinitus U9-1 during SSC with sugarcane bagasse without inducer addition, with a productivity of 188 U L−1 h−1 (fifth cultivation day) and maximum productivity on the 10th day of 250 U L−1 h−1. The presence of soluble polyphenols in sugarcane bagasse [38] and its use in SSC could contribute to the anticipation of L. crinitus laccase production compared with other cultivation conditions, since these compounds may increase the amount of produced laccase or expressed isoenzyme variety [40]. There are numerous laccase isoforms which could be expressed at different phases of the metabolism or under diverse cultivation conditions [1] and that could explain the distinct patterns of L. crinitus laccase production in various media.
The crude enzymatic extract obtained from SSC with sugarcane bagasse was able to decolorize anthraquinone (RBBR) as well as azo dyes (RB220 and RB5); a capacity attributed to laccase since MnP and LiP activity was not detected when nitrogen was added to the cultivation medium. The azo dyes had the highest color reduction, and the decolorization efficiency of these dyes increased with the cultivation time. This is the first report of decolorization of azo dye RB5 by L. crinitus laccase. RB220 was rapidly decolorized with the maximum decolorization from the 6th to the 10th cultivation day. RB5 only showed significant decolorization from the 6th cultivation day and maximum decolorization on the 10th day, but it was the one that presented greater color reduction percentage (95%). Decolorization rates can be affected by molecular structures of the dyes. Dyes with simpler structures and lower molecular mass can present higher rates of color removal, whereas highly substituted and higher molecular mass dyes can be decolorized in a lower rate [41]. RB220 is a monoazo dye and RB5 is a diazo dye, and RB5 is highly substituted (two vinyl sulfone groups) and has a bigger and more complex structure (molecular mass = 991.82 g/mol), while RB220 has a simplest structure (one vinyl sulfone group) and is a smaller molecule (molecular mass = 733.1 g/mol), which could help explain the greater decolorization rate of the RB220 [42, 43]. Our results are in accordance with the ones described by Niebisch et al. [29], who reported that 94% of the decolorization of azo dye RB220 by L. crinitus laccase; however, this result was obtained after cultivation medium optimization (solid medium with 10 g/L ammonium oxalate and glucose at 28 °C and pH 5.5) and after 15 cultivation days. Munari et al. [44] grew P. sajor-caju (sin. Lentinus sajor-caju) in SSC with sawdust from Pinus sp. and wheat bran, and reported total decolorization of RB220 in just 30 min, but with the extract obtained on the 20th cultivation day.
Decolorization of anthraquinone dye RBBR was less efficient than the decolorization of the azo dyes. In our study, there was significant decolorization from the 5th cultivation day and maximum decolorization on the 9th day. However, while the decolorization of azo dyes was greater than 90%, the discoloration of the RBBR was of 77%. Although smaller than the observed decolorization of azo dyes, the discoloration of RBBR was significant. Our results are in accordance with the ones described by Almeida et al. [28] who utilized the extract obtained in the submerged cultivation of L. crinitus U9-1 with coffee husks (6th cultivation day) or citric pulp (12th cultivation day) and reported decolorization of RBBR of 74% and 76%, respectively.
The differences observed in the degradation of azo and anthraquinone dyes could be explained by different factors as difference of redox potential between laccases and dyes, the structural complexity of dyes, and specific characteristics of the active site of the enzyme. The difference of the redox potential between the enzyme and the substrate seems to have a determining role in the oxidation of bigger substrates such as dyes and laccases with high redox potential oxide bigger substrates more efficiently [45]. Laccases are known by their non-specific oxidation capacity, but decolorization of high redox potential dyes may require the use of redox mediators [46]. It is important to emphasize that the observed decolorization in our study were obtained without the utilization of additional redox mediators and without the enzyme purification. Structural differences in substrate binding sites may also affect decolorization as the presence of specific amino acids residues in the laccase active sites seems to determine the catalytic efficiency of the enzyme [45]. There is no information yet on L. crinitus laccase structure, but there are indications that the structural laccase characteristics of this species favor the oxidation of azo dyes.
