Abstract
This work examines the antibacterial and antifungal activity of chitosans with a deacetylation degree of 85% and molecular weights (MWs) in a wide range of values (5, 10, 50, 150 kDa), as well as copper complexes obtained on their basis. It was found that the studied chitosan activity has a certain dependence on the chitosan MW and concentration. It has been shown that the biocidal activity of copper complexes at a concentration of 0.02–01%, does not depend on the chitosan MW, but the complexes are characterized by a higher antibacterial and antifungal activity as compared to chitosan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The polysaccharide chitosan, which is widely distributed in nature, still attracts the attention of researchers due to its unique physical and chemical properties and biological activity, as well as its complete safety for the environment. [1–4]. Special methods are used to obtain chitosan from various natural sources; thus, it is characterized by structural heterogeneity in many parameters [2]. These include the molecular weight (MW), the quantitative ratio of acetylated and deacetylated units in the chain, and the nature of their location in the polymer chain. It is these features of chitosan that determine the diversity of its biological properties, which has been confirmed by numerous studies in this field [5, 6].
Most of the works related to the study of the mechanisms of the antibacterial and antifungal action of this biopolymer indicate that the biocidal activity of chitosan is associated with its polycationic structure and the ability to bind to negatively charged cell surface structures [5, 7]. The mechanism of the manifestation of such activity is still not clear, but there is clear evidence that the interaction of chitosan and its derivatives with cell membranes at the molecular level leads to cell death [8–10]. This is the so-called “antibacterial effect” of chitosan [8]. The first target of the chitosan polymer in the case of gram-negative bacteria is lipopolysaccharide, which is negatively charged and is part of the outer membrane. In gram-positive bacteria, the main target for chitosan may be teichoic acids, which are negatively charged by numerous phosphoric acid residues. In both cases, such interaction disrupts the normal functioning of the metabolic processes of the cell with the external environment and changing the permeability of the cytoplasmic membrane, resulting in an increase in the outflow of substances from the cell.
It should be noted that, while some authors have found differences in the sensitivity of gram-negative and gram-positive bacteria to chitosan, a number of studies have concluded that the structural differences in the bacterial cell wall are not decisive in their interaction with the polysaccharide [11].
Ilyina et al. [12] stated that the antibacterial activity of chitosan against gram-negative and gram-positive microorganisms may depend not only on its MW weight but also on the degree of deacetylation (DD). When testing chitosans with an MW of 4 kDa but with varying DD values (55, 73, 78, and 86%), there was a tendency toward an increase in the level of cell death with an increase in the DD of the polymer. According to the authors, a high concentration of positive charges in the chitosan chain with the maximum DD led to the formation of the strongest bond with the surface of the cell wall of the microorganisms.
The authors came to the same conclusion in works [13] on the antibacterial activity of a number of chitosan samples, the MW of which was changed from 2 to 224 kDa and the DD from 16 to 84%. The activity of the samples was assessed according to growth inhibition and membrane integrity based on the example of Bacillus cereus, Escherichia coli, and Salmonella typhimurium. The authors concluded that chitosans with a high DD showed an increase in antibacterial activity [13].
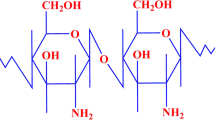
Structure of chitosan–metal complexes [28].
An unambiguous correlation between the MW of chitosan and its biological properties has not yet been established in the literature [7, 13]. It was shown [14] that an increase in the chitosan MW upon exposure to the growth of Bacillus cereus led to an increase in the antimicrobial effect in the series of oligosaccharides up to samples with an MW of 628 kDa. This was explained by the increase in the number of amino groups capable of firmly binding to the surface structures of the cell. Other researchers [15] come to the opposite conclusion about the higher antibacterial activity of low-MW chitosans. The authors attribute the biocidal effect of these polymers to the fact that such samples have a greater ability to penetrate the bacterial cell wall. This disrupts their functioning and affects the physiological processes occurring inside the cells, which leads to cell death. The authors of [16] came to the same conclusion when studying bacteria such as Staphylococcus aureus, Bacillus cereus, Klebsiella pneumoniae, and Escherichia coli. It was established that the antibacterial effect increased proportionally with a decrease in the chitosan MW. Molecules of low-MW chitosan can bind to DNA, penetrating the cell nucleus and suppressing mRNA synthesis [6]. An analysis of the influence of various factors on the antibacterial activity of chitosan was presented in recent reviews [7, 17].
