Abstract
The palladium-catalysed Suzuki–Miyaura cross-coupling (SMC) is currently the most commonly used reaction to construct carbon–carbon bonds in the pharmaceutical industry. Typical methods require the use of a base, which limits the substrate scope. To mitigate this shortcoming, substantial effort has been made to develop base-tolerant organoboron reagents, efficient catalysts and reaction conditions that do not require external bases. Still, many boronic acids cannot be used or must be independently protected, and many Lewis-basic functional groups poison the catalyst. Here we report a conceptually different SMC reaction that can proceed even under acidic conditions, with a broad substrate scope. Key to this advance is the formation of an acid-stable, palladium-based ion pair between the reaction partners that does not require base for subsequent productive transmetallation. Boronic acids that cannot be used directly in other SMC reactions, such as 2-pyridylboronic acid and boronic acids with strong Lewis bases, can now be used successfully.

Similar content being viewed by others
Main
Aryl(pseudo)halides and arylboronic acids serve as the common coupling partners for the Suzuki–Miyaura cross-coupling (SMC)1,2. Many arylboronic acids3 are less toxic, more stable and easier to store and handle than other aryl nucleophiles, which contributes to the popularity of the SMC reaction. However, many heteroarylboronic acids are insufficiently stable4,5, especially under the basic reaction conditions required for productive transmetallation6,7,8,9 of the aryl substituent from the arylboronic acid to the intermediate arylpalladium(II) (pseudo)halide complex (Fig. 1a)6,8,9. The low stability of heteroarylboronic acids is especially problematic because heteroarenes such as pyridine, imidazole and thiophene are some of the most prevalent substructures in pharmaceuticals and agrochemicals10,11,12. For example, the 2-pyridyl problem is a well-recognized challenge13,14; it results in the unproductive protodeboronation of 2-pyridylboronic acid under the reaction conditions4. This problem has been tackled by independent protection of the boronic acid as its cyclic triolborate15 or N-methyliminodiacetic acid (MIDA) boronate derivatives16. These derivatives slowly hydrolyse to the boronic acids under basic reaction conditions for subsequent transmetallation to palladium with the aid of a copper co-catalyst15,16,17. No successful SMC protocol that uses 2-pyridylboronic acid directly is currently available. A successful approach to circumvent fast decomposition of other heteroaryl4 or otherwise also problematic polyfluoroaryl5 boronic acids include catalyst development18,19,20 to accelerate the rate of transmetallation, development of SMC under neutral reaction conditions21,22,23 and the use of aryl electrophiles other than common (pseudo)halides21,23. For example, the Buchwald group developed a specific Pd-XPhos precatalyst that allowed for effective cross-coupling of 2-thiophenyl boronic acid in more than 90% yield18. The Sanford group succeeded in developing a nickel-catalysed SMC under neutral reaction conditions for benzoyl fluorides, in which an intermediate nickel fluoride is basic enough to engage in transmetallation, without the need for exogenous base (Fig. 1b)21. The Niwa group approached the problem from a similar angle, in which the zinc hydroxide complex [(TMEDA)Zn(OH)(OTf)]3 exhibits sufficient halophilicity to allow for transmetallation from aryltrifluoroborates, and also under otherwise neutral reaction conditions (Fig. 1c)22. The Carrow group developed a SMC from aryldiazonium salts that can also proceed without exogenous base23. Finally, the Liotta group reported a SMC of aryl halides at pH 3−7 via a hydroxo palladium intermediate, which shows Lewis basic group tolerance for several substrates24,25. All of these modern advances have substantially increased the substrate scope in arylboronic acid derivatives compared with the otherwise robust, conventional SMC reaction. However, despite all of the advances so far, the 2-pyridyl problem, for example, remains unsolved because 2-pyridylboronic acid is unstable towards protodeboronation even under neutral conditions4,21,22,23; the rate constant for protodeboronation from pH 4‒10 under SMC reaction conditions is 10−2 s‒1, as determined by the Lloyd-Jones group4. Moreover, reactions under both conventional basic and modern neutral conditions fail in the presence of a variety of functional groups with high Lewis basicity, probably due to catalyst poisoning upon coordination to the transition metal catalyst26,27. Despite the advances made on catalyst improvement18,19,20 and conditions in acidic buffer24,25, challenges in heteroaryl SMC persist.
a, Traditional SMC of aryl halides. b, Nickel-catalysed SMC of benzoyl fluoride21. c, Zinc-hydroxide-mediated SMC of aryl halides22. d, This work: SMC of arylthianthrenium salts with acid. e, Comparison of substrate scope and Lewis-basicity tolerance under basic, neutral and acidic conditions. L, ligand; TT, thianthrene; Tf, trifluoromethansulfonyl.