Conclusion
In general, the addition of 1.0 g/L N mixture (peptone and urea) in SSC with sugarcane bagasse and nutritional solution promotes the best laccase activity along L. crinitus cultivation days. MnP and LiP activities are not favored by the addition of N sources in the substrate. The 10th cultivation day produces the highest laccase activity (6555 U/g dry mass). The laccase production from the 6th to the 10th cultivation day promotes decolorization of 90% of RB220, at the 9th day promotes decolorization of 77% of RBBR, and on the 10th day decolorization of 94% of RB5. L. crinitus crude extract obtained from SSC with bagasse is an alternative to decolorize anthraquinone dyes and especially azo dyes.
References
Othman AM, Elsayed MA, Elshafei AM, Hassan MM (2018) Purification and biochemical characterization of two isolated laccase isoforms from Agaricus bisporus CU13 and their potency in dye decolorization. Int J Biol Macromol 113:1142–1148. https://doi.org/10.1016/j.ijbiomac.2018.03.043
Vikrant K, Giri BS, Raza N, Roy K, Kim K-H, Rai BN, Singh RS (2018) Recent advancements in bioremediation of dye: current status and challenges. Bioresour Technol 253:355–367. https://doi.org/10.1016/j.biortech.2018.01.029
Leite LDS, Maselli BDS, Umbuzeiro GDA, Nogueira RFP (2016) Monitoring ecotoxicity of disperse red 1 dye during photo-Fenton degradation. Chemosphere 148:511–517. https://doi.org/10.1016/j.chemosphere.2016.01.053
Xiang X, Chen X, Dai R, Luo Y, Ma P, Ni S, Ma C (2016) Anaerobic digestion of recalcitrant textile dyeing sludge with alternative pretreatment strategies. Bioresour Technol 222:252–260. https://doi.org/10.1016/j.biortech.2016.09.098
Ali H (2010) Biodegradation of synthetic dyes - a review. Water Air Soil Pollut 213:251–273. https://doi.org/10.1007/s11270-010-0382-4
Rawat D, Mishra V, Sharma RS (2016) Detoxification of azo dyes in the context of environmental processes. Chemosphere 155:591–605. https://doi.org/10.1016/j.chemosphere.2016.04.068
Forootanfar H, Rezaei S, Zeinvand-Lorestani H, Tahmasbi H, Mogharabi M, Ameri A, Faramarzi MA (2016) Studies on the laccase-mediated decolorization, kinetic, and microtoxicity of some synthetic azo dyes. J Environ Health Sci Eng 14:7–9. https://doi.org/10.1186/s40201-016-0248-9
Joo DJ, Shin WS, Choi J-H et al (2007) Decolorization of reactive dyes using inorganic coagulants and synthetic polymer. Dyes Pigments 73:59–64. https://doi.org/10.1016/j.dyepig.2005.10.011
Perlatti B, da Silva MFG, Fernandes JB, Forim MR (2012) Validation and application of HPLC–ESI-MS/MS method for the quantification of RBBR decolorization, a model for highly toxic molecules, using several fungi strains. Bioresour Technol 124:37–44. https://doi.org/10.1016/j.biortech.2012.08.032
Kaushik P, Malik A (2009) Fungal dye decolourization: recent advances and future potential. Environ Int 35:127–141. https://doi.org/10.1016/j.envint.2008.05.010
Rodríguez-Couto S (2011) Production of laccase and decolouration of the textile dye Remazol brilliant blue R in temporary immersion bioreactors. J Hazard Mater 194:297–302. https://doi.org/10.1016/j.jhazmat.2011.07.098
Martínková L, Kotik M, Marková E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere 149:373–382. https://doi.org/10.1016/j.chemosphere.2016.01.022
Maté DM, Alcalde M (2016) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467. https://doi.org/10.1111/1751-7915.12422
Soccol CR, Costa ESFD, Letti LAJ, Karp SG, Woiciechowski AL, Vandenberghe LPDS (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1:52–71. https://doi.org/10.1016/j.biori.2017.01.002
Hernández C, Silva A-MFD, Ziarelli F, Perraud-Gaime I, Gutiérrez-Rivera B, García-Pérez JA, Alarcón E (2016) Laccase induction by synthetic dyes in Pycnoporus sanguineus and their possible use for sugar cane bagasse delignification. Appl Microbiol Biotechnol 101:1189–1201. https://doi.org/10.1007/s00253-016-7890-0