To date, it has been established that chitosan has a direct fungistatic effect, which depends on its physicochemical properties and the type of microorganism [18]. Fungi and oomycetes containing a small amount of chitosan in their cell walls are sensitive to chitosan, while zygomycetes containing a large amount of chitosan in their cell walls are resistant to its action. Entomopathogenic fungi with high chitinolytic activity are resistant to the action of chitosan.
A number of works [17, 19–21] also obtained contradictory data on the correlation between the antifungal activity and the chitosan MW. The results of the study of the process of chitosan inhibition of ten plant pathogenic fungi (Colletotrichum gloeosporioides, Fusarium oxysporum f. sp. cubense, Colletotrichum capsici, Pythium aphanidermatum, Phytophthora parasitica, Curvularia lunata, Rhizoctonia solani, Helminthosporium oryzae, Sphaceloma ampelinum, and Fusarium graminearum) showed that low-MW polymers had a higher degree of mycelium inhibition than high-MW chitosans [17, 21]. Conversely, it was found [19] that an increase in the MW led to an increase in antifungal activity against candida albicans, Candida krusei, and candida glabrata.
It must be added that the type of microorganism also determines the susceptibility to chitosan with different MWs, as shown in [20]. The mushrooms Puccinia asparagi and Fusarium oxysporum were more sensitive to low-MW chitosan, while high-MW chitosan was more effective against Stemphylium solani.
Another important factor in the manifestation of antifungal activity is the degree of chitosan deacetylation: with an increase in the DD, the antifungal activity also increases [22]. The variation of two characteristics of chitosan (DD and MW) makes it possible to find a combination that leads to the maximum result in this parameter. The conclusion that the antifungal activity is greater at a high DD and a low MW has been confirmed in various types of pathogens: candida albicans [22], Aspergillus fumigatus [23], and Aspergillus flavus [24].
The mechanism of antifungal action of chitosan, by analogy with bacteria, is associated with violation of the structure of the cell wall. This leads to a change in the mycelium morphology, spore viability, and violation of the integrity of the fungal cytoplasmic membrane, which leads to the release of cytoplasmic contents from the cells. The electrostatic interaction of positively charged free amino groups of chitosan with negatively charged phospholipids of fungal cell membranes is confirmed by its dependence on pH, which is different for low- and high-acetylated chitosan with the same molecular mass and concentration in the medium [18–24]. Thus, chitosans with a higher DD (86–90%) showed a stronger fungistatic effect in all cases. This parameter plays a decisive role in the adhesion of chitosan to mycelial cells and fungal spores. Thus, it can be considered established that any biological activity of chitosan is primarily determined by the presence of a positive charge on its macromolecules.
In the last two decades, there have been many studies on the potential to increase the chitosan activity by increasing the positive-charge density via the addition of biologically active substances to its composition, which leads to an intentional enhancement of its biocidal activity. From this point of view, it is of great interest that chitosan can form complexes with various metals that themselves, on the one hand, have biocidal activity and, on the other hand, can be used by plants as catalysts for biochemical processes. The most common strategy to increase the antimicrobial properties of chitosan is its use in combination with metals and their nanoparticles [25–27].
We previously [28] obtained complexes of chitosan (ММ = 3–150 kDa) with metals (Cu, Zn, Fe) showing high antifungal activity against F. oxysporum. The complexes of chitosan with copper turned out to be the most active.
The purpose of this work is a comparative study of the antibacterial and antifungal activities of chitosan with an 85% DD and different MWs, as well as copper complexes obtained on their basis.
MATERIALS AND METHODS
The method of oxidative degradation [29] of high-MW chitosan Chit-150 (MW = 150 kDa, DD = 85%) was used to obtain three samples with MWs of 5, 10, and 50 kDa, respectively, Chit-5, Chit-10, and Chit-50. The DD value determined for all samples via potentiometric titration [30] was 85%.
