Abstract
One of the urgent problems of plant protection from pests and diseases is the creation of environmentally safe biocontrol agents whose use would not be accompanied by the resistance of insect pests. Microorganisms have a great potential in this regard. The most promising group are endophytes, which inhabit the internal tissues of plants and are involved in the formation of the phenotype of plants. Bacteria of the genus Bacillus are of particular interest due to their wide distribution in nature, the safety of many species for humans, and the relative ease with which biocontrol means based on Bacillus sp. can be obtained. The review considers the properties of B. thuringiensis as follows: endophytic, insecticidal, and antibiotic activity; production of growth regulators and mobilization of plant nutrients; resistance induction; and the possibility of constructing new strains using genetic engineering methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pathogens and pests cause significant crop yield losses. The use of crop protection chemicals (CPCs) to control pests in fields, especially insects, has become a conventional practice in modern crop production. Although CPCs have benefits, protecting agricultural plants and ensuring agricultural efficiency, their use has led to an increase in consumer concern about the safety for humans and animals of both the CPCs themselves and the resulting food products, as well as the development of pest resistance to existing chemicals and the need to solve these problems.
Biological products based on various types of entomopathogenic microorganisms, including Bacillus thuringiensis Berliner (Bt) bacteria, are considered an alternative to CPCs. The study of the protective properties of Bt was started in Japan by the silkworm engineer Ishivata [1], who isolated a bacterium that was named Bacillus sotto silkworms from dead bombyx mori caterpillars. The bacterium was identified as the causative agent of a deadly infection of this insect, Sotto’s disease. Later, it was classified as the pathovar Bacillus thuringiensis subsp. sotto. Several years later, in 1911, the German scientist Ernst Berliner [2] in Thuringia isolated a bacterium from dead larvae of the millworm Anagasta kuehniella, which was named Bacillus thuringiensis in honor of this area. Then, in 1938, the first commercial product of the Bitoxibacillin family appeared in France. It was called Sporene and was intended primarily to combat barn moths [3]. In the 1950s, almost simultaneously and independently, the first commercial insecticides based on Bt began to appear in the USSR and the United States. In the USSR, the production of the first such insecticide, Entobacterin, was done at the Berdsk (Russia) and Stepnogorsk (Kazakhstan) plants [4]. In 1949, the microbiologist E.V. Talalaev isolated a bacterium called Bacillus dendrolimus from the dead larvae of the Siberian silkworm. Later, this bacterium became the active principle of the biopreparation Dendrobacilin, which was successfully used to protect forests from pests. At the same time, in the United States, the Bacillus thuringiensis subsp. kurstaki strain was used to produce the Thurincide bioinsecticide. In 1970, the Bacillus thuringiensis var. alesti strain HD-1 (serotype H3a3b, subsp. kurstaki) was isolated from caterpillars of the cotton moth Pectinophora gossypiella Saund. This bacterium was almost 20 times more effective in protecting plants from target insects than other bioinsecticides available at that time. This led to the creation in 1983 of the biopreparation Lepidocide based on this strain.
Today, Bt strains of subspecies (subsp.) kurstaki, aizawai, israelensis, tenebrionis, and thuringiensis are most often used in the world as the basis of bioinsecticides. Due to their high specificity with respect to various pests and environmental friendliness, many researchers consider bt-based products an efficient and environmentally friendly alternative to CPCs [5]. Moreover, bt-preparations are allowed for use in organic farming both in Russia and abroad.
The bt-based pesticides account for up to 75% of the global bioinsecticide sales market and approximately 4% of all insecticides. The following biological products have been registered in Russia: Bitoxibacillin, Leptocid, and Insetim (based on Bt subsp. thuringiensis); Lepidobactocide (based on Bt subsp. kurstaki); and Bioslip (based on Bt subsp. toumanoffi) [6]. In Belarus, products based on Bt subsp. dendrolimus (Dendrolin) and subsp. darmstadiensis (Baciturin) are known [7].
However, despite the fairly detailed studies of the properties of bacteria Bt, the prevalence of preparations based on them, and the high efficiency of some of them for protecting plants against certain insect species, there are a number of debatable issues. The analysis of the current trends in the strategy of increasing the resistance to phytophages and phytopathogens and the productivity of agricultural crops suggests the need to identify the promising directions for the further use of microorganisms of this species in crop production.
Controversial issues of Bt taxonomy. Due to the active use of Bt-based products, pests acquire resistance to the most commonly used strains, which threatens their effectiveness and requires the search for new strains and toxins with different mechanisms of action and high activity. In this regard, problems of strain identification arise, and the taxonomy of some Bacillus species, including Bt, is still under discussion.
Studies of the morphology of cells and spores, as well as their physiological and biochemical properties using immunochemical and molecular genetic methods, made it possible to conclude that Bt serovars (= subspecies) exist and to perform an intraspecific taxonomic classification based on the analysis of the bacterial flagellar H-antigen. According to this classification, the species Bt includes 69 antigenic groups and 13 subgroups [8]. At the same time, this distribution of serogroups had no a clear correlation with the specific insecticidal activity of strains. However, it is also of interest that bacteria of this species with genes for insectotoxic proteins with up to 90% homology encode proteins with different toxic specificity for different insect species. For example, although the cry1Aa and cry1Ac genes are 84% identical to each other; only the cry1Aa protein is toxic to the silkworm (Bombyx mori L.). Conversely, the cry3Aa and cry7Aa genes are only 33% identical to each other and both their products show toxicity against the Colorado potato beetle Leptinotarsa decemlineata Say [9].
Molecular genetic methods for identifying Bacillus representatives made it possible to divide them into clusters and, in accordance with the evolutionary genetic distance tree, include the Bt species in one cluster with such species as B. anthracis, B. cereus, B. medusa, B. mycoides, B. maroccanus, B. simplex, and B. psychrosaccharolyticus. Other researchers, in determining the phylogenetic relationship between 40 species of the genus Bacillus and using the nucleotide sequences of 16S rDNA and 16S–23S internal transcribed spacer, included only B. anthracis, B. cereus, and B. mycoides into the same cluster with the Bt species [10].
According to Liu et al. [11], Bt, as insectotoxin producers, are included in the supraspecific group of B. cereus bacteria, also called B. cereus sensu lato (sl), which includes 21 closely related species. Among them, B. anthracis, B. cereus, Bt, B. mycoides, B. weihenstephanensis, B. pseudomycoides, as well as the recently identified B. gaemokensis, B. manliponensis, B. cytotoxicus, B. toyonensis, B. bingmayongensis, and B. wiedmannii are widely known. Based on the phenotypic and phylogenetic data, the authors identified nine new bacterial species: Bacillus paranthracis sp. nov., B. pacificus sp. nov., B. tropicus sp. nov., B. albus sp. nov., B. mobilis sp. nov., B. luti sp. nov., B. proteolyticus sp. nov., B. nitratireducens sp. nov., and B. paramycoides sp. nov.
Despite the recognition of Bt as a separate species by many authors, there are alternative opinions on whether Bt is a real species and a separate member of the B. cereus group; whether Bt belongs to the bacterial group B. cereus sensu lato or B. cereus sensu stricto; and whether Bt should be considered as an independent species or as a B. cereus subspecies carrying only the corresponding plasmid [12]. Especially many questions arose after the whole-genome sequencing of Bt DNA, when an exceptionally high genetic similarity of this species with B. cereus was found [13], which can lead to the difficulty of their identification and separation in food products. In another study based on the sequencing of the 16SRNA gene of Bt ATCC 10792T (ACNF01000156) and B. paranthracis Mn5T (KJ812420) the greatest genetic similarity between them was shown [13]. However, one of the clearest markers to distinguish the species Bt from B. cereus may be the transcriptional regulator XRE, which controls the production of the majority of protein crystalline toxins in Bt [14]. This allowed some authors to attribute B. cereus sl strains producing insecticidal crystals to the species Bt [15]. Given the above, in this review we discuss B. cereus along with Bt.
The Ecological niches of Bt. Microorganisms of the genus Bacillus are classified as typical representatives of soils, and Bt bacteria, similarly to B. subtilis, are considered “ubiquitous.” Since the isolation of the first Bt strain, researchers have obtained many pathovars of this bacterial species from insect corpses and from the environment of their mass accumulation, from the surface of plant leaves, from water, animal feces, soil, as well as from flour mills and grain storage facilities [16].
The occurrence of Bt, as well as the closely related species B. cereus in various natural objects, the insufficient number of studies that clearly reveal the pathways of circulation of bacteria of this species in nature, similarly to the above disagreements on taxonomy, lead to disagreements in determining the ecological niche of this bacterium species. Some authors consider Bt a cosmopolitan soil bacterium with occasional insecticidal activity [17], while others attribute the niche of these bacteria to the phylloplane, considering them to be mutualists in relation to plants due to their insecticidal activity [18]. Some researchers tend to believe that Bt are commensals of the intestinal microbiota of insects, which cause no obvious diseases and accidentally get on/into plants [19]. There are two possible scenarios: in the case of a strict insecticidal activity, dead insects falling on the soil surface become an additional reserve of the so-called infection for plants; however, in the evolutionary aspect, this path gradually reduced the probability of successful distribution of Bt in the environment. It can be assumed that it is preferable for bacteria to be released into the environment with insect excrement. However, according to this scenario, the population of insects that destroy plants increases, which, in feedback, may lead to a reduction in food resources for phytophages. To maintain balance in food chains, it may be “feasible” to insert insecticidal plasmids into the bacterial genome. When, where, and how this occurs and whether it occurs in plant tissues is not known.
The “feasibility” of the interaction of Bt bacteria with plants to retain their properties and population in nature can be demonstrated by the increase in the insecticidal activity of Bt strains under the influence of plant components. For example, pectin and xylan modulated the formation of a biofilm of Bt strains and contributed to an increase in their insecticidal activity [20]. The formation of a biofilm with the involvement of plant exudates was observed during repeated, artificial, adaptive evolution of Bt in the Arabidopsis root system [21]. In this regard, the study of Bt representatives that endophytically and mutualistically interact with plants is relevant not only from the standpoint of the practical use of these bacteria as a potential basis for bioinsecticides but also for understanding the holistic picture of the relationship of the above-mentioned triad (plant–insect–Bt).
Endophyticity. Plant tissues can be a very effective natural habitat for bacterial populations, including Bt. Therefore, the probability of discovering new economically promising strains of microorganisms (both prokaryotes and eukaryotes), including Bt, among the diversity of plant endophytic microbiome is fairly high.
Until recently, little was known about the endophyticity of Bt, although the facts of the discovery of Bt populations on the leaves of plants, including trees [22], suggested this property. For example, on the leaves of clover Trifolium hybridum, Bt cells were detected after the plants grew from seeds on soil treated with a cell suspension of this bacterium [23]. More recent studies have shown that some of the “non-commercial” Bt strains not only are found in plant tissues but also are capable of producing crystalline insecticidal proteins and lipopeptides in this state [24], as well as phytohormones and substances that induce the immune potential of plants against pathogens [25]. These data raise natural questions: how does Bt colonize plants, how did bacteria of this species evolve to kill phytophagous insects and why, and how do Bt cells move within plants from the underground to the aboveground part.
There is an opinion that insects and plants can be considered as peculiar intermediate hosts that maintain the Bt life cycle [26]; the presence of a plasmid encoding the Bt toxin can periodically facilitate the passage of this cycle in nature. According to the study cited above, in the soil Bt cells are able to penetrate the plant through the rhizosphere or otherwise and spread over the tissues. When insects feed on plants inhabited by such bacteria, the bacteria enter the intestines of insects, and then, together with excrement, again enter the soil. It is assumed that in this way a selective cycle occurs that maintains the population of this bacterial species in the cenosis. The possibility of Bt circulation in nature is confirmed by the study by Monnerat et al. [27], who isolated bacteria of this species from the internal tissues of cotton plants grown in the field where no Bt-based biopesticides were ever used.