Karp SG, Faraco V, Amore A, Birolo L, Giangrande C, Soccol VT, Pandey A, Soccol CR (2012) Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol 114:735–739. https://doi.org/10.1016/j.biortech.2012.03.058
Madeira JV Jr, Contesini FJ, Calzado F, Rubio MV, Zubieta MP, Lopes DB, Melo RR (2017) Agro-industrial residues and microbial enzymes: an overview on the eco-friendly bioconversion into high value-added products. In: Brahmachari G, Demain AL, Adrio JL (eds) Biotechnology of microbial enzymes: production, biocatalysis and industrial applications. Elsevier/AP, Tokyo, pp 475–511
CONAB Companhia Nacional de Abastecimento (2018) Brazilian crop 2017/18. Available at http://www.conab.gov.br. Accessed 23 november 2018
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2009) Laccases: a never-ending story. Cell Mol Life S 67:369–385. https://doi.org/10.1007/s00018-009-0169-1
D'Agostini ÉC, Mantovani TRD, Valle JS, Paccola-Meirelles LD, Colauto NB, Linde GA (2011) Low carbon/nitrogen ratio increases laccase production from basidiomycetes in solid substrate cultivation. Sci Agric 68:295–300. https://doi.org/10.1590/s0103-90162011000300004
Marim RA, Oliveira ACC, Marquezoni R et al (2016) Use of sugarcane molasses by Pycnoporus sanguineus for the production of laccase for dye decolorization. Genet Mol Res 15. https://doi.org/10.4238/gmr15048972
Drechsler-Santos ER, Wartchow F, Coimbra VRM, Gibertoni TB, Cavalcanti MAQ (2012) Studies on lentinoid fungi (Lentinus and Panus) from the semi-arid region of Brazil. J Torrey Bot Soc 139:437–446. https://doi.org/10.3159/torrey-d-12-00019.1
Vargas-Isla R, Ishikawa NK (2008) Optimal conditions of in vitro mycelial growth of Lentinus strigosus, an edible mushroom isolated in the Brazilian Amazon. Mycoscience 49:215–219. https://doi.org/10.1007/s10267-007-0404-2
Umeo S, Souza G, Rapachi P, Garcia DM, Paccola-Meirelles LD, Valle JS, Colauto NB, Linde GA (2015) Screening of basidiomycetes in submerged cultivation based on antioxidant activity. Genet Mol Res 14:9907–9914. https://doi.org/10.4238/2015.august.19.25
Valle JS, Vandenberghe LPS, Santana TT, Almeida PH, Pereira AM, Linde GA, Colauto NB, Soccol CR (2014) Optimum conditions for inducing laccase production in Lentinus crinitus. Genet Mol Res 13:8544–8551. https://doi.org/10.4238/2014.october.20.31
Cambri G, Sousa MMLD, Fonseca DDM, Marchini FK, Silveira JLMD, Paba J (2016) Analysis of the biotechnological potential of a Lentinus crinitus isolate in the light of its secretome. J Proteome Res 15:4557–4568. https://doi.org/10.1021/acs.jproteome.6b00636
Marim RA, Avelino KV, Linde GA, Colauto NB, Valle JS (2018) Lentinus crinitus strains respond differently to cultivation pH and temperature. Genet Mol Res 17. https://doi.org/10.4238/gmr16039885
Almeida PH, Oliveira ACC, Souza GPN, Friedrich JC, Linde GA, Colauto NB, Valle JS (2018) Decolorization of Remazol brilliant blue R with laccase from Lentinus crinitus grown in agro-industrial by-products. Ann Braz Acad Sci 90:3463–3473. https://doi.org/10.1590/0001-3765201820170458
Niebisch CH, Malinowski AK, Schadeck R, Mitchell DA, Kava-Cordeiro V, Paba J (2010) Decolorization and biodegradation of reactive blue 220 textile dye by Lentinus crinitus extracellular extract. J Hazard Mater 180:316–322. https://doi.org/10.1016/j.jhazmat.2010.04.033
Valle JS, Vandenberghe LPDS, Santana TT, Linde GA, Colauto NB, Soccol CR (2014) Optimization of Agaricus blazei laccase production by submerged cultivation with sugarcane molasses. Afr J Microbiol Res 8:939–946. https://doi.org/10.5897/ajmr2013.6508
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250. https://doi.org/10.1016/0014-5793(84)80327-0