Based on the obtained samples, complexes of chitosan with copper (Chit + Cu) were synthesized as described in [28]. To do this, 0.5 g of chitosan was dissolved in 100 mL of 4% acetic acid; 0.77 g of CuSO4· 5H2O and an alkali solution (10% KOH) were added, and the pH was adjusted to 5.6. The mixture was stirred at 20°C for 3 h, and the product was then precipitated with acetone, centrifuged, washed with ethanol, filtered, and dried in vacuum. The yield of the complex was 60–80%, depending on the chitosan MW.
The copper content in premineralized samples of chitosan complexes was determined photometrically on a UV-2600 Shimadzu device (Japan) according to the well-known method used earlier in [28] based on the interaction of Cu ions with an organic reagent (picramin-epsilon) with the formation of colored complex connections. The infrared (IR) spectra of chitosan and its complexes were recorded on a Spectrum BX Fourier spectrometer (Perkin Elmer Inc., United States) in tablets with KBr.
The following samples were obtained and tested: chitosan (Chit-5; Chit-10; Chit-50; Chit-150) and copper complexes based on them (Chit-5 + Cu; Chit-10 + Cu; Chit-50 + Cu; Chit-150 + C).
In all experiments on the evaluation of the biological activity of chitosans and their complexes, 0.1% succinic acid, which does not have antimicrobial activity, was used for dissolution.
The antibacterial activity of chitosan samples was evaluated based on their ability to suppress the growth of bacterial cultures that cause crop diseases: Bacilus polymyxa (Prazmowski 1880) Mace 1989, which causes bacterial rot of potato tubers; Pseudomonas syringae pv tomato, which causes tomato spotting; and Erwinia carotovora (syn. Pectobacterium carotovorum), which causes “black leg,” diseases of potatoes and other plants.
The test cultures were obtained from the collections of type cultures of the All-Russia Research Institute of Agricultural Microbiology and the laboratory of the micro-biomethod of the All-Russia Institute for Plant Protection (Russia). The antimicrobial activity of chitosan samples was assessed via diffusion into agar (the well method) of a solution of the test compound [31]. The method is based on a comparison of the inhibition of the growth of a test microorganism by the test chitosan solution with respect to the control (distilled water) and determination of the biological activity by the zone of growth inhibition of the test microorganism (the radius of the zone of inhibition of the growth of the test organism in mm).
Potato-glucose agar cooled to 45°C was poured into sterile Petri dishes. After solidification, 0.2 mL of a suspension of the tested bacteria with a titer of 106 cfu/mL was applied to the agar surface. After the seeding of the agar, the dishes were left for 1–2 h to absorb the inoculum. Then, holes 6 mm in diameter were made on the agar surface with a sterile drill. A solution of the test samples (0.2 mL) at a concentration of 0.2% and a standard solution were simultaneously added to the wells of each dish. A 0.1% solution of succinic acid served as a standard. The cups were left for 1–2 h at room temperature and then placed in a thermostat and kept at 25°С for 48 h. The zones of inhibition of growth of the test strain of microorganisms in mm (radius) were measured for each working solution.
The antifungal activity of preparations was studied with the method of agar blocks [32]. For the test cultures, we used Fusarium oxysporum (Schlecht.) f. sp. lycopersici (Sacc.), which causes Fusarium wilt of tomato, Slerotinia sclerotiorum, which causes white rot in cucumbers, tomatoes, and other crops, and Cochliobolus sativus (S. Ito & Kurib.) Drechsler ex Dastu, the causative agent of dark-brown wheat blotch. When testing with the block method, solutions of the test samples of chitosan and copper complexes based on it were added to the warm agar Czapek medium, the final concentration in the medium was calculated. After solidification of the medium, agar blocks of a 7-day test culture were placed on its surface and cut out with a sterile cork drill. The Petri dishes were then placed in a thermostat at 22–25°C. Fungistatic activity was assessed by the diameter of growth suppression of the test culture on the fifth day of cultivation. Cups with Czapek’s medium without test substances served as controls.