When discussing the prospects of using endophytic representatives of Bt to completely protect agricultural crops, the question arises about their prevalence in the tissues of cultivated and wild plants. Some authors who studied the endophytic microbiome reported the isolation of representatives of Bt or the closely related species B. cereus along with the bacterial species such as B. subtilis, B. megaterium, etc., which are often found in plant tissues [28]. Bacteria belonging to the B. cereus s.l. group, including Bt, were isolated from the surface of sterilized seeds of beans, peas, corn, pumpkins, radishes, rye, oats, barley, soybeans, sweet peppers, and alfalfa, as well as seeds and fruits of a number of trees and shrubs [28]. According to the cited authors, among the 43 species of bacteria isolated from plant tissues, the highest frequency of occurrence was shown for B. cereus, along with B. megaterium. Bt cells were isolated from the surface-sterilized vegetatively growing tissues of cabbage, peanut [29], cotton, maize [30], coffee [31], ginseng Panax notoginseng [32], banana [33], Arabidopsis [34], Physalis [35], and fragrant manjack [36]. Manyunata et al. [37] isolated Bt isolates, along with other types of bacteria, from millet Pennisetum glaucum L. stem tissues. Of the total biodiversity of species of the genus Bacillus isolated from the endosphere of sugarcane plants, the Bt species accounted for up to 8.9% [38]. Of 23 endophytic isolates of maize seed bacteria, Pal et al. [39] identified 19 isolates belonging to Bacillus sp., including one isolate classified as B. cereus ZMS6. Isolate B. cereus N5 with a high level of growth-stimulating activity with respect to plants was isolated from the endosphere of maize plants irrigated with industrial and municipal wastewater [40]. B. cereus and Bt were identified in isolates obtained from the flowers, berries, and seeds of the grapevine cultivar Zweigelt clone GU9 [41] and from the internal tissues of the leaves of the tea plant Camellia sinensis (L.) O. Kuntze [42]. Endophytic Bt strains 58-2-1, 37-1, and YC-1 were isolated from winter wheat plants in China [43]; Bt strain GS1 was isolated from tissues of bracken (Pteridium aquilinum) [44]. Together with the B. amyloliquefaciens strain P5, Bt strain C3 was isolated from cassava Manihot esculenta plants [24]. Bacteria belonging to the B. cereus s.l. group were isolated from field mustard and cauliflower seeds after their strict surface disinfection [45].
In terms of environmental friendliness, it is of interest that B. cereus isolates (NCBI CP034551) were found among the isolates obtained from tomato seeds and identified as species of the genus Bacillus, which accounted for up to 13% of the entire microbiome [46]. During the analysis of the microbiome of 26 plant species of Mexico, the bt strain LBIT-1250L was isolated from the lavender (Lavandula angustifolia) tissues, and the bt strain LBIT-1251P was isolated from milkweed (poinsettia) (Euphorbia pulcherrima). These strains belong to the israelensis and kurstaki serotypes, respectively, and are characterized by insecticidal activity against the larvae of the mosquito Aedes aegypti and the Carolina sphinx moth Manduca sexta [47]. The Bt subsp. kurstaki strain HD-1 isolated from cotton successfully and repeatedly populated this plant and persists for a long time in tissues and has toxicity with respect to the caterpillars of the cutworm Spodoptera frugiperda [27] and the cabbage moth Plutella xylostella on cabbage [48].
Antibiotic activity of Bt. In addition to insecticidal activity, many Bt strains are able to synthesize antibiotics and fungistatics, which makes it possible to create biopreparations with complex biological activity based on such bacteria. Kamenek et al. [49] studied the effect of the toxin from the Bt strain 202 from the collection of the All-Russian Research Institute of Genetics and Breeding of Microorganisms (Russia) on the resistance of the Nevsky potato variety to the late blight pathogen Phytophthora infestans (Mont.) de Bary. It was found that a sample containing purified δ-endotoxin of this Bt strain more effectively slowed the development and spread of late blight on plants of the Nevsky variety in the field as compared to the chemical fungicide Metamil. δ-Endotoxin also successfully suppressed the development of tuber disease, and its efficiency was similar to the protective effect of fludioxonil, the active ingredient of fungicide Maxim produced by Syngenta (Russia).
B. cereus strains that showed a high level of fungicidal activity against the fungus Fusarium verticillioides, which causes ear rot in maize stalks, were isolated from the maize rhizosphere [50]. Gorilyuk et al. [51] isolated 11 Bt isolates from tissues of the celandine Chelidonium majus L., including those capable of suppressing the growth of fungi in vitro. For example, Bt isolate no. 6 inhibited the growth of Alternaria alternata, Chaetomium sp., Paecilomyces variotii, Aureobasidium pullulans, and Exophiala mesophila. A high antagonistic activity against the fungi Fusarium oxysporum f. sp cubense and Colletotrichum guaranicola was characteristic of the Bt strain isolated from vegetating banana plants [33]. It was found that the endophytic isolate B. cereus REN 3 (from rice Oryza sativa plants) exhibited antibiotic activity against many rice pathogens (Fusarium fujikuroi, F. proliferum, F. verticillioides, Magnaporte grisea, and M. salvinii) [52]. The Bt subsp. darmstadiensis H10 is an interesting strain that is the basis of the Russian biopreparation Batsikol, which, along with a high level of insecticidal activity, exhibits antagonism with respect to a number of phytopathogenic fungi [53]. Endophytic specimens of Bacillus spp. isolated from tissues of melon Cucumis melo L. fruits, including those identified as B. cereus, showed a high degree of antagonism with respect to a number of phytopathogenic fungi [54]. The tomato rhizosphere-associated strain Bt B2, which produces bacillomycin, exhibited a high level of antagonistic activity with respect to the causative agent of rhizoctoniosis [55].
B. cereus strain YN917 was proposed as a means that effectively stimulates the growth of rice plants and protects them from the fungus M. oryzae [56]. Thirty isolates of endophytic bacteria were isolated from the surface-sterilized leaves of the Indian barberry Berberis lycium, which were identified on the basis of 16S rRNA as B. cereus and Bt and exhibited a high level of fungicidal activity against the fungi Aspergillus niger (60%) and Aspergillus flavus (56%) [57]. Bt strains isolated from wheat tissues protected plants from the causative agent of the flag smut of wheat Urocystis tritici [45]. The treatment of soil and rhizomes of turmeric Curcuma longa L. with a cell suspension of a strain of growth-stimulating bacteria B. cereus (strain RBacDOB-S24) reduced the degree of plant damage by the pathogens Pythium aphanidermatum (Edson) Fitzp. and Rhizoctonia solani Kuhn., which cause root and leaf rot [58]. The authors showed the ability of this strain to effectively penetrate into the tissues of the rhizomes of this culture. Kim et al. [59] isolated the Bt strain CMB26, which showed fungicidal activity against the causative agent of pepper anthracnose Colletotrichum gloeosporioides, from the soil. The lipopeptide isolated from the bacterium had a cyclic structure similar to that of fengycins and exhibited not only fungicidal but also insecticidal activity, as well as toxicity with respect to the cabbage butterfly (Pieris rapae crucivora) larvae.
The properties of the Bt bacteria described above and their ability to stimulate growth and increase crop yields make it possible to use biopreparations based on them in practice for comprehensive preventive plant protection not only from pests and fungal pathogens but also to control bacterial diseases. For example, Bt strains exhibited antibiotic activity against causative agents of cucumber and oat bacteriosis [60] and tomato bacterial rot [25]. Islam et al. [61] demonstrated the possibility of using Bt strains isolated from yew (Taxus brevifolia) leaves to protect mandarin Citrus unshiu plants from bacterial rot caused by Xanthomonas citri subsp. citri. These properties of bt bacteria are presumably associated with their ability to synthesize antibiotics of various families. The Bt strain KL1 isolated from the leaves of the medicinal plant Andrographis paniculata Nees from India exhibited a high level of antimicrobial activity due to the production of a fengycin-like lipopeptide [62]. Bt BAC3151 strains isolated from bean Phaseolus vulgaris plants, together with Microbacterium testaceum BAC1065, BAC1100, BAC2153, and Rhodococcus erythropolis BAC2162, exhibited antimicrobial activity and suppressed the quorum-sensing effects of Pseudomonas syringae and Hafnia alvei [63]. The lactonase-producing Bt KMCL07 isolate obtained from the endemic plant Madhuca insignis (India) was able to suppress the quorum-sensing effects of phytopathogenic bacteria P. syringae [64]. Root-associated Bt strains effectively protected tomato plants from the fungi Verticillium dahliae and V. longisporum [65]; the authors attributed this effect to their ability to synthesize not only the antibiotic bacilliobactin but also chitinases. Endophytic bt CHGP12 isolate synthesized the antibiotics fengycin, surfactin, iturin, bacillaene, bacillibactin, plantazolicin, and bacilisin and showed a high level of fungicidal activity against the fungus F. oxysporum f. sp. ciceris (FOC), which causes chickpea wilt [66]. The B. cereus T4S isolate that stimulates the growth and productivity of sunflower was isolated and identified from the endosphere of sunflower roots (South Africa). In this isolate, the genes encoding petrobactin, bacillibactin, bacitracin, zwitterycin, and fengycin, as well as those responsible for phosphate mobilization and nitrogen fixation, were identified [67].
At the same time, it is known that Bt bacteria produce antibiotics that suppress the reproduction of other nonphytopathogenic bacteria, including Bt strains [68]. For example, Favre and Euston [69] showed that bacteria of the serovar Bt subsp. thuringiensis (HD-2) produced the antibiotic turicine, which exhibited activity against 48 out of 56 Bt strains, as well as B. megaterium, B. polymyxa, and B. sphaericus species, but did not suppress the growth of B. licheniformis and B. macerans strains and Gram-negative bacteria. A. Sherif et al. [70] extracted a novel bacteriocin, entomocin 110, from the metabolites of Bt subsp. entomocidus HD110, which inhibited the growth of several Gram-positive bacteria, including Listeria monocytogenes, Paenibacillus, and Bacillus species. It was shown that antibiotics produced by Bt may differ in the selectivity of their effect. Pike et al. [71] purified bacteriocin tochicin from Bt subsp. tochigiensis HD868 culture, which showed a bactericidal effect against the majority of 20 typical Bt strains and B. cereus strain, but not against yeast. Turicine 17, a subclass IId bactriocin with a molecular weight of 3.162 kDa, which was synthesized by the Bt strain NEB17 isolated from soybean nodules, showed a high level of antibacterial activity against a wide range of different strains of rhizospheric pathogens, but not against rhizobial bacteria, as well as mutualist bacteria Serratia proteomaculans 1-102, 2-68, Pseudomonas putida, Bacillus licheniformis Alfa-Rhiz, B. subtilis NEB 5, and NEB4 [72]. The treatment of soybean plants with cells of this Bt strain or with turicine 17 promoted stimulation of their growth and more effective adaptation to changing environmental conditions. Given the ability of turicine 17 to stimulate the adaptive potential of plants and the presence of antimicrobial activity, Liu et al. [73] proposed this bacteriocin as a basis for creating an effective universal dual-purpose agent.
