Abstract
Endophytic bacteria play a key role in the biocontrol of phytopathogenic microorganisms. In this study, genotypic diversity was analyzed via repetitive element PCR (rep-PCR) of endophytic isolates of the phylum Actinobacteria that were previously collected from leaves of cultivars of common bean (Phaseolus vulgaris). Considerable variability was observed, which has not been reported previously for this phylum of endophytic bacteria of the common bean. Furthermore, the ethanol extracts from cultures of various isolates inhibited the growth of pathogenic bacteria in vitro, especially Gram-positive pathogens. Extracts from cultures of Microbacterium testaceum BAC1065 and BAC1093, which were both isolated from the ‘Talismã’ cultivar, strongly inhibited most of the pathogenic bacteria tested. Bean endophytic bacteria were also demonstrated to have the potential to inhibit the quorum sensing of Gram-negative bacteria. This mechanism may regulate the production of virulence factors in pathogens. The ability to inhibit quorum sensing has also not been reported previously for endophytic microorganisms of P. vulgaris. Furthermore, M. testaceum with capacity to inhibit quorum sensing appears to be widespread in common bean. The genomic profiles of M. testaceum were also analyzed via pulsed-field gel electrophoresis, and greater differentiation was observed using this method than rep-PCR; in general, no groups were formed based on the cultivar of origin. This study showed for the first time that endophytic bacteria from common bean plants exhibit high variability and may be useful for the development of strategies for the biological control of diseases in this important legume plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria that colonize the interior of the host plant and cause no apparent damage to the plant are known as endophytic bacteria [5]. A variety of interactions between endophytic bacteria and plants as well as a large richness of endophytic species has been described [12]. Although the endophytic bacterial community of seeds and roots has been reported in common bean (Phaseolus vulgaris) [6], little is known about the endophytic bacteria of the shoot tissues of P. vulgaris. Costa et al. [2] described the culturable endophytic bacteria from leaves of the ‘Vermelhinho,’ ‘Talismã,’ and ‘Ouro Negro’ cultivars of P. vulgaris and reported isolates of Proteobacteria (36.7 %), Firmicutes (32.9 %), Actinobacteria (29.7 %), and Bacteroidetes (0.6 %). Those results also revealed differences in the structure of the endophytic bacterial community across different bean cultivars.
Microbial diseases of common bean are a leading cause of reduced yields. Xanthomonas axonopodis pv. phaseoli, Curtobacterium flaccumfaciens pv. flaccumfaciens, and Pseudomonas syringae pv. tabaci are responsible for common bacterial blight (and its variant, fuscous blight), bacterial wilt, and wildfire disease, respectively, which are among the most important bacterial diseases of bean plants. Several phytopathogenic bacteria regulate the production of virulence factors through the population density-dependent gene expression control mechanism known as quorum sensing, which is mediated by self-produced molecules, typically N-acyl-homoserine lactone (AHL) for Gram-negative bacteria, such as Pseudomonas [4]. However, endophytic bacteria have the capacity to reduce the symptoms of diseases caused by phytopathogens through several mechanisms, including quorum sensing inhibition of phytopathogenic bacteria [10].
This study aimed to (1) analyze the genotypic diversity of leaf endophytic actinobacteria from three cultivars (i.e., ‘Talismã’, ‘Ouro Negro,’ and ‘Vermelhinho’) of P. vulgaris using repetitive element PCR (rep-PCR); (2) assess the antimicrobial activity of the obtained isolates against pathogenic bacteria; (3) evaluate the potential of the obtained isolates to inhibit the quorum sensing of Gram-negative bacteria, including P. syringae; and (4) analyze the genomic profile of endophytic Microbacterium testaceum using pulsed-field gel electrophoresis (PFGE).