The effect of chitosan samples on the germination of C. sativus conidia was also carried out in a drop (200 μL) on glass slides in the dark at 22°C in a humid chamber for 24, 48 and 72 h. An ascomycete spore suspension (0.1 mL, 104) was added to 0.1 mL of a 0.05% sample solution. The germination of conidia was assessed via microscopy of at least 100–200 spores in the experimental variant and in the control (in water). The germination frequency was expressed as a percentage of the total number of spores examined in the control and experiment [33].
All experiments were performed in triplicate, and the data obtained were processed with descriptive statistics methods (based on standard errors of means ±SEM). The least significant difference (LSD) was used to compare means at R < 0.05.
RESULTS AND DISCUSSION
It is known that, due to the presence of functional groups, chitosan is a good chelating agent and easily forms complexes with metal ions with variable valence [34]. As a rule, spectral methods are used to prove the formation and confirm the structure of chitosan–metal complexes [35].
It was previously shown [28] that, as a result of coordination between the metal (Cu) and amine groups of chitosan, the IR spectra shifted the absorption maxima of stretching and bending vibrations of NH bonds towards low frequencies and a maximum appeared at 1560 cm–1 due to the bending vibrations of NH bonds in the amine groups of chitosan bound to the metal. Based on these data, the structure of chitosan-metal complexes was proposed, which was subsequently confirmed by other researchers [6].
The copper content in the complexes, which was calculated at the absorption maximum at 551 nm, was 10 ± 1%. This corresponds to the molar ratio of copper : chitosan—1 : 4.
It is known that the mechanism of chitosan action on microorganisms is associated with violation of the integrity of the outer membrane [5, 10].
Due to the different structures of bacterial cell walls, gram-negative (P. syringae, E. carotovora) and one gram positive (B. polymyxa) bacteria were taken to test the direct antibacterial activity.
Table 1 shows the experimental data on the antifungal and antibacterial activities of chitosan and copper complexes derived from them.
It should be noted that, when studying the antibacterial and antifungal activity of chitosan, most researchers use 0.2% hydrochloric or acetic acid as a solvent [36], which have antimicrobial activity of their own. In this work, to dissolve chitosan samples, we used an aqueous solution of succinic acid, which does not affect the linear growth of test cultures (Table 1). It was shown that all chitosan samples had high antifungal activity; they inhibited the growth of fungal mycelium. F. oxysporum by 79.1–83.1%, and S. sclerotiorum by 66.6–83.3%. For example, on the fifth day of cultivation, the antifungal activity of chitosans with an MW of 5–50 kDa practically did not change in relation to the mushroom S. sclerotiorum. Upon an increase in the chitosan MW to 150 kDa, it decreased to 66.6%. As expected, the copper complexes of chitosans had a higher degree of inhibition of fungal mycelium growth as compared to chitosans.
Table 1 shows that all samples of chitosan with a MW from 5 to 150 kDa had antibacterial activity, both against gram-positive and gram-negative bacteria. The strain Pseudomonas syringae was less sensitive to chitosan action, which is most likely due to its characteristics.
A model experiment to assess the effect of chitosan Chit-5 on the germination of C. sativus conidia showed that a 50% inhibitory effect was achieved at a concentration of 0.05% on the second day and it gradually increased with increases in the polymer concentration (Table 2).
Table 3 presents the results of the evaluation of the antifungal activity of chitosan with different MWs in relation to C. sativus. The following trend was noted: the antifungal activity increased as the MW decreased. The increase in antifungal activity with increases in the sample concentration is natural. For example, the radial growth of C. sativus was suppressed from 58.7 to 68.6% on the fifth day of incubation with an increase in the concentration of Chit-5 chitosan from 0.05 to 0.1%. At a lower concentration (0.02%), chitosan could inhibit only 48.0% of the radial growth of fungal mycelium on day 5 of incubation.
The introduction of copper with the formation of the complex significantly increased the biocidal activity as compared to the activity of the original chitosan, which is associated with an increase in the positive charge of chitosan in the presence of metal ions. In addition, this increased the ability of the polycation to be adsorbed on the negatively charged surface of the microbial cell. The results presented in Table 3 show that the copper complexes of chitosan, regardless of the initial characteristics, showed high antifungal activity, inhibiting the growth of fungal mycelium. C. sativus by 85.8–88.6% at a concentration of 0.1%. The increased antifungal activity of the complexes was preserved when they were used at lower concentrations. At a concentration of 0.02–0.1%, the biocidal activity of the complexes practically did not depend on the MW of the initial chitosan. The same pattern was revealed in an experiment to assess the effect of the test samples (0.05%) on the germination of conidia of the fungus C. sativus.