The fungicidal and bactericidal properties of Bt can be associated with the ability to synthesize not only antibiotics but also other compounds that inhibit the growth of phytopathogens. A number of authors have noted that extracellular chitinases and chitosanases of Bt enhance the target activity of insectotoxic proteins; however, so far there is no evidence that they actually function synergistically [74]. Chitinase activity of the Bt subsp. dendrolimus (HD-548) isolate inhibited the growth of fungi B. cinerea, Alternaria solani, and Aspergillus sp. The insecticidal properties of the bacteria were retained due to the production of entomotoxic proteins Cry1Ab and Cry1Ac [53]. Aktuganov et al. [75] showed that chitin and chitosan oligosaccharides formed with the involvement of extracellular chitinases and chitosanases of Bt subsp. dendrolimus B-387 possessed high antimicrobial and fungicidal activities. Muhammad et al. [76] showed that Bt bacteria, as chitinase producers, exhibited a high level of antagonistic activity against the fungi C. gloeosporioides and Curvularia affnis. Pleban et al. [45] observed growth inhibition of phytopathogenic fungi R. solani, Pythium ultimum, and Sclerotium rolfsii on cotton plants after the treatment of seedlings with B. cereus isolate cells isolated from field mustard seeds. It was assumed that this property could manifest itself in the bacteria due to the presence of chitinase activity.
Viable endophyte cells in cotton tissues remained for 72 days at a level of 2.8 × 105–5 × 104 CFU/g fresh weight. The presence of high chitinase activity explains the ability of the endophytic B. cereus strain XB177R to protect eggplant plants from bacterial wilt caused by Ralstonia solanacearum [77]. An isolate of the endophytic bacterium Bt GS1 from the leaves of the fern Pteridium aquilinum was characterized by the production of chitinase and induced resistance to the fungus R. solani KACC 40111 (RS) in cucumber plants, differentially activating other defense proteins in plants (e.g., the specific isoforms of guaiacol peroxidase, ascorbate peroxidase, and polyphenol oxidase [44].
Interaction of Bt with the phytobiome. In connection with the suppression of the growth of pathogenic and nonpathogenic bacteria, the question arises of whether Bt can interact with beneficial microorganisms, for example, with rhizobia, which provide symbiotic nitrogen fixation by legumes. The analysis of papers in this field of study of insecticidal bacilli indicates a possible “triple” mutualism of bt. It has been established that endophytic Bt can colonize the roots of legumes [78], stimulating their growth and increasing the number of nodules on them [79]. For example, Bai et al. [80] showed the possibility of increasing the indices of nodulation, growth, and yield of soybean when plants are co-inoculated with the Bt strain NEB17 and the Bradyrhizobium japonicum strain 532C, isolated from soybean root nodules. Bt strains were isolated from the nodules of several legumes (Glycine max, Vigna umbellata, Macrotyloma uniflorum, and Phaseolus vulgaris) with a frequency of up to 21.4 × 10–4 CFU in tissues [78–80]. The authors of [26] showed that Bt bacteria can colonize the internal tissues of clover plants with a population density of up to 1000 CFU/g leaf in sterile soil and up to 300 CFU/g leaf in nonsterile soil when co-sowing seeds treated with spores. Selvakumar et al. [81] in the study of the nodular microbiome of Pueraria thunbergiana, a forage and cover crop in the northwestern region of India, obtained the KR-1 isolate, which was attributed to the Bt species. Bt VRB1 and Bt VLG15 isolates with cry-1 and cry-2 genes, which caused complete death of the first-instar Spilososma obliqua larvae in experiments, were found and isolated in legume root nodules [79]. Four endophytic Bt strains isolated from nodules of leguminous plants growing in the Himalayas showed different degrees of tissue colonization depending on the species of legumes (Lens culinaris, Glycine max L., Vigna umbellate, Macrotyloma uniflorum) and their age. Among the microorganisms isolated from the seeds of chickpea Cicer arietinum L., the Bt strain Y2B was identified, which showed not only a high level of insecticidal activity but also growth-stimulating activity associated with the synthesis of siderophores, hydrogen cyanide (HCN), indole-3-acetic acid (IAA), and phosphate solubilizers [82].
The above information suggests that Bt-based biological preparations and genetically modified Bt cultures can change the structure of the microbial population in the endo-, rhizo-, and phyllosphere of plants, as well as in their environment, directly or indirectly through plants [83]. For example, the possibility of destroying the Nod factor of the soybean symbiont B. japonicum under the influence of chitinase produced by Bt subsp. pakistani HD 395 was shown, which may prevent the formation of root nodules [84]. On the basis of the fact that a number of chitinases produced by Bt strains exhibit fungicidal effects against the pathogenic fungi F. solani, F. oxysporum, F. proliferatum, Colletotrichum sp., Rhizoctonia cerealis, Rh. solani, V. dahlia, and Bipolaris papendorfii [85], it was assumed that arbuscular mycorrhizal fungi may be damaged by Bt strains [86]. At the same time, there are observations in the scientific literature that generally rule out significant and adverse effect of Bt bacteria [87], as well as genetically modified Bt cultures, on the agrocenosis microbiome [88]. No effect of the cry1Ah protein on the coefficient of colonization of 33-7 line maize plants by the endophytic bacterium B. subtilis B916 gfp was shown in [89]. Similarly, no significant change in the rhizosphere microbiome structure was observed during the cultivation of Bt-maize line 2A-7, which expresses the Cry1Ab and Cry2Ab proteins [90].
The inconsistency of these views is probably associated with the use of different subspecies (and even, perhaps, strains of the same Bt subspecies) for plant treatment. Taking these data into account, it can be concluded that to increase the effectiveness of plant protection with specialized harmful insects and, at the same time, to be able to use Bt-based preparations as polyfunctional ones, it is necessary to search for endophytic strains that would exhibit not only insecticidal but also fungicidal and bactericidal effects against typical pathogens of the target crop or would be able to directly or indirectly (through the plant defense system) regulate the functioning of pathogen virulence factors, for example, those belonging to the quorum sensing (QS) system.
One of the key molecules of the QS system is N-acetyl homoserine lactone, which is synthesized by plant pathogens such as Erwinia carotovora, Pantoea stewartii, Agrobacterium tumefaciens, Pectobacterium carotovorum, and Pseudomonas syringae [91]. The factors leading to plant disease are the abundant synthesis of extracellular hydrolytic enzymes, capsular exopolysaccharides that clog the xylem of the host plant, and other substances. It has been shown that the synthesis of virulence factors of phytopathogenic bacteria and, accordingly, the degree of plant damage depends on the presence and level of N-acetyl-homoserine lactone. Mutants deficient in the synthesis of this molecule were less virulent than the wild-type forms [91].
It was found that Bt representatives are able to disrupt communications in the microbial community due to the ability to destroy certain signaling molecules, including QS. It was reported that the Bt enzyme that degrades N-acetyl-homoserine lactone effectively suppressed the virulence of phytopathogenic bacteria due to the ability to disrupt communication in their population (quorum). To assess the ability of Bt to suppress the virulence of phytopathogenic bacteria [92], a mutant line with a defect in the synthesis of the enzyme that degrades N-acetyl-homoserine lactone was constructed. The mutant Bt bacteria were found to be less effective in suppressing the symptoms of soft rot caused by the bacterium E. carotovora. The authors concluded that the Bt enzymes that provide the destruction of N-acetyl-homoserine lactone are important in quorum suppression in Gram-negative bacteria without changing the density and structure of their population. In fact, the transfer of the N-acyl-homoserine lactonase (aiiA) gene, which inhibits the production of quorum-sensitive signals, from Bt into the genome of the endophytic Burkholderia sp. strain KJ006 promoted a decrease in the incidence of infection of rice plants in situ with the bacterium B. glumae [93].
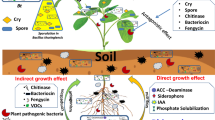
Stimulation of growth and regulation of the hormonal balance of plants. In addition to the Bt properties described above, researchers pay considerable attention to its ability to stimulate plant growth and produce phytohormones. The B. cereus (ECL1) and Bt (ECL2) lines that exhibited plant growth-stimulating activity were isolated from the rhizomes of Curcuma longa L. plants [94]. The auxin-producing endophytic strain Bt RZ2MS9 isolated from the Paullinia cupana guarana roots exhibited a high level of growth-stimulating activity against soybean and maize plants, as well as tomatoes [95]. Armada et al. [96] revealed growth stimulation of lavender Lavandula dentata plants treated with spores of Bt bacteria and arbuscular mycorrhizal fungi Archaespora trappei, Glomus versiforme, and Paraglomus ocultum. Auxin-producing endophytic Bt strains were found in tissues of the tropical plant Withania somnifera [97] and haricot [98]. The Bt strain A1B3, capable of producing auxin in large amounts (up to 27.7 µg/mL) in a liquid medium, was isolated from Adhatoda vasica plants growing in Southeast Asia [99]. The ability not only to produce auxins but also to fix nitrogen was characteristic of the endophytic Bt isolate obtained from rice Oryza sativa L. seedlings [100].
The fact that Bt bacteria have genes responsible for IAA synthesis is evidenced by the work of Figueired et al. [101], in which the knockout in Bt RZ2MS9 strain cells of the ipdC gene, which is responsible for the tryptophan pathway of IAA synthesis, resulted in a drop in the level of IAA synthesized by the Bt RZ2MS9 ΔipdC mutant, a decrease in its growth-stimulating activity in maize plants, and a decrease in the population density in plant tissues. The involvement of bacterial auxins in the plant growth-stimulating effect of bacteria was shown directly using a diageotropic (insensitive to effects of exogenous auxins) mutant (dgt) of tomato (Solanum lycopersicum) cultivar Micro-Tom and the auxin-producing Bt strain RZ2MS9 [95]. Auxin-producing Bt bacteria toxic to cutworms Spodoptera frugiperda and Plutella xylostella were isolated from cotton and cabbage plants, respectively [102]. The authors of the cited work assumed that the ability to successfully colonize plant tissues with Bt cells may be associated with the efficient functioning of genes encoding indolepyruvate decarboxylase, which regulates the IAA production by plants [102]. The obtained data suggest that the auxins synthesized by Bt are necessary not only for the life activities of the plant as an ecological niche for bacteria but also for the colonization of plant tissues by such bacteria.
Studies discussing the control of the levels of phytohormones (e.g., ethylene) in plants by endophytic Bt strains are also of interest [103]. For example, it was found that strains Bt SNKr10 (isolated from spinach phyllosphere) [104] and B. cereus AKAD A1-1 (isolated from soybean rhizosphere) [105] are capable of synthesizing 1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase) and, thus, affect ethylene synthesis in plant tissues. It was found that the Bt strain PM25 converted 1-aminocyclopropane-1-carboxylic acid (ACC) into ammonia and α-ketobutyrate in plant roots and thus restricted ethylene synthesis [106].
Bt bacteria are able not only to regulate the level of ethylene production but also to influence the production of other volatile compounds by plants and synthesize their own compounds. For example, changes in the profile of plant volatile organic compounds were found when maize plants were treated with Bt RZ2MS9 cells [107]. Volatile compounds produced by an endophytic Bt strain isolated from cherry tomato fruits collected in Tebulba (Sahel region, Tunisia), exhibited fungicidal activity against the causative agent of gray rot of fruits [108]. It was shown that dimethyl disulfide produced by the B. cereus strain C1L exhibited the properties of an elicitor and protected tobacco and maize plants from the fungi B. cinerea and Cochliobolus heterostrophus [109].
Formation of plant resistance to abiotic stressors and environmental remediation using Bt. An extensive field of application of endophytes is also associated with the need for an environmentally friendly enhancement of the adaptive potential of plants to rapidly changing environmental conditions, phytoremediation of soil contaminated with heavy metals, and degradation of organic toxic compounds in soil, plants, and air. Despite the fact that the exact mechanisms of the increase in plant adaptability to abiotic environmental factors by growth-stimulating bacteria, including the endophytic ones, remain largely speculative, the explanations for this effect are as follows: (1) the production of plant growth-regulating hormones, such as abscisic acid, gibberellic acid, cytokinins, and auxins; (2) the synthesis of ACC deaminase to reduce ethylene levels in plants; (3) the induction of plant systemic resistance by compounds synthesized by bacteria; and (4) the formation of a bacterial biofilm, i.e., an extracellular matrix. The adaptive potential is probably formed by plants under the influence of bacteria in real time, and bacterial strains isolated from plants subjected to certain stress (e.g., drought [110]) and overcoming their effect are more effective. It was reported that bacteria Bacillus sp., including the Bt isolated from pearl millet (Pennisetum glaucum L.), sunflower (Helianthus annuus L.), and maize (Zea mays L.), promoted an increase in the resistance of maize plants to drought [111]. The endophytic B. cereus strain AKAD A1-1 isolated from soybean roots (identified by 16S RNA), which synthesizes ACC deaminase, enhanced plant resistance to osmotic stress [105].