Magalhães DB, Carvalho MEAD, Bon E, Neto JSA, Kling SH (1996) Colorimetric assay for lignin peroxidase activity determination using methylene blue as substrate. Biotechnol Tech 10:273–276. https://doi.org/10.1007/bf00184028
Conceição TA, Koblitz MGB, Kamida HM, Góes-Neto A (2017) Study of the production of Lentinus crinitus (L.) Fr. Lignolytic enzymes grown on agro-industrial waste. Adv Biosci Biotechnol 8:259–272. https://doi.org/10.4236/abb.2017.88019
Elisashvili V, Kachlishvili E (2009) Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J Biotechnol 144:37–42. https://doi.org/10.1016/j.jbiotec.2009.06.020
Silva CMMS, Melo ISD, Oliveira PRD (2005) Ligninolytic enzyme production by Ganoderma spp. Enzyme Microb Tech 37:324–329. https://doi.org/10.1016/j.enzmictec.2004.12.007
Martani F, Beltrametti F, Porro D, Branduardi P, Lotti M (2017) The importance of fermentative conditions for the biotechnological production of lignin modifying enzymes from white-rot fungi. FEMS Microbiol Lett 364. https://doi.org/10.1093/femsle/fnx134
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol 74:69–80. https://doi.org/10.1016/s0960-8524(99)00142-x
Vijayalaxmi S, Jayalakshmi SK, Sreeramulu K (2015) Polyphenols from different agricultural residues: extraction, identification and their antioxidant properties. J Food Sci Technol 52:2761–2769. https://doi.org/10.1007/s13197-014-1295-9
Kachlishvili E, Metreveli E, Elisashvili V (2014) Modulation of Cerrena unicolor laccase and manganese peroxidase production. SpringerPlus 3:463. https://doi.org/10.1186/2193-1801-3-463
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genomics 12:104–112. https://doi.org/10.2174/138920211795564331
Kachlishvili E, Asatiani M, Kobakhidze A, Elisashvili V (2016) Trinitrotoluene and mandarin peels selectively affect lignin-modifying enzyme production in white-rot basidiomycetes. SpringerPlus 5:1–9. https://doi.org/10.1186/s40064-016-1895-0
Ramírez-Montoya LA, Hernández-Montoya V, Montes-Morán MA, Jáuregui-Rincón J, Cervantes FJ (2015) Decolorization of dyes with different molecular properties using free and immobilized laccases from Trametes versicolor. J Mol Liq 212:30–37. https://doi.org/10.1016/j.molliq.2015.08.040
Patel VR, Bhatt NS, B̀bhatt H (2013) Involvement of ligninolytic enzymes of Myceliophthora vellerea HQ871747 in decolorization and complete mineralization of reactive blue 220. Chem Eng J 233: 98–108. https://doi.org/10.1016/j.cej.2013.07.110
Munari FM, Gaio TA, Calloni R, Dillon AJP (2007) Decolorization of textile dyes by enzymatic extract and submerged cultures of Pleurotus sajor-caju. World J Microbiol Biotechnol 24:1383–1392. https://doi.org/10.1007/s11274-007-9621-2
Glazunova O, Trushkin N, Moiseenko K, Filimonov I, Fedorova T (2018) Catalytic efficiency of basidiomycete laccases: redox potential versus substrate-binding pocket structure. Catalysts 8:152. https://doi.org/10.3390/catal8040152
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes – a review. Int Biodeterior Biodegradation 104:21–31. https://doi.org/10.1016/j.ibiod.2015.04.027
Funding
The authors thank the Universidade Paranaense, Programa de Pós-graduação em Biotecnologia Aplicada à Agricultura (Probiot), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, finance code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 307953/2017-3) for the financial support and fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Cyntia Canedo Silva
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavares, M.F., Avelino, K.V., Araújo, N.L. et al. Decolorization of azo and anthraquinone dyes by crude laccase produced by Lentinus crinitus in solid state cultivation. Braz J Microbiol 51, 99–106 (2020). https://doi.org/10.1007/s42770-019-00189-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00189-w