It was found that, for samples of chitosan at a concentration of 0.05%, the difference in the inhibition of germination of C. sativus conidia was determined by the exposure time but not by the MW value of the polymer (Table 4). At the same time, the data in Table 4 show that the copper complex based on chitosan Chit-5 completely suppressed the germination of conidia of the fungus C. sativus within 2 days and led to a significant decrease in the number of germinated ascomycete spores (up to 10%) as compared with the control (100%) on the third day of incubation.
The original data from these studies made it possible to identify general patterns in the manifestation of the biological activity of chitosans with different MWs but with the same DD (85%), as well as their copper complexes, on all test objects. Thus, an increase in MW from 5 to 50 kDa led to an increase in antimicrobial and antibacterial activities, which was followed by a tendency to decrease. The inclusion of copper in chitosan with the formation of a chelate complex (Chit + Cu) contributed to an increase in biological activity, regardless of the MW value and the type of microorganism. This opens up the potential for the successful use of complexes of chitosan with copper in agricultural practice against a wide range of plant pathogens.
REFERENCES
El Hardrami, A., Adam, L.R., El Hadrami, I., and Daayf, F., Mar. Drugs, 2010, vol. 8, no. 4, pp. 968–987.
Khitozan (Chitosan), Skryabin, K.G, Mikhailov, S.N., and Varlamov, V.P., Eds., Moscow: Tsentr Bioinzheneriya Ross. Akad. Nauk, 2013.
Kabanov, V.L. and Novinyuk, L.V., Food Syst., 2020, no. 3, pp. P. 10–15.
Wang, W., Xue, C., and Mao, X., Int. J. Biol. Macromol., 2020, vol. 164, pp. 4532–4546.
Varlamov, V.P., Il’ina, A.V., Shagdarova, B.Ts., Lun’kov, A.P., and Mysyakina, I.S., Usp. Biol. Khim., 2020, vol. 60, pp. 317–368.
Ardean, C., Davidescu, C.M., Nemes, N.S., Negrea, A., Ciopec, M., Duteanu, N., Negrea, P., Duda-Seiman, D., and Musta, V., Int. J. Mol. Sci., 2021, vol. 22, no. 14, p. 7449.
Jianhui, Li. and Shaoling, Z., Eur. Polym. J., 2020, vol. 138. https://doi.org/10.1016/j.eurpolymj.2020.109984
Hosseinnejad, M. and Jafari, S.M., Int. J. Biol. Macromol., 2016, vol. 85, pp. 467–475.
Verlee, A., Mincke, S., and Stevens, C.V., Carbohydr. Res., 2017, vol. 164, pp. 268–283.
Matica, M.A., Aachmann, F.L., Tondervik, A., Sletta, H., and Ostafe, V., Int. J. Mol. Sci., 2019, vol. 20, p. 5889. https://doi.org/10.3390/ijms20235889
Kong, M., Chen, X.G., Xing, K., and Park, H.J., Int. J. Food Microbiol., 2010, vol. 144, pp. 51–63.
Il'ina, A.V. and Varlamov, V.P., App. Biochem. Microbiol., 2003, vol. 39, no. 3, pp. 239–242.
Mellegård, H., Strand, S.P., Christensen, B.E., Granum, P.E., and Hardy, S.P., Int. J. Food Microbiol., 2011, vol. 148, pp. 48–54.
Fernandes, J.C., Eaton, P., Gomes, A.M., Pimtado, M.E., and Xavier Malcata, F., Ultramicroscopy, 2009, vol. 109, no. 8, pp. 854–860.
Kumar, A.B.V., Varadaraj, M.C., and Tharanathan, R.N., Biomacromoleculers, 2007, vol. 2, no. 2, pp. 566–572.