The studies [110] in which the Bt strain AZP2, an endophyte of the roots of the yellow pine Pinus ponderosa (Arizona, United States), which was grown under conditions of especially severe nutrient deficiency and drought, heat, and ultraviolet stress, stimulated resistance to moisture deficiency in wheat plants are of particular interest. The Bt GDB-1 isolate, which was isolated from Scots pine Pinus sylvestris roots, stimulated the growth of Japanese green alder Alnus firma under conditions of high levels of soil pollution with heavy metals (As, Cu, Pb, Ni, and Zn), facilitating the accumulation of ions in plants, which can be used for remediation of polluted areas [112]. The use of endophytic Bt bacteria on rice crops growing on soils contaminated with arsenic facilitated plant tolerance and decreased the level of accumulation of ions of this toxic metal in grains [113]. The development of tolerance of Brassica nigra plants to Cr3+ ions under the influence of the bacterium B. cereus on soils contaminated with chromium was noted [114]. The inoculation of Broussonetia papyrifera L. plants with a suspension of B. cereus HM5 and Bt HM7 cells increased the uptake of manganese ions by plants, contributing to the maintenance of the physiological functions of the roots and reducing the severity of oxidative stress [115]. Complex treatment of pepper C. annum plants with the Bt strain IAGS 199 and putrescine reduced cadmium-induced phytotoxicity [116]. A high antioxidant activity (e.g., against 1,1-diphenyl-2-picrylhydracyl radicals) was exhibited by the exopolysaccharide of the endophytic B. cereus strain SZ-1 isolated from Artemisia annua L. plants. The treatment of plants with this exopolysaccharide reduced the degree of DNA damage in PC12 cells by hydrogen peroxide [117]. It was found that the halotolerant Bt strain PM25 promoted growth of maize plants on saline soils, including that due to the high antioxidant activity of bacterial metabolites [106]. The endophytic bacterium B. cereus SA1, which promotes plant growth, increased thermotolerance in soybean.
The discovery of the ability of endophytic B. cereus strains to purify the air from ozone [118], formaldehyde [119], and ethylbenzene [120] in the plant–microorganism metabiome system is of interest. Under hydroponic conditions, Dracaena sanderiana plants inoculated with a composition of Bt and Pantoea dispersa cells removed bisphenol A, one of the most common compounds that damage the endocrine system [121]. The ability of the soil B. cereus strain CB4 from Sichuan Province (China) to degrade glyphosate, one of the common herbicides actively used in the field, is also of interest [122]. This opens prospects for the discovery of endophytic non-soil-contaminating forms of bacteria that would help relieve herbicide stress in cenoses. An isolate of a bacterium identified as B. cereus, which was isolated from the leaves of Garcinia xanthochymus, not only exhibited antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhi, and Klebsiella pneumoniae, but was also able to form silver nanoparticles [123]. Biocompatible silver nanoparticles formed with the use of Bt kurstaki showed a high level of complex insecticidal activity against Trichoplusia ni (Hübner) larvae completely and against Agrotis ipsilon [124]. The inoculation of plants with the strain led to overexpression of the stress-responsive GmLAX3 and GmAKT2 SA1 genes, reduced the level of ROS generation, and modulated the factors that form plant resistance to temperature stress [125]. Given the visually observed antistress effect of a number of endophytic Bt strains on plants, some authors even suggest their use for acclimatization of banana seedlings [126]. It was shown that the rhizospheric and endophytic strains of bacteria B. cereus CSR-B-1 and Bt CSRB-3 at a high sodium concentration can be effective growth regulators of gladiolus and function as bioameliorants, in particular, due to the induction of superoxide dismutase, phenylalanine lyase, catalase, and peroxidase by microbial cells in plants [127].
Insectotoxic proteins. In 1956, it was shown [128] that the insecticidal properties of Bt bacteria are manifested due to the presence of crystalline protein inclusions in them, the best known of which are insectotoxic parasporal δ-endotoxins of the family of cytolytic (Cyt) and crystalline (Cry) proteins, which cause the death of more than 3000 species of insects from 16 orders, as well as mites, protozoans, helminths, and nematodes. Currently, at least 700 sequences of genes encoding insecticidal Cry proteins, which are their carriers in the plasmid genome of various Bt strains, have been identified. The products of these genes usually accumulate in the compartments of bacterial cells with the formation of crystalline inclusions, which may account for 20–30% of the dry mass of sporulating cells [128]. Large-scale studies of the properties of Cry proteins made it possible to reveal the selective insectotoxicity of some of them against representatives of insects of the orders Lepidoptera, Diptera, Coleoptera, Rhabditida, Hemiptera, Hymenoptera, as well as against Gastropoda, which served as one of the criteria for their classification. For example, CryI proteins were toxic for Lepidoptera; CryII, for Lepidoptera and Diptera; CryIII, for Coleoptera; CryIV, exclusively for Diptera; and CryV, for Lepidoptera and Coleoptera [129].
Bt bacteria also produce toxins that are synthesized during the vegetative phase of cell growth and are called Vip (vegetative insecticidal proteins) and Sip (secreted insecticidal proteins). Originally, Vip proteins were classified into four families (Vip1, Vip2, Vip3, and Vip4). According to the new classification system, Vip1 and Vip4 were renamed Vpb1 and Vpb4, respectively, and the Vip2 protein was assigned to the Vpa protein group. Vip3 proteins with a multidomain structure were classified as Vip. Thus, the previously classified four types of Vip proteins were divided into three more classes as Vpa, Vpb, and Vip [130]. The Vip1, Vip2, and Sip proteins contain conserved signal sequences that are cleaved before secretion or after its completion. Vip1 and Vip2 toxins exhibit a high level of insecticidal activity against a number of species of beetles (Coleoptera) and aphids (Hemiptera), whereas Vip3 proteins are lethal to Lepidoptera. The insecticidal activity of Vip4 proteins has not been studied in detail. These proteins are known to be phylogenetically close to Vip1 proteins, in comparison with representatives of Vip2 and Vip3. Sip proteins are toxic to beetle larvae [131].
In addition to the described toxic compounds, Bt bacteria actively synthesize exotoxins that are detrimental to a number of eukaryotes: α-exotoxin (phospholipase C); non-protein thermostable β-exotoxin, or thuringeinsin, which is toxic to insect and mammalian cells, including humans; γ-exotoxin (toxic to sawfly insects); the louse death factor exotoxin (active only against lice); and the mouse death factor exotoxin (toxic to mice and lepidopterans) [132]. The toxicity of thermostable β-exotoxins limits the use of Bt strains [133]. However, there are modern technologies for Bt genome editing based on the CRISPRCas9 platform [134], which can be proposed to switch off the synthesis of such toxins.
In view of the above, the presence of strains among the endophytic Bt representatives that are toxic, for example, to nematodes, is of interest. B. cereus BCM2 and B. cereus SZ5 strains exhibiting a high level of nematicidal activity against Meloidogyne incognita on tomato plants were isolated from strawberries Fragaria ananassa and oriental persimmon Diospyros kaki L. [135]. The treatment of tomato plants with the endophytic Bt strain AK08 caused mortality of 95.46% of the nematodes Meloidogyne sp. According to the authors, this effect was due to the synthesis of cholest-5-en-3-ol(3.beta.)-carbonochloridate, which exhibits nematicidal activity [136]. Liang et al. [137] showed that the high nematicidal activity of Bt GBAC46 and Bt NMTD81 strains isolated from plants growing on the Qinghai–Tibetan Plateau (China) can be explained by both the properties of the bacteria themselves and by the induction under their influence of the defense system of rice plants against the nematode Aphelenchoides besseyi.
The use of the composition of strains B. amyloliquefaciens FR203A, B. megaterium FB133M, Bt FS213P, Bt FB833T, B. weihenstephanensis FB25M, B. frigoritolerans FB37BR, and Pseudomonas fluorescens FP805PU in the protection of Cabernet Sauvignon grape plants against the nematodes Xiphinema index and Meloidogyne ethiopica showed an efficiency comparable to that of a chemical nematicide [138]. It was shown that the nematicidity of Bt for the second-instar juveniles of the rhizospheric nematode Meloidogyne hapla, which worsens their subsequent reproductive properties and the efficiency of penetration into tomato roots, was associated with the synthesis of the Cry6A protein [139]. The nematicidity of the Bt strain BRC-XQ12 for the pine wood nematode Bursaphelenchus xylophilus is due to the synthesis of the Cry1Ea11 protein [140].
By the early 1980s, the genes encoding crystalline Bt-toxins had been found on transmissible plasmids. The determination of the nucleotide sequence of the genes encoding insecticidal Bt proteins made it possible to form another direction in plant protection against pests, that is, the use of the genetic material of bacteria of this species to create transgenic crops resistant to target insects.
Schnepf and Whiteley [141] were the first to clone the gene for the Cry crystalline toxin from Bt subsp. kurstaki and express it in Escherichia coli. Since 1996, plants that were genetically modified using Bt culture have been grown in fields, which led to a “genetic” revolution in crop production. By the beginning of the 21st century, Bt-maize, Bt-cotton, Bt-eggplants, and Bt-potatoes had been actively cultivated all over the world, which made it possible to significantly reduce the amount of used CPCs in a number of countries. Plants modified to express insecticidal proteins from Bt (called Bt-protected plants) are believed to provide a safe and highly effective insect control method that allows obtaining high yields of, for example, cotton or corn grains without using high doses of pesticides [142]. Currently, the United States is the world leader in the cultivation of Bt crops, and China and India are the countries that are most rapidly introducing them into crop production.
The insertion of a bacterial gene encoding insecticidal Bt proteins into the plant cell genome makes it possible to form the resistance of plants to pests throughout the growing season and avoid the competitive elimination of Bt bacteria by other types of microorganisms when viable spores and cells of these bacilli are used as insecticides and the destruction of the crystalline toxin under the influence of various environmental factors (in particular, solar ultraviolet radiation) when the crystalline toxin alone is used [143].
Despite the successful distribution of Bt-crops around the world, the issues of biosafety of crop production with their use, as well as the emergence of resistant forms of harmful insects, have long been discussed [144]. At the same time, for a clear separation of Bt-products from the products of traditional crop varieties, special biotechnological labeling is required in the majority of countries [145]. For example, to overcome the resistance of insects, the need to introduce GM plants containing more than two genes encoding insecticidal proteins into the production cycle is discussed. For example, the Bollgard cotton variety limits the viability of pests such as Pectinophora gossypiella and Helicoverpa zea due to the insertion of the Bt Cry1Ac gene into the cotton genome. Plants of the Bollgard II variety, which express two Bt endotoxins, became more resistant to a wider range of insects, expanding the range of protection against lepidopteran pests [146]. Bt-cotton with three protective genes (1Ac + Cry2Ab + Vip3A), (Cry1Ab + Cry2Ac + Vip3Aa19), or (Cry1Ac + Cry1F + Vip3A) was cultivated in Australia in the 2016–2017 season on more than 90% of the area, occupied by this culture [147].