Materials and Methods
Isolated and Bacterial Strains
Endophytic bacterial isolates, which were previously collected from surface sterilized leaves of the ‘Talismã,’ ‘Ouro Negro,’ and ‘Vermelhinho’ cultivars of P. vulgaris [2], and Brevibacillus sp., which was isolated from bean pods and used as positive control in the in vitro antimicrobial activity assays, are included in the bacterial collection of the Laboratory of Microbial Molecular Genetics of the Federal University of Viçosa (Universidade Federal de Viçosa (UFV)). Staphylococcus aureus ATCC 25923, Salmonella Typhimurium ATCC 14028, and Escherichia coli MG1655 were obtained from the American Type Culture Collection. X. axonopodis pv. phaseoli, C. flaccumfaciens pv. flaccumfaciens, and P. syringae pv. tabaci were provided by the Laboratory of Plant Bacteriology at UFV. Chromobacterium violaceum CV026 [8] and E. coli pSB403 [16] were used as reporter strains and are capable of detecting AHL via violacein production and bioluminescence, respectively. Hafnia alvei 071 was provided by Viana et al. [15]. Enterobacter cloacae 067T [7] was the reference strain in the quorum quenching assay.
rep-PCR Fingerprinting
Total DNA was extracted from endophytic actinobacteria using a previously described protocol [2]. Genomic fingerprints were generated using the primers BOXA1R (5′-CTACGGCAAGGCGACGCTGACG-3′) and ERIC1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′). PCR was performed using 50 ng of total DNA in a final volume of 25 µl, as described previously [14]. The reactions were incubated at 95 °C for 7 min for an initial denaturation, followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 50 °C (BOX-PCR) or 52 °C (ERIC-PCR) for 1 min, extension at 72 °C for 8 min, and a final extension at 72 °C for 8 min. The PCR products were separated in a 1.5 % agarose gel containing ethidium bromide (0.2 µl/ml). The band pattern was analyzed using Bionumerics software version 6.0 (Applied Maths, Inc., Austin, TX, USA) and the Jaccard similarity coefficient, and clustering was performed using the UPGMA clustering algorithm for a combined gel.
In Vitro Antimicrobial Activity
The isolates were cultured at 28 °C for 72 h in 10 % trypticase soy agar (TSA) and nutrient broth media. The supernatant was extracted with ethanol, and the organic solvent was evaporated under reduced pressure to yield the ethanol extract. The ethanol extract suspension was filtered through a 0.22-µm membrane and used for antimicrobial activity screening against pathogenic bacteria using the paper-disk agar-plate method. The inhibition zone diameter was measured after 24 h. A 25-µl volume of ethanol was used as a negative control. The test was performed with three independent replicates. The mean inhibition zone diameter values were subjected to analysis of variance (ANOVA) and the Scott-Knott test with a significance level of P < 0.05 using SAEG software version 9.1 (UFV).
Quorum Quenching Test
The AHL produced by H. alvei 071 and P. syringae pv. tabaci was extracted as described previously [11]. The growth cultures of endophytic isolates in 10 % TSA were diluted 1:5 in fresh medium, and 3-ml aliquots were added to 100 µl of AHL and incubated for 18 h. Then, the decrease of AHL in the supernatant was evaluated using the biosensors C. violaceum CV026 and E. coli pSB403 inoculated in plates. The diameter of the inhibition zones of the biosensors was determined after a 24-h incubation. The test was performed with three independent replicates.
PFGE
The overnight cultures were rinsed three times in a 0.85 % NaCl solution, and the cell suspension was standardized to an optical density of 1.2 (OD 620 nm) by mixing 500 µl of the cell suspension with an equal volume of 2 % low melting agarose (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cell suspension was then allowed to set in molds (Bio-Rad Laboratories). The cells were lysed in situ by incubating the plugs in a lysozyme solution (2 mg lysozyme/ml, 0.05 % N-lauryl sarcosine, and 50 mM EDTA, pH 8.0) for 4–5 h at 37 °C. The plugs were then rinsed three times in 50 mM EDTA, pH 8.0, and incubated overnight at 50 °C in NDS buffer (10 mM Tris–HCl, 1 % sodium dodecyl sulfate (SDS), and 50 mM EDTA, pH 8.0) with 2 mg proteinase K/ml. Following the incubation, the plugs were rinsed again from five to seven times in 50 mM EDTA, pH 8.0, for at least 30 min at room temperature. The plugs were equilibrated for 30 min in Tris–EDTA (TE) buffer prior to electrophoresis, and DNA digestion was performed overnight using 30 U of XbaI (Promega). PFGE was performed using the CHEF-DR III system (Bio-Rad Laboratories) and 1 % agarose gel electrophoresis in ×0.5 Tris–Borate-EDTA (TBE) buffer at 12 °C. A voltage of 6.0 V/cm was applied at a 120° angle with 1- to 15-s pulse times for 24 h. Following the PFGE, the gel was stained in an ethidium bromide solution for 30 min and photographed using a molecular imaging system (Loccus Biotecnologic L-Pix Chemi, São Paulo, SP, Brazil). The obtained restriction profiles were compared using the Bionumerics 6.0 software, and an analysis of Dice similarity coefficients was performed based on the UPGMA algorithm to generate a dendrogram. Profiles with Dice similarity coefficients of at least 80 % were clustered together.