Hafdani, F. and Sadeghinia, N., World Acad. Sci. Eng. Technol., 2011, vol. 74, pp. 257–261; Int. J. Pharm. Pharm. Sci., 2011, vol. 5, no. 2, pp. 46–50.
Attjioui, M., Gillet, D., El Gueddari, N.E., and Moerschbacher, B.M., MPMI, 2021, vol. 34, no. 7, pp. 770–778.
Matica, A., Menghiu, G., and Ostafe, V., New Front. Chem., 2017, vol. 26, no. 1, pp. 55–63.
Seyfarth, F., Schliemann, S., Elsner, P., and Hipler, U.C., Int. J. Pharm., 2008, vol. 353, nos. 1–2, pp. 139–148.
Li, R., Guo, Z., and Jiang, P., Carbohydr. Res., 2010, no. 345, pp. 1896–1900.
Singburaudom, N., Piasai, O., and Dethaub, T., Kasetsart J. (Nat. Sci.), 2011, vol. 45, pp. 644–655.
Hongpattarakere, T. and Riyaphan, O., J. Sci. Technol., 2008, no. 30, pp. 1–9.
Cé, R., Marchi, J.G., Bergamo, V.Z., Fuentefria, A.M., Lavayen, V., Guterres, S.S., and Pohlmann, A.R., Colloids Surf. A: Physicochem. Eng. Aspects, 2016, no. 511, pp. 153–161.
de Oliveira, PedroR., Takaki, M., Gorayeb, T.C.C., Bianchi, V.L.D., Thomeo, J.C., Tiera, M.J., and de Oliveira Tiera, V.A., Microbiol. Res, 2013, vol. 168, no. 1, pp. 50–55.
Choudhary, M.K., Joshi, A., and Saharan, V., Int. J. Curr. Microbiol. Appl. Sci., 2017, vol. 6, no. 11, pp. 1335–1350.
Al-Dhabaan, F.A., Shoala Ali, A.A.M., Alaa, M., and Abd-Elsalam, K., Int. J. Agric. Technol., 2017, vol. 13, no. 5, pp. 753–769.
Gritsch, L., Lovell, C., Goldmann, W., and Boccaccini, A., Carbohydr. Res., 2018, vol. 179, pp. 370–378.
Vlasov, P.S., Kiselev, A.A., Domnina, N.S., Popova, E.V., and Tyuterev, S.L., Zh. Prikl. Khim., 2009, vol. 82, no. 9, pp. 1571–1575.
Riccardo, A.A., Chitin, Oxford: Pergamon Press, 1977.
Kong, X., Carbohydr. Res., 2012, no. 88, pp. 336–341.
Kuleshova, S.I., Vedomosti Nauchn. Tsentra Ekspert. Sredstv Med. Primen., 2015, no. 3, pp. 13–17.
Metodicheskie rekomendatsii po ispytaniyu khimicheskikh veshchestv na fungitsidnuyu aktivnost' (Guidelines for Testing Chemicals for Fungicidal Activity) Andreeva, E.I. and Kartomyshev, V.S., Eds., NIITEKhIM, 1990, pp. 4−5.
Palma-Guerrero, J., Jansson, H.-B., Salinas, J., and Lopez-Llorca, L.V., J. Appl. Microbiol., 2008, vol. 104, no. 2, pp. 541–53.
Divya, K., Vijayan, S., George, T.K., and Jisha, M.S., Fibers Polim., 2017, vol. 18, no. 2, pp. 221–230.
Kurek, D.V., in Khitozan (Chitosan), Skryabin, K.G., Mikhailov, S.N., and Varlamov, V.P., Eds., Moscow: Tsentr Bioinzheneriya Ross. Akad. Nauk, 2013, pp. 61–70.
Abdeltwab, W.M., Abdelaliem, Y.F., Metry, W.A., and Eldeghedy, M., J. Adv. Lab. Res. Biol., 2019, vol. 10, no. 1, pp. 8–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Popova, E.V., Kovalenko, N.M. & Domnina, N.S. Fungicidal and Bactericidal Activity of Chitosans with Different Molecular Weights and Copper Complexes Based on Them. Appl Biochem Microbiol 58, 322–328 (2022). https://doi.org/10.1134/S0003683822030115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822030115