Currently, to increase the effectiveness of biological plant protection against pests, intensive work is performed to create GM plants carrying not only the Cry or similar genes but also other nucleotide sequences. Recently, the US Environmental Protection Agency approved the SmartStaxPRO transgenic maize that expresses the Cry3Bb1 protein against the Western corn rootworm Diabrotica virgifera and dsRNA targeting the DvSnf7 vacuolar protein, whose use increased the mortality of the target pest up to 80–95% [144]. In another study, a vector containing information on the dsRNA targeting the juvenile hormone (acid methyltransferase) gene of the cotton bollworm Heliothis armigera was introduced into the genome of transgenic Bt-cotton, which helped to protect crops and delay the development of pest resistance to Bt, in contrast to the plants expressing only insectotoxic proteins [148].
Thus, the classic use of Bt genes for the plant genome modification is gradually replaced by their combination with other nucleotide sequences, or, as reported below, by modifications in the genome of the representatives of this bacterial species.
Recombinant endophytic bacteria. One of the promising directions in the creation of modern complex active biological products, as an alternative to the cultivation of GM plants, can be the construction of bacterial hybrid recombinant insectotoxins with a wide range of action and increased toxicity to the target object using the method of site-directed mutagenesis, for example, by replacing the Bt domain III in the Cry protein with a similar toxin with target specificity. Currently, recombinant genetically engineered constructs make it possible to transfer/supplement/modify target insectotoxin genes into another Bt strain or a strain of another bacterial species. The Cry toxins, which in the wild-type form exhibit a low specificity to, for example, the mall mottled willow moth Spodoptera exigua, including Cry1Ab, Cry1Ac, Cry1Ba, and Cry1Ea, became highly toxic as a result of such genetic-modification recombination [149]. Site-directed substitution of amino acid residues in the 450–612 a.a. region of the Cry1Aa toxin with similar ones from the Cry1Ac toxin resulted in an almost 300-fold increase in the toxicity of Cry1Aa to the tobacco moth Heliothis virescens [150]. A mutation (H168R) in the α-5 helix of domain I of the Cry1Aa toxin resulted in a three-fold increase in the toxicity of this protein to the Caroline sphinx moth Manduca sexta larvae [151] compared to the initial value. Two mutations, N372A or N372G, in domains II and III of the toxin led to an eight-fold increase in the insect toxicity of Cry1Ab to the gypsy moth Lymantria dispar, and the triple amino acid substitution N372A, A282G, and L283S increased this toxicity 36 times [152].
The insecticidal activity of Bt toxins can be enhanced by obtaining recombinants that produce these proteins. For example, an increase in the insecticidal activity of the entomopathogenic bacterium Photorhabdus temperata K122 against the mill moth Ephestia kuehniella and African cotton leafworm Spodoptera littoralis as a result of heterologous expression of the Btvip3LB gene [153] or to the olive moth Prays oleae as a result of expression of the Btcry1Aa and Btcry1Ia genes was shown [154]. Jan et al. [155] showed that the accumulation of the Cry1Ac–Av3 chimeric protein (Anemonia viridis neurotoxin) increased the insecticidal activity against H. armigera 2.6 times compared to the original Cry1Ac. In another study, the chimeric protein obtained from Cry1Ac and the peptide toxin HWTX-XI from the spider Ornithoctonus huwena venom enhanced the insecticidal activity against H. armigera and S. exigua compared to Cry1Ac [156].
Before the efficient transformation of Bt cells with additional proteins expanding the range of biopreparations based on them became available, genes encoding Cry proteins were introduced into the cells of bacteria E. coli, B. subtilis, B. megaterium, and Pseudomonas fluorescens [157]. The range of effectiveness of biological products can be expanded by the insertion into the genome of one of Bt strains that is already the basis of an effective biological product of the genes encoding proteins that increase resistance to other types of biotic and abiotic stresses. It is of interest to create endophytes (Bt or other species) that contribute to their greater activity against target pests by populating the internal tissues of plants and remaining there.
The genes encoding Bt-toxins were transfected into the bacteria E. coli, B. megaterium, B. subtilis, P. fluorescens, Clavibacter xyli, Herbaspirillum seropedicae, and R. leguminosarum [128, 141, 158]. Such bacterial strains, enhanced by the transformation of the genes encoding Bt Cry-toxins into their genomes, are actively used abroad as the basis of biological preparations Agree and Desine (Thermo Triligy, United States); Condor, Cutlass, CRYMAX, Leptinox, and Raven (Ecogen, United States). At the end of the 20th century, biological preparations M-CapTMb, MVP®b, M-One Plusb, MattchTM, and М-Press® (Mycogen Corporation, United States) were created using P. fluorescens recombinants [159]. A recombinant strain of P. fluorescens became the basis for the CellCapTM biopesticide (Mycogen Corporation). The latter contains encapsulated Cry toxins, which are better preserved in the environment than the original ones [160]. Using recombinant E. coli strains (BL21C+), Cry2Ac7 toxin crystals that exhibited insecticidal activity against the cotton moth Helicoverpa armigera were obtained [161]. Roch et al. [162] obtained a recombinant strain of Bacillus brevis carrying the Btcry 11a gene, which exhibited insecticidal and antimicrobial activities. The cry1Ac7 gene of the Bt strain 234 was used to transform the endophytic bacterium H. seropedica inhabiting sugar cane tissues, which resulted in effective protection against the larvae of the African sugar-cane borer Eldana saccharina.
Bacteria of the genus Bradirhizobium carrying the Bt toxin gene protected plant roots from Rivellia angulata [163]. The gene encoding the Cry protein of Bt subsp. tenebrionis (65 kDa), which is toxic to beetles, was inserted into R. leguminosarum. The cell extract of these bacteria was toxic to the larvae of the green dock beetle Gasterophysa viridula and the clover root weevil Sitona lepidus. Inoculation of pea and white clover roots with this bacterium reduced damage from soil insects [164].
Employees of Monsanto Company (United States) used tn5 transposons to transfer the Bt сry1Aa gene from Bt ssp. kurstaki HD-1 into the chromosomal genome of the bacterium P. fluorescens [165]. They showed that a thus-obtained strain on maize plants is similar to the donor strain in terms of insecticidal activity against the dark sword-grass Agrotis ipsilon. The insertion of the Btcry218 gene into the endophyte Burkholderia pyrrocinia JKSH007 genome and subsequent treatment of mulberry with this strain resulted in the death of almost 80% of Bombyx mori caterpillars [166]. Under the same conditions, the original bacterial strain had no effect on insects.
In [167], the Btсry1Ia gene from Bt B-5351 was introduced into the commercially active B. subtilis strain 26D using the pDG1662 integrative plasmid conjugated with the B. subtilis amylase gene. It was shown that the Btcry1Ia gene mRNA efficiently accumulated in the B. subtilis 26DCryChS cells. The insertion of the Btcry1Ia gene into the B. subtilis 26D chromosome promoted the manifestation of insecticidal (aphicidal) activity in the B. subtilis strain 26DCryChS, which was comparable to that in the donor strain Bt B-5351 cells. The insertion of the Btcry1Ia gene encoding the Cry1 protein into the endophytic B. subtilis strain 26D did not result in the loss of endophyticity in the recombinant strain [167, 168]. A similar result was obtained earlier when Bt-maize plants were treated with the endophytic bacterium B. subtilis B916-gfp [89]. These facts have been confirmed by the work of Bizzari and Bishop [26], who studied Bt strains deficient in the synthesis of the crystalline protein and showed that they had no significant role in the endophyticity of strains. It can be assumed that the production of insecticidal Cry proteins by both the bacteria themselves and Bt-cultures did not affect the endophyticity of the former and the ability of the latter to be colonized by microorganisms.
As a supplement to the genetically modified plants and recombinant bacteria, the use of preparations for plant protection against pests using RNA interference mechanisms, including those based on the Bt bacterium, is being actively developed [169]. It is believed that with the increase in insect resistance to the products based on the active principle of Bt, its use as a platform for the dsRNA expression can help in pest control with the Bt + RNAi strategy [170].
The Bt-based dsRNA production platform has some advantages over other platforms. The sporulation-dependent Cry gene promoter was used to express double-stranded (ds)RNAs, and this dsRNA could be produced during the sporulation phase of Bt. Moreover, no inductor is required when Bt is used, whereas other microbial species (such as E. coli, B. subtiis, and S. cerevisiae) require an inducer (IPTG or others) for dsRNA expression. Finally, since Bt cells can undergo autolysis by enzymes after sporulation, cell lysis is not required for dsRNA extraction [171]. For example, the use of Bt as an expressing host for dsRNA production was proposed in [171]. Another team of researchers [182] created a recombinant Bt strain containing a 325-bp expression-active DNA fragment from the conserved region of the arginine kinase gene of the cabbage moth P. xylostella, which exhibited a high level of insecticidal activity against the larvae of the target pest in comparison with the original strain. In all cases, dsRNA was expressed using convergent promoters flanking the target dsRNA: Park et al. [171] used the cyt1Aa promoter, and Jiang et al. [172] used the Pro3a promoter.
The target activity against the fourth- and fifth-instar larvae of Spodoptera littoralis significantly increased due to obtaining a Bt-based biopesticide composition (XenTari, Belgium) in the case of the combined use of dsRNA-Bacs targeted against the Sl 102 gene, which is responsible for insect cell aggregation and encapsulation to protect against infection. Similarly, the effectiveness of biopreparations based on live Bt bacteria was enhanced when used together with dsRNA-Bac targeting the Pxfused gene of P. xylostella [170], which forms insect resistance to the Cry1Ac toxin. This combination of the conventional methods of plant treatment with the Bt-based biopreparations and the RNA interference technology opens new vistas for the successful protection of agricultural crops from pests and pathogens.
Thus, the transfer of “useful” insect toxin genes from other economically important Bt species into endophytic strains, as well as the construction of their effective consortia, should contribute to the creation of a new generation of biological preparations for comprehensive plant protection from both pathogens and pests on their basis. The ability of endophytic bacteria to produce proteins with fungicidal and insecticidal activity, priming phytoimmune reactions, and long-term coexistence in plant tissues will contribute to avoiding the use of transgenic plants that produce the corresponding proteins.
***
Thus, scientific literature data indicate that the endophytic forms of entomopathogenic microorganisms exist in nature. This natural property can contribute to the search for strains with complex protective activities and opens prospects for the improvement of the existing endophytes by genetic engineering methods for their use in crop production (e.g., seed treatment), which can be an inexpensive and reliable way to increase plant resistance to pests and diseases. The multidirectional effects of Bt bacteria on plants increase the efficiency of using this method. As follows from the works cited above, some strains can significantly enhance the activity of the formation of nodules by nitrogen-fixing bacteria. Suppression of fungal growth due to the synthesis of antibiotics and chitinases by Bt contributes to the acquisition of additional properties of biofungicides and fungistatics by these bacteria. However, it is also necessary to assess the possibility of suppressing the growth of mycorrhizal fungi by these endophytic bacteria. Other practically valuable properties of endophytic Bt are the ability to synthesize phytohormones (plant growth stimulants), suppress the synthesis of ethylene, and enhance the growth of crops under adverse environmental conditions. Another valuable property is the synthesis of siderophores and the opportunity to mobilize poorly soluble plant nutrients in the soil. However, along with all these and other properties that are important for protecting and increasing productivity of plants, mutual integration with the host plant, based on endophyticity without loss of other economically useful qualities, is especially important.
The mechanisms and pathways of penetration of endophytes, in our opinion, can be different and independent of any damage of the surface plant tissues, as well as through stomata. Having once populated plant tissues, insecticidal Bt-endophytes can avoid competition with other epiphytic microorganisms, have access to food sources in the form of plant metabolites and substances entering plants through vessels, and be protected from solar radiation. After entering the intestines of an insect with plant food particles they can reduce the attractiveness of plant tissues as a food source and even lead to the death of the phytophage. Of course, such insecticidal endophytes are of interest only if they do not pose a danger to humans and animals.