Results and Discussion
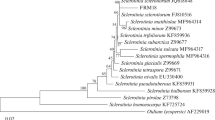
The BOX- and ERIC-PCR patterns were used to distinguish the different isolates of Actinobacteria (Fig. 1), a phylum of Gram-positive bacteria which includes the most economically important prokaryotes, due to production of many bioactive compounds described. The fingerprints obtained using BOX-PCR showed DNA bands ranging from 100 to 4000 bp, while the products amplified by ERIC-PCR had an estimated size of approximately 100–3000 bp. The clustering analysis generated 18 groups (A to R) with at least 65 % similarity. The main isolates were M. testaceum and they formed groups B to M, with groups B, C, D, F, G, H, K, and M consisting of only one or two isolates that were collected exclusively from the ‘Talismã’ or ‘Ouro Negro’ cultivars. Groups E, I, and J included M. testaceum isolates from both cultivars, while group L was formed by isolates from the ‘Talismã,’ ‘Ouro Negro,’ and ‘Vermelhinho’ cultivars. The other groups consisted of other actinobacteria species that were represented by fewer isolates. Our results revealed significant genotypic diversity among the analyzed isolates. Furthermore, most isolates were not clustered according to the cultivar of origin. These findings revealed a genotypic diversity higher than that found by Yuan et al. [17] for other species of endophytic actinobacteria from different host plants using BOX-PCR and other methods.
Dendrogram generated from the band profiles of the BOX and ERIC-PCR fingerprints of endophytic actinobacteria. The dendrogram was constructed using the Bionumerics 6.0 software. The UPGMA algorithm and the Jaccard similarity coefficient were applied to the resulting matrix, and the clusters were set at a similarity level ≥65 %
A variation in the microbial antagonism was observed when the isolates were grown in 10 % TSA and nutrient broth media (Table 1). The antimicrobial activity of most ethanol extract against X. axonopodis pv. phaseoli exhibited no difference when the cells were grown on 10 % TSA or nutrient broth. However, the extracts from 11 cultures that were grown in 10 % TSA showed greater antagonism than those from other cultures in the same medium against C. flaccumfaciens pv. flaccumfaciens. Furthermore, 22, 18, and 17 extracts from cultures that were grown in 10 % TSA showed greater S. aureus ATCC 25923, E. coli MG1655, and S. Typhimurium ATCC 14028 growth inhibition than the extracts from other cultures in the same medium, respectively. However, most extracts from cultures that were grown in nutrient broth exhibited no difference in antagonism against S. aureus ATCC 25923, and only 9 and 12 extracts exhibited greater antimicrobial activity than the extracts from remaining cultures in this medium against E. coli MG1655 and S. Typhimurium ATCC 14028, respectively. Those results demonstrated that the isolates were more efficient against Gram-positive pathogens, particularly when grown in 10 % TSA. As the 10 % TSA medium has lower concentration nutrients than the nutrient broth medium, this suggested that the reduced availability of nutrients may stimulate the production of compounds which inhibit the growth of other microorganisms. M. testaceum BAC1065 and M. testaceum BAC1093, which were both isolated from the ‘Talismã’ cultivar, were considered promising antagonists because their culture extract strongly inhibited most of the pathogenic bacteria tested. The antimicrobial action of endophytic bacteria can occur by mechanisms such as synthesis of antibiotics [1].