The use of endophytic Bt in the form of a so-called slow-acting insecticidal “mine” that enters insects together with plant food is attractive not only in terms of protecting plants from phytophages but also from the standpoint of the possibility of acquiring additional useful properties by plants. For example, their use can make it possible to successfully control the group of pests with piercing and sucking mouthparts or parasites of the internal tissues of plants, against which CPCs, as well as biological preparations based on commercial nonendophytic Bt strains, are ineffective.
The endophytic Bt strains can be used to develop a completely different strategy for plant protection, where they can serve as natural insecticides of long-term preservation in plant tissues. This can help, on the one hand, to reduce the frequency of treatments with biopesticides and, on the other hand, to expand the range of not only pests but also pathogens. The possibility and efficiency of biocontrol by transformed endophytic strains with edited genes, as well as plants genetically modified by target genes of endophytic Bt, have been studied to a lesser extent compared with the natural but selected bacterial strains. The assessment of the possibility of using the endophytic microflora in general, regardless of the species of the microorganism, natural or “engineered,” is still at the very early stages. Side effects adverse for the environment and humans that may occur when using products based on modified or edited endophytes, including Bt, have not been studied. A versatile plant metabiome that protects plants from pathogens and pests can be convenient for growing plants in hydroponics, where contact between plants and soil microbiome is excluded. It should be noted that soil-less hydroponic farming is becoming more commercially popular in the world, eliminating the problems associated with soil pollution and other negative consequences of traditional farming. According to published data, the global market of hydroponic systems in 2020 was estimated at approximately 9.5 billion and, according to forecasts, by 2025 it will reach 16.6 billion dollars, increasing by 11.9% every 5 years [173].
Currently, research and plant protection technologies based on RNA interference are being actively developed. Despite the obvious advantages of RNA preparations and/or the insertion into the plant genome of genes encoding small RNA molecules that are lethal for insects, the combined use of artificial (dsRNA preparations in the form of sprays) and natural mechanisms for regulating the size of pest populations using natural “controllers” (among which endophytic Bt bacteria are promising) is attractive. In terms of the natural phenomenon of RNA interference, the use of Bt as a dsRNA biocontrol expression system is still in its infancy compared to other well-established technologies and requires further research of the possibility of its use as a practical plant protection against insect pests because active genetically engineered microorganisms for generating targeted dsRNAs and inexpensive purified dsRNAs will become available in the foreseeable future, which will lead to greener agriculture without the use of chemical pesticides to protect plants from insects and microbial infections.
REFERENCES
Ishiwata, S., Rept. Assoc. Seric., 1905, vol. 160, pp. 1–8.
Berliner, E., J. Appl. Entomol., 1915, vol. 2, no. 1, pp. 29–56.
Lord, J.C., J. Invertebr. Pathol., 2005, vol. 89, no. 1, pp. 19–29.
Dolzhenko, T.V., Biol. Rast. Sadovod.: Teor., Innov., 2021, no. 3 (160), pp. 50–62.
Peterson, J.A., Ode, P.J., Oliveira-Hofman, C., and Harwood, J.D., Front. Plant Sci., 2016, vol. 7, p. 1794. https://doi.org/10.3389/fpls.2016.01794
Sheina, N.I., Budanova, E.V., Myalina, L.I., Sazonova, L.P., and Kolesnikova, V.V., Toksikol. Vestn., 2018, no. 1 (148), pp. 35–37.
Priscepa, L., Stankeviciene, A., and Sneskiene, V., Miestu Zeldynu Formavimas, 2016, no. 1 (13), pp. 315–322.
Lecadet, M.-M., Frachon, E., Cosmao Dumanoir, V., Ripouteau, H., Hamon, S. Laurent, P., et al., J. Appl. Microbiol., 1999, vol. 86, pp. 660–672.
Atsumi, S., Mizuno, E., Hara, H., Nakanishi, K., Kitami, M., Miura, N., et al., Appl. Environ. Microbiol., 2005, vol. 71, no. 7, pp. 3966–3977.
Flores, A., Diaz-Zamora, J.T., Orozco-Mosqueda, M.D.C., Chavez, A., de Los, Santos., Villalobos, S., Valencia-Cantero, E., et al., 3 Biotech, vol. 10, no. 5, p. 220. https://doi.org/10.1007/s13205-020-02209-1
Liu, Y., Du, J., Lai, Q., Zeng, R., Ye, D., Xu, J., and Shao, Z., Int. J. Syst. Evol. Microbiol., 2017, vol. 67, no. 8, pp. 2499–2508.
Carroll, L.M., Cheng, R.A., Wiedmann, M., and Kovac, J., Crit. Rev. Food. Sci. Nutr., 2022, vol. 62, no. 28, pp. 7677–7702.
Muigg, V., Cuenod, A., Purushothaman, S., Siegemund, M., Wittwer, M., Pfluger, V., and Schmidt, K.M., New Microb. New Infect., 2022, vol. 26, pp. 49–50.
Wei, S., Chelliah, R., Park, B.-J., Kim, S.-H., Forghani, F., Cho, M.S., et al., Front. Microbiol., 2019, vol. 10.
Schoch, C.L., Ciufo, S., Domrachev, M., Hotton, C.L., Kannan, S., Khovanskaya, R., and Leipe, D., Database (Oxford), 2020, vol. 2020, p. baaa062. https://doi.org/10.1093/database/baaa062
Rahman, M.-M., Lim, S.-J., and Park, Y.-C., Animals, 2022, vol. 12.
Martin, P.A.W. and Travers, R.S., Appl. Environ. Microbiol., 1989, vol. 55, pp. 2437–2442.
Elliot, S.L., Sabelis, M.W., Janssen, A., van der Geest, L.P.S., Beerling, E.A.M., et al., Ecol. Lett., 2000, vol. 3, pp. 228–235.
Raymond, B., Elliot, S.L., and Ellis, R.J., J. Invertebr. Pathol., 2008, vol. 98, pp. 307–313.
Li, M., Shu, C., Ke, W., Li, X., Yu, Y., Guan, X., and Huang, T., Front. Microbiol., 2021, vol. 12, p. 676146
Lin, Y., Alstrup, M., Pang, J.K.Y., Maroti, G., Er-Rafik, M., Tourasse, N., et al., mSystems, 2021, vol. 6, no. 5, p. e0086421. https://doi.org/10.1128/mSystems.00864-21
Smith, R.A. and Barry, J.W., J. Invertebr. Pathol., 1998, vol. 71, no. 3, pp. 263–267.
Bizzarri, M.F. and Bishop, A.H., J. Invertebr. Pathol., 2007, vol. 94, no. 1, pp. 38–47.
Perez, K.J., Viana, J.D.S., Lopes, F.C., Pereira, J.Q., dos Santos, D.M., Oliveira, J.S., et al., Front. Microbiol., 2017, vol. 8, p. 61. https://doi.org/10.3389/fmicb.2017.00061
Takahashi, H., Nakaho, K., Ishihara, T., Ando, S., Wada, T., Kanayama, Y., et al., Plant Cell Rep., vol. 33, pp. 99–110.
Bizzarri, M.F. and Bishop, A.H., Microb. Ecol., 2008, vol. 56, no. 1, pp. 133–139.
Monnerat, R.G., Soares, C.M., Capdeville, G., Jones, G., Martins, É.S., Praca, L., et al., Microbiol. Biotechnol., 2009, vol. 2, no. 4, pp. 512–520.
Mundt, J.O. and Hinkle, N.F., Appl. Environ. Microbiol., 1976, vol. 32, no. 5, pp. 694–698.
Subrahmanyan, P., Reddy, M.N., and Rao, A.S., Seed Sci. Technol., 1983, vol. 11, pp. 267–272.
McInroy, J.A. and Kloepper, J.W., Plant Soil, 1995, vol. 173, pp. 337–342.
Miguel, P.S.B., Delvaux, J.C., De Oliveira, M.N.V., Monteiro, L.C.P., Costa, M.D., Totola, M.R., et al., Afr. J. Microbiol. Res., 2013, vol. 7, no. 7, pp. 586–594.
Ma, L., Cao, Y.H., Cheng, M.H., Huang, Y., Mo, M.H., Wang, Y., et al., Antonie van Leeuwenhoek, 2013, vol. 103, no. 2, pp. 299–312.
Souza, A., Cruz, J.C., Sousa, N.R., Procopio, A.R., and Silva, G.F., Genet. Mol. Res., 2014, vol. 13, no. 4, pp. 8661–8670.
Hong, Z., Chen, W., Rong, X., Cai, P., Tan, W., and Huang, Q., Chem. Geol., 2015, vol. 416, pp. 19–27.
Hernandez-Pacheco, C.E., Orozco-Mosqueda, M.D.C, Flores, A., Valencia-Cantero, E., and Santoyo, G., Curr. Res. Microbiol. Sci., 2021, vol. 2, p. 100028.
Sharma, M. and Mallubhotla, S., Front. Microbiol., 2022, vol. 13, p. 879386. https://doi.org/10.3389/fmicb.2022.879386
Manjunatha, B.S., Paul, S., Aggarwal, C., Bandeppa, S., Govindasamy, V., Dukare, A.S., et al., Microb. Ecol., 2019, vol. 77, pp. 676–688.
Rocha, F.Y.O., Negrisoli, JuniorA.S., de Matos, G.F., Gitahy, P.M., Rossi, C.N., Vidal, M.S., et al., Front Microbiol., 2021, vol. 12, p. 659965. https://doi.org/10.3389/fmicb.2021.659965
Pal, G., Kumar, K., Verma, A., and Verma, S.K., Microbiol. Res., 2022, vol. 255, p. 126926. https://doi.org/10.1016/j.micres.2022.127201
Abedinzadeh, M., Etesami, H., and Alikhani, H.A., Biotechnol. Rep. (Amst.), 2019, vol. 21, p. e00305. https://doi.org/10.1016/j.btre.2019.e00305
Compant, S., Mitter, B., Colli-Mull, J.G., Gangl, H., and Sessitsch, A., Microb. Ecol., 2011, vol. 62, pp. 188–197.
Wahlang, B., Sen, S., and Roy, J.D., Indian J. Appl. Pure Biol., 2022, vol. 37, no. 2, pp. 438–448.
Tao, A., Panga, F., Huang, S., Yu, G., Li, B., and Wang, T., Biocontrol Sci. Technol., 2014, vol. 24, pp. 901–924.
Seo, D.J., Nguyen, D.M., Song, Y.S., and Jung, W.J., J. Microbiol. Biotechnol., 2012, vol. 22, no. 3, pp. 407–415.
Pleban, S., Ingel, F., and Che, I., Eur. J. Plant Pathol., 1995, vol. 101, pp. 665–672.
Thomas, P. and Shaik, S.P., Microb. Ecol., 2020, vol. 79, no. 4, pp. 910–924.
García-Suárez, R., Verduzco-Rosas, L.A., and Ibarra, J.E., FEMS Microbiol. Ecol., 2021, vol. 97, no. 7, p. fiab080. https://doi.org/10.1093/femsec/fiab080
Praça, L.B., Menezes, Mendes., Gomes, A.C., Cabral, G., Martins, É.S., Sujii, E.R., and Monnerat, R.G., Bt Res., vol. 3, no. 3, pp. 11–19.
Kamenek, L.K., Satarova, T.A., Kamenek, D.V., and Terpilovskii, M.A., S-kh. Biol., 2011, no. 1., pp. 112–117.
Mirsam, H., Suriani, A.M., Azrai, M., Efendi, R., Muliadi A., Sembiring, H., et al., Heliyon, 2022, vol. 8, no. 12, p. e11960. https://doi.org/10.1016/j.heliyon.2022.e11960
Goryluk, L.A., Rekosz-Burlaga, H., B£aszczyk, M., Pol. J. Microbiol., 2009, vol. 58, no. 4, pp. 355–361.
Etesami, H. and Alikhani, H.A., Eur. J. Plant Pathol., 2017, vol. 147, pp. 7–14.
Grishechkina, S.D., S-kh. Biol., 2015, vol. 50, no. 5, pp. 685–693.
Glassner, H., Zchori-Fein, E., Compant, S., Sessitsch, A., Katzir, N., Portnoy, V., et al., FEMS Microbiol. Ecol., 2015, vol. 91, no. 7, p. fiv074. https://doi.org/10.1093/femsec/fiv074
Ben Abdeljalil, N., Renault, D., Gerbore, J., Vallance, J., Rey, P., and Daami-Remad, M., J. Microb. Biochem. Technol., 2016, vol. 8, pp. 110–119.
Zhou, H., Ren, Z.H., Zu, X., Yu, X.Y., Zhu, H.J., Li, X.J., et al., Front. Microbiol., 2021, vol. 12, p. 684888. https://doi.org/10.3389/fmicb.2021.684888
Nisa, S., Shoukat, M., Bibi, Y., Al Ayoubi, S., Shah, W., Masood, S., et al., Saudi J. Biol. Sci., 2022, vol. 29, no. 1, pp. 287–295.
Vinayarani, G. and Prakash, H.S., Plant Pathol. J., 2018, vol. 34, no. 3, pp. 218–235. https://doi.org/10.5423/PPJ.OA.11.2017.0225
Kim, P.I., Bai, H., Chae, H., Ching, S., Kim, Y., Park, R., et al., J. Appl. Microbiol., 2004, vol. 97, pp. 942–949.
Kamenyok, L.K., Levina, T.A., Teriokhin, D.A., and Minacheva, L.D., Biotechnol. Russ., 2005, no. 1, pp. 81–93.
Islam, M.N., Ali, M.S., Choi, S.J., Hyun, J.W., and Baek, K.H., Plant Pathol. J., 2019, vol. 35, no. 5, pp. 486–497.
Roy, S., Yasmin, S., Ghosh, S., Bhattacharya, S., and Banerjee, D., Microbiol. Insights, 2016, vol. 9, pp. 1–7.
Lopes, R.B.M., Costa, L.E.O., Vanetti, M.C.D., Araujo, E.F., and Queiroz, M.V., Curr. Microbiol., 2015, vol. 71, pp. 509–516.
Anandan, K. and Vittal, R.R., Microb. Pathog., 2019, vol. 132, pp. 230–242.
Hollensteiner, J., Wemheuer, F., Harting, R., Kolarzyk, A.M., Diaz Valerio, S.M., Poehlein, A., et al., Front. Microbiol., 2017, vol. 7, p. 2171. https://doi.org/10.3389/fmicb.2016.02171
Fatima, R., Mahmood, T., Moosa, A., Aslam, M.N., Shakeel, M.T., Maqsood, A., et al., Pest Manage. Sci., 2023, vol. 79, no. 1, pp. 336–348.
Adeleke, B.S., Ayangbenro, A.S., and Babalola, O.O., Plants (Basel), 2021, vol. 10, no. 9, p. 1776.
Mercado, V. and Olmos, J., Probiotics Antimicrob. Proteins, 2022, vol. 14, pp. 1151–1169.
Favret, M.E. and Youston, A.A., J. Invertebr. Pathol., 1989, vol. 53, pp. 206–216.
Cherif, A., Rezgui, W., Raddadi, N., Daffonchio, D., and Boudabous, A., Microbiol. Res., 2008, vol. 163, no. 6, pp. 684–692.
Paik, H.D., Bae, S.S., Park, S.H., and Pan, J.G., J. Industr. Microbiol. Biotechnol., 1997, vol. 19, pp. 294–298.
Nazari, M. and Smith, D.L., Front Plant Sci., 2020, vol. 11, p. 916. https://doi.org/10.3389/fpls.2020.00916
Lyu, D., Backer, R., Subramanian, S., and Smith, D.L., Front. Plant Sci., 2020, vol. 11.
Martínez-Zavala, S.A., Barboza-Pérez, U.E., Hernández-Guzmán, G., Bideshi, D.K., and Barboza-Corona, J.E., Front. Microbiol., 2020, vol. 10, p. 3032. https://doi.org/10.3389/fmicb.2019.03032
Aktuganov, G.E., Safina, V.R., Galimzianova, N.F., Gilvanova, E.A., Kuzmina, L.Yu., Melentiev, A.I., et al., J. Microbiol. Biotechnol., 2022, vol. 38, p. 167. https://doi.org/10.1007/s11274-022-03359-5
Muhammad, A., Nisa, R.M., and Aris, T.W., Res. J. Microbiol., 2014, vol. 9, pp. 265–277.
Achari, G.A. and Ramesh, R., Proc. Natl. Acad. Sci. India, Sect. B: Biol. Sci., 2018, vol. 89, pp. 585–593.
Tanuja, R., Bisht, S.C., and Mishra, P.K., Eur. J. Soil Biol., 2013, vol. 56, pp. 56–64.
Mishra, P.K., Bisht, S.C., Ruwari, P., Subbanna, A.R.N.S., Bisht, J.K., Bhatt, J.Ch., et al., Ann. Microbiol., 2017, vol. 67, pp. 143–155.
Bai, Y., Zhou, X., and Smith, D.L., Crop Sci., 2003, vol. 43, no. 5, p. 1774. https://doi.org/10.2135/cropsci2003.1774
Selvakumar, G., Kundu, S., Gupta, A.D., Shouche, Y.S., and Gupta, H.S., Curr. Microbiol., 2008, vol. 56, pp. 134–139.
Laranjeira, S.S., Alves, I.G., and Marques, G., Curr. Microbiol., 2022, vol. 79, no. 9, p. 277. https://doi.org/10.1007/s00284-022-02942-1
Li, Y., Wang, C., Ge, L., Hu, C., Wu, G., Sun, Y., Song, L., et al., Plants (Basel), 2022, vol. 11, no. 9, p. 1212. https://doi.org/10.1007/s00284-022-02942-1
Yung, W.J., Mabood, F., Souleimanov, A., Park, R.D., and Smith, D.L., Microbiol. Res., 2008, vol. 163, no. 3, pp. 345–349.
Djenane, Z., Nateche, F., Amziane, M., Gomis-Cebolla, J., El-Aichar, F., Khorf, H., et al., Toxins (Basel), 2017, vol. 9, no. 4, p. 139. https://doi.org/10.3390/toxins9040139
Belousova, M.E., Malovichko, Y.V., Shikov, A.E., Nizhnikov, A.A., and Antonets, K.S., Toxins (Basel), 2021, vol. 13, no. 5, p. 355. https://doi.org/10.3390/toxins13050355
Wang, X., Xue, Y., Han, M., Bu, Y., and Liu, C., Chemosphere, 2014, vol. 108, pp. 258–264.
Chen, Y., Pan, L., Ren, M., Li, J., Guan, X., and Tao, J., GM Crops Food, 2022, vol. 13, no. 1, pp. 1–14.
Sun, C., Geng, L., Wang, M., Shao, G., Liu, Y., Shu, C., et al., Microbiol. Open, 2017, vol. 6, no. 1, p. e00404. https://doi.org/10.1016/j.jip.2015.02.005
Yang, S., Liu, X., Xu, X., Sun, H., Li, F., Hao, C., et al., Plants (Basel), 2022, vol. 11, no. 17, p. 2218. https://doi.org/10.3390/plants11172218
Versluys, M., Ende, W., and Oner, E.T., in Quorum Sensing. Molecular Mechanism and Biotechnological Application, Tommonaro, G., Ed., Chap: Academic, 2019, pp. 127–149.
Park, S.-J., Park, S.-Y., Ryu, C.-M., Park, S.-H., and Lee, J.-K., J. Microbiol. Biotechnol., 2008, vol. 18, no. 9, pp. 1518–1521.
Cho, H.S., Park, S.Y., Ryu, C.M., Kim, J.F., Kim, J.G., and Park, S.H., FEMS Microbiol. Ecol., 2007, vol. 60, pp. 14–23.
Kumar, A., Singh, R., Yadav, A., Giri, D.D., Singh, P.K., and Pandey, K.D., 3 Biotech, vol. 6, no. 1, p. 60. https://doi.org/10.1007/s13205-016-0393-y
Batista, B.D., Dourado, M.N., Figueredo, E.F., Hortencio, R.O., Marques, J.P.R., Piotto, F.A., et al., Arch. Microbiol., 2021, vol. 203, no. 7, pp. 3869–3882.
Armada, E., Probanza, A., Roldan, A., and Azcon, R., J. Plant Physiol., 2016, vol. 192, pp. 1–12.
Ali, M.M. and Vora, D., Int. Res. J. Environ. Sci., 2014, vol. 3, no. 9, pp. 27–31.
Ismail, M.A., Amin, M.A., Eid, A.M., Hassan, S.E., Mahgoub, H.A.M., Lashin, I., et al., Cells, 2021, vol. 10, no. 5, p. 1059. https://doi.org/10.3390/cells10051059
Vyas, P. and Kaur, R., J. Soil Sci. Plant Nutr., 2019, vol. 19, pp. 290–298.
Ahumada, G.D., Gomez-Alvarez, E.M., Dell’Acqua, M., Bertani, I., Venturi, V., Perata, P., et al., Front. Plant Sci., 2022, vol. 13, p. 908349. https://doi.org/10.3389/fpls.2022.908349
Figueredo, E.F., Cruz, T.A.D., Almeida, J.R., Batista, B.D., Marcon, J., Andrade, P.A.M., et al., Microbiol. Res., 2023, vol. 266, p. 127218. https://doi.org/10.1016/j.micres.2022.127218
Vidal-Quist, J.C., Rogers, H.J., Mahenthiralingam, E., and Berry, C., FEMS Microbiol. Ecol., 2013, vol. 86, pp. 474–489.
Azizoglu, U., Curr. Microbiol., 2019, vol. 76, pp. 1379–1385.
Sharma, N. and Saharan, B.S., Microbiol. Res. J. Int., 2016, vol. 16, pp. 1–10.
Dubey, A., Saiyam, D., Kumar, A., and Hashem, A., Abd_Allah, E.F., and Khan, M.L., Int. J. Environ. Res. Public Health, 2021, vol. 18, p. 931. https://doi.org/10.3390/ijerph18030931
Ali, B., Hafeez, A., Ahmad, S., Javed, M.A., Sumaira, Afridi, M.S., et al., Front. Plant Sci., 2022, vol. 13, p. 921668. https://doi.org/10.3389/fpls.2022.921668
de Almeida, J.R., Bonatelli, M.L., Batista, B.D., Teixeira-Silva, N.S., Mondin, M., Dos, SantosR.C., et al., Environ. Microbiol. Rep., vol. 13, no. 6, pp. 812–821.
Chaouachi, M., Marzouk, T., Jallouli, S., Elkahoui, S., Gentzbittel, L., Ben, C., et al., Postharvest Biol. Technol., 2021, vol. 172, p. 111389. https://doi.org/10.1016/j.postharvbio.2020.111389
Huang, C.J., Tsay, J.F., Chang, S.Y., Yang, H.P., Wu, W.S., and Chen, C.Y., Pest Manage. Sci., 2012, vol. 68, no. 9, p. 1306-10. https://doi.org/10.1002/ps.3301
Timmusk, S., Abd El-Daim, I.A., Copolovici, L., Tanilas, T., Kannaste, A., Behers, L., et al., PLoS One, 2014, vol. 9, no. 5, p. e96086. https://doi.org/10.1371/journal.pone.0096086
Vardharajula, S., Ali, S.Z., Grover, M., Reddy, G., and Bandi, V., J. Plant Interact., 2011, vol. 6, no. 1, pp. 1–14.
Babu, A.G., Kim, J.-D., and Oh, B.-T., J. Hazard. Mater., 2013, vols. 250–251, pp. 477–483.
Dolphen, R. and Thiravetyan, P., Chemosphere, 2019, vol. 223, pp. 448–454.