Furthermore, the isolates exhibited difference in quorum quenching potential against Gram-negative bacteria, as revealed by difference in the ability to inhibit the response of AHL reporter strains to P. syringae pv. tabaci or Hafnia alvei 071 (Table 1). The isolates with greater quorum quenching activity were M. testaceum BAC1065, BAC1100, and BAC2153, Bacillus thuringiensis BAC3151, and Rhodococcus erythropolis BAC2162. The ability of M. testaceum [9] and R. erythropolis [13] to cleave AHL has been reported. Further, Dong et al. [3] demonstrated that lactonase-producing B. thuringiensis strains suppress the quorum sensing-dependent virulence of the bacterial pathogen Pectobacterium carotovorum. Those results revealed the potential of antagonists that inhibit bacterial cellular communication mechanisms for disease control. Moreover, because most M. testaceum isolates exhibited moderate or strong quorum quenching of the tested bacteria, it is possible that M. testaceum with quorum quenching potential may also be widespread in common bean.
Because most isolates used in this study were M. testaceum and because some of those isolates had greater antimicrobial activity and exhibited greater quorum quenching potential, we analyzed the genomic profiles of isolates of that species using PFGE, which is a more accurate molecular typing method. The profiles generated using this approach revealed 13–21 bands per isolate, ranging from 48.5 to 436.5 kb. The resulting dendrogram showed the presence of 11 clusters (I to XI) (Fig. 2). Our results demonstrated that the M. testaceum isolates exhibit greater differentiation via PFGE than via rep-PCR and fail to cluster according to the cultivar of origin, confirming the high variability of this species in the endophytic P. vulgaris community. To our knowledge, the genomic profiles of M. testaceum have never been evaluated by PFGE and no reports of the macro-restriction profiles of these bacteria are available.
This paper is the first report of the variability of endophytic actinobacteria from common bean plants and the potential of these endophytes for the biocontrol of phytopathogenic bacteria of common bean and other types of pathogens. The results of this study will contribute to the development of new strategies for the biological control of diseases of P. vulgaris and to the reduction of the use of agrochemicals, which are harmful to the environment.
References
Christina A, Christapher V, Bhore SJ (2013) Endophytic bacteria as a source of novel antibiotics: an overview. Pharmacol Rev 7:11–16
Costa LEO, Queiroz MV, Borges AC, Moraes CA, Araújo EF (2012) Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz J Microbiol 43:1562–1575
Dong YH, Xu JL, Li XZ, Zhang LH (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. P Natl Acad Sci USA 497:4154–4158
Elasri M, Delorme S, Lemanceau P, Stewart G, Laue B, Glickmann E, Oger PM, Dessaux Y (2001) Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl Environ Microbiol 67:1198–1209
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
López-López A, Rogel MA, Ormeño-Orrillo E, Martínez-Romero J, Martínez-Romero E (2010) Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. Syst Appl Microbiol 33:322–327
Martins ML (2007) Caracterização de protease e lipase de Pseudomonas fluorescens e quorum sensing em bactérias psicrotróficas isoladas de leite. Thesis, Federal University of Viçosa
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711
Morohoshi T, Someya N, Ikeda T (2009) Novel N-acylhomoserine lactone-degrading bacteria isolated from the leaf surface of Solanum tuberosum and their quorum-quenching properties. Biosci Biotechnol Biochem 73:2124–2127
Newman KL, Chatterjee S, Ho KA, Lindow SE (2008) Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Mol Plant Microbe Interact 21:326–334
Ravn L, Christensen AB, Molin S, Givskov M, Gram L (2001) Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J Microbiol Methods 44:239–251
Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19:827–837
Uroz S, Oger P, Chapelle E, Adeline MT, Faure D, Dessaux Y (2008) A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl Environ Microbiol 74:1357–1366
Versalovic J, Schneider M, Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Method Mol Cell Biol 5:25–40
Viana ES, Campos MEM, Ponce AR, Mantovani HC, Vanetti MCD (2009) Biofilm formation and acyl homoserine lactone production in Hafnia alvei isolated from raw milk. Biol Res 42:427–436
Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GSAB (1998) Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163:185–192
Yuan HM, Zhang XP, Zhao K, Zhong K, Gu YF, Lindstrom K (2008) Genetic characterisation of endophytic actinobacteria isolated from the medicinal plants in Sichuan. Ann Microbiol 58:597–604
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopes, R.B.M., de Oliveira Costa, L.E., Vanetti, M.C.D. et al. Endophytic Bacteria Isolated from Common Bean (Phaseolus vulgaris) Exhibiting High Variability Showed Antimicrobial Activity and Quorum Sensing Inhibition. Curr Microbiol 71, 509–516 (2015). https://doi.org/10.1007/s00284-015-0879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0879-6