Akhtar, N., Ilyas, N., Yasmin, H., Sayyed, R.Z., Hasnain, Z., Elsayed, E.A., et al., Molecules, 2021, vol. 26, p. 1569. https://doi.org/10.3390/molecules26061569
Huang, H., Zhao, Y., Fan, L., Jin, Q., Yang, G., and Xu, Z., Chemosphere, 2020, vol. 260, p. 127614. https://doi.org/10.1016/j.chemosphere.2020.127614
Shah, A.A., Bibi, F., Hussain, I., Yasin, N.A., Akram, W., Tahir, M.S., et al., Plants (Basel), 2020, vol. 9, no. 11, p. 1512. https://doi.org/10.3390/plants9111512
Zheng, L.P., Zou, T., Ma, Y.J., Wang, J.W., and Zhang, Y.Q., Molecules, 2021, vol. 21, no. 2, p. 174. https://doi.org/10.3390/molecules21020174
Autarmat, S., Treesubsuntorn, C., and Thiravetyan, P., Environ. Exp. Bot., 2022, vol. 194, p. 104761. https://doi.org/10.1016/j.envexpbot.2021.104761
Khaksar, G., Treesubsuntorn, C., and Thiravetyan, P., Environ. Exp. Bot., 2016, vol. 126, pp. 10–20.
Daudzai, Z., Treesubsuntorn, C., and Thiravetyan, P., Ecotoxicol. Environ. Saf., 2018, vol. 164, pp. 50–60.
Suyamud, B., Thiravetyan, P., Panyapinyopol., B., and Inthorn, D., Ecotoxicol. Environ. Saf., 2018, vol. 157, pp. 318–326.
Fan, J., Yang, G., Zhao, H., Shi, G., Geng, Y., Hou, T., et al., J. Gen. Appl. Microbiol., 2012, vol. 58, no. 4, pp. 263–271.
Sunkar, S. and Nachiyar, C.V., Asian Pac. J. Trop. Biomed., 2012, vol. 2, no. 12, pp. 953–959.
Sayed, A.M.M., Kim, S., and Behle, R.W., Biocontrol Sci. Technol., 2017, vol. 27, pp. 1308–1326.
Khan, M.A., Asaf, S., Khan, A.L., Jan, R., Kang, S.M., Kim, K.M., et al., BMC Microbiol., 2020, vol. 20, p. 175. https://doi.org/10.1186/s12866-020-01822-7
Araújo, R.C., Rodrigues, F.A., Nadal, M.C., Ribeiro, M.S., Antônio, C.A.C., Rodrigues, V.A., et al., Microbiol. Res., 2021, vol. 248, p. 126750. https://doi.org/10.1016/j.micres.2021.126750
Damodaran, T., Rai, R.B., Jha, S.K., Kannan, R., Pandey, B.K., Sah Vijayalaxmi, et al., J. Plant Interact., 2014, vol. 9, no. 1, pp. 577–584.
Schnepf, E., Crickmore, N., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., et al., Microbiol. Mol. Biol. Rev., 1998, vol. 62, pp. 775–806.
Chakrabarty, S., Chakraborty, P., Islam, T., Islam, A.K.M.A., Datta, J., Bhattacharjee, T., et al., in Bacilli and Agrobiotechnology, Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., and Aeron, A., Eds., Cham: Springer, 2022.
Crickmore, N., Berry, C., Panneerselvam, S., Mishra, R., Connor, T.R., and Bonning, B.C., J. Invertebr. Pathol., 2020, p. 107438. https://doi.org/10.1016/j.jip.2020.107438
Palma, L., Munoz, D., Berry, C., Murillo, J., and Caballero, P., Toxins (Basel), 2014, vol. 6, no. 12, pp. 3296–3325.
Chattopadhyay, P. and Banerjee, G., 3 Biotech, vol. 8, no. 4, p. 201. https://doi.org/10.1007/s13205-018-1223-1
Liu, X., Ruan, L., Peng, D., Li, L., Sun, M., and Yu, Z., Toxins, 2014, vol. 6, pp. 2229–2238.
Soonsanga, S., Luxananil, P., and Promdonkoy, B., Biotechnol. Lett., 2020, vol. 42, no. 4, pp. 625–632.
Hu, H.-J., Chen, Y.-L., Wang, Y.-F., Tang, Y.-Y., Chen, S.-L., and Yan, S.-Z., Plant Dis., 2017, vol. 101, no. 3, pp. 448–455.
Maulidia, V., Soesanto, L., Syamsuddin, KhairanK., Hamaguchi, T., Hasegawa, K., et al., Biodiversitas, 2020, vol. 21, pp. 5270–5275.
Liang, Z., Ali, Q., Wang, Y., Mu, G., Kan, X., Ren, Y., et al., Int. J. Mol. S ci., 2022, vol. 23, no. 15, p. 8189. https://doi.org/10.3390/ijms23158189
Aballay, E., Prodan, S., Correa, P., and Allende, J., Crop Protect., 2020, vol. 131, p. 105103. https://doi.org/10.3390/ijms23158189
Yu, Z., Xiong, J., Zhou, Q., Luo, H., Hu, S., Xia, L., et al., J. Invertebr. Pathol., 2015, vol. 125, pp. 73–80.
Huang, T., Lin, Q., Qian, X., Zheng, Y., Yao, J., Wu, H., et al., Phytopathology, 2018, vol. 108, pp. 44–51.
Schnepf, H.E. and Whiteley, H.R., Proc. Natl. Acad. Sci. U. S. A., 1981, vol. 78, no. 5, pp. 2893–2897.
Peng, Q., Yu, Q., and Song, F., Appl. Microbiol. Biotechnol., 2019, vol. 103, no. 4, pp. 1617–1626.
Zhou, X.-Y., Li, H., Liu, Y.-M., Hao, J.-Ch., Liu, H.-F., and Lu, X.-Z., Adsorption Sci. Technol., 2018, vol. 36, nos. 5–6, pp. 1233–1245.
Reinders, J.D., Reinders, E.E., Robinson, E.A., Moar, W.J., Price, P.A., Head, G.P., et al., PLoS One, 2022, vol. 17, no. 5, p. e0268902. https://doi.org/10.1371/journal.pone.0268902
Maghari, B.M. and Ardekani, A.M., J. Med. Biotechnol., 2011, vol. 3, no. 3, pp. 109–117.
Jost, P., Shurley, D., Culpepper, S., Roberts, P., Nichols, R., Reeves, J., et al., Agron. J., 2008, vol. 100, no. 1, pp. 42–51.
Tabashnik, B.E. and Carriere, Y., J. Econ. Entomol., 2020, vol. 113, no. 2, pp. 553–561.
Ni, M., Ma, W., Wang, X., Gao, M., Dai, Y., Wei, X., et al., Plant Biotechnol. J., 2017, vol. 15, pp. 1204–1213.
de Maagd, R.A., van der Klei, H., Bakker, P.L., Stiekema, W.J., and Bosch, D., Appl. Environ. Microbiol., 1996, vol. 62, pp. 1537–1543.
Mudoz-Garay, C. and Porta, H., Peptides, 2009, vol. 30, no. 3, pp. 589–595.
Wu, D. and Aronson, A.I., J. Biol. Chem., 1992, vol. 267, pp. 2311–2327.
Rajamohan, F., Alzate, O., Cotrill, J.A., Curtiss, A., and Dean, D.H., Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, pp. 14338–14343.
Jamoussi, K., Sellami, S., Abdelkefi-Mesrati, L., Givaudan, A., and Jaoua, S., Mol. Biotechnol., 2009, vol. 43, no. 2, pp. 97–103.
Tounsi, S., Aoun, A.E., Blight, M., Rebai, A., and Jaoua, S., J. Invertebr. Pathol., 2006, vol. 91, no. 2, pp. 131–135.
Yan, F., Cheng, X., Ding, X., Yao, T., Chen, H., Li, W., et al., Curr. Microbiol., 2014, vol. 68, pp. 604–609.
Sun, Y., Fu, Z., He, X., Yuan, C., Ding, X., and Xia, L., J. Invertebr. Pathol., 2016, vol. 135, pp. 60–62.
Gawron-Burke, C. and Baum, J.A., Genet. Eng. (NY), 1991, vol. 13.
Azizoglu, U., Jouzani, G.S., Yilmaz, N., Baz, E., and Ozkok, D., Sci. Total Environ., 2020, vol. 734, p. 139169. https://doi.org/10.1016/j.scitotenv.2020.139169
Wozniak, C.A., McClung, G., Gagliardi, J., Degal, M., and Matthews, K., in Regulation of Agricultural Biotechnology: The United States and Canada, Wozniak, C.A. and McHughen, A., US Government, 2012, chapter 4, p. 57.
Hernandez-Rodriguez, C.S., de Escudero, I.R., Asensio, A.C., Ferre, J., and Caballero, P., Biol. Control., 2013, vol. 66, pp. 159–165.
Saleem, F. and Shakoori, A.R., Toxins (Basel), 2017, vol. 9, no. 11, p. 358.
Roh, J.Y., Kim, Y.S., Wang, Y., Liu, Q., Tao, X., Xu, H.G., et al., J. Asia-Pac. Entomol., 2010, vol. 13, no. 1, pp. 61–64.
Nambiar, P.T.C., MaS, W., and Aiyer, V.N., Appl. Environ. Microbiol., 1990, vol. 56, pp. 2866–2869.
Skot, L., Harrison, S.P., Nath, A., Mytton, L.R., and Clifford, B.C., Plant Soil, 1990, vol. 127, pp. 285–295.
Obukowicz, M.G., Perlak, F.J., Kusano, K.K., Mayer, E.J., and Watrud, L.S., Gene, 1986, vol. 45, pp. 327–331.
Li, Y., Wu, Ch., Xing, Zh., Gao, B., and Zhang, L., Biotechnol. Biotechnol. Equip., 2017, vol. 31, no. 6, pp. 1167–1172.
Maksimov, I.V., Blagova, D.K., Veselova, S.V., Sorokan, A.V., Burkhanova, G.F., Cherepanova, E.A., et al., Biol. Control, 2020, vol. 144, p. 104242. https://doi.org/10.1016/j.biocontrol.2020.104242
Sorokan, A., Benkovskaya, G., Burkhanova, G., Blagova, D., and Maksimov, I., Plants, 2020, vol. 9, p. 1115. https://doi.org/10.3390/plants9091115
Price, D.R. and Gatehouse, J.A., Trends Biotechnol., 2008, vol. 26, no. 7, pp. 393–400.
Gong, L., Kang, Sh., Zhou, J., Sun, D., Guo, L., Qin, J. et al., Toxins (Basel), 2020, vol. 12, no. 2, p. 76. https://doi.org/10.3390/toxins12020076
Park, M.G., Kim, W.J., Choi, J.Y., Kim, J.H., Park, D.H., Kim, J.Y., et al., Pest Manage. Sci, 2020, vol. 76, pp. 1699–1704.
Jiang, Y.X., Chen, J.Z., Li, M.W., Zha, B.H., Huang, P.R., Chu, X.M., et al., Int. J. Mol. Sci., 2021, vol. 23, no. 1, p. 444. https://doi.org/10.3390/ijms23010444
Azizoglu, U., Yilmaz, N., Simsek, O., Ibal, J.C., Tagele, S.B., and Shin, J.-H., 3 Biotech, 2021, vol. 11, p. 382. https://doi.org/10.1007/s13205-021-02941-2
Funding
This work was performed under the state assignment no. AAAA-A16-116020350027-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Khairullin, R.M., Sorokan, A.V., Gabdrakhmanova, V.F. et al. The Perspective Properties and Directions of Bacillus thuringiensis Use for Plant Protection. Appl Biochem Microbiol 59, 408–424 (2023). https://doi.org/10.1134/S0003683823040075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823040075