Herein we report a conceptually different SMC that can also tolerate acidic conditions (Fig. 1d); it solves both the difficulty in engaging otherwise fragile coupling partners, including heteroarylboronic acids such as 2-pyridylboronic acid, and also provides a general solution to avoid catalyst poisoning caused by Lewis-basic functional groups (Fig. 1e). Acid prevents transmetallation in other SMC reactions, basic and neutral, because the Lewis-basic groups that are required for productive transmetallation—such as the uncharged palladium hydroxide in conventional SMC (Fig. 1a)6, palladium fluorides7,28, Pd‒O‒B pre-transmetallation intermediates9 from anionic aryltrihydroxyborates (Supplementary Figs. 25–27), and possibly also the nickel fluoride and zinc hydroxide shown in Fig. 1—are protonated. Our goal was to develop a reaction in which an ion pair, stable to acid, can function as a pre-transmetallation intermediate and thereby enable transmetallation even in the presence of acid (Fig. 2). Our group previously disclosed arylthianthrenium salts29,30,31—which are more readily accessible selectively in late-stage functionalization than halides or diazonium salts—as versatile coupling partners for cross-coupling chemistry. Upon oxidative addition to Pd(0), arylthianthrenium reagents deliver cationic Ar-[PdII]+ complexes without strongly coordinating anions31. We anticipated that aryltrifluoroborates might form a suitable ion pair with the cationic palladium intermediates obtained from arylthianthrenium salts. Aryltrifluoroborates exhibit high stability to acid32,33 and can be formed by B‒X bond metathesis34 from arylboronic acids and HBF4, as well as external BF4‒ ions directly, which seemed ideal, given that arylthianthrenium salts are most commonly obtained as tetrafluoroborates salts29.
a, Robustness screening. b, Pre-transmetallation mechanism in traditional SMC. c, The formation of an acid-tolerant ion pair B in the SMC of arylthianthrenium salts. d, The effects of counter-anion and arylboron source. aYield determined from 19F NMR spectroscopic analysis with fluorobenzene as an internal standard. bHBF4·Et2O (4.0 equiv.). c60 °C, 10 min. Non-covalent interactions are depicted with dashed bonds.
Results and discussion
Reaction development
A commercially available Pd(0) complex catalyses the SMC between phenylboronic acid and arylthianthrenium tetrafluoroborate 1-BF4, both in the absence and presence of HBF4 (Fig. 2a). Acid is not required for productive cross-coupling, but it is tolerated. Such tolerance enables reactions in the presence of Lewis-basic functional groups because they are in situ protected by protonation where they otherwise would not be under basic or neutral conditions (Fig. 2a)35. Previous work has demonstrated tolerance of several basic functional groups under pH 3−7 when aryl halides are used as substrates24,25. Yet, various heterocycles that failed under previous conditions25 are tolerated in our acidic SMC of arylthianthrenium salts (Supplementary Table 2). Basic intermediates that are required for other SMC reactions (for example, the pre-transmetallation intermediate shown in Fig. 2b) are not required in the SMC with arylthianthrenium salts: although oxidative addition generates a cationic arylpalladium complex A, the corresponding aryltrifluoroborate counter-ion is generated from the arylboronic acid, and either the tetrafluoroborate counter-ion of the thianthrenium salt under neutral conditions, or HBF4 under acidic conditions (Fig. 2c and Supplementary Fig. 3–9). Both ions result in the formation of ion pair B. The anion's aryl π system and the cationic palladium may engage in a cation−π interaction36,37 that could both facilitate formation of B while being geometrically appropriate for ensuing transmetallation under both neutral and acidic conditions. [Pd(tBu3P)2], with sterically hindered monodentate ligands38, is the optimal palladium catalyst, consistent with ion pair formation (Supplementary Table 3); a second tBu3P is reluctant to coordinate to A due to steric repulsion38, leaving a coordinating site for the cation–π interaction of substrates. Past research has demonstrated boron-to-palladium transmetallation via cationic arylpalladium intermediate in the presence of zinc hydroxide22, but the process fails in the presence of acid (Supplementary page 15). The reaction reported here provides a transmetallation pathway for SMC that can tolerate stoichiometric strong acids.
To better probe the potential relevance of ion pair B, we evaluated different acids and thianthrenium counter-ions that would not allow formation of similar ion pairs (Fig. 2d). For example, when HCl was used instead of HBF4, less than 5% conversion was observed, consistent with chloride binding to A, which should preclude ion pair formation (Fig. 2d, entry 2). Likewise, no arylboronate anion should form when the BF4‒ counter-ion is swapped for the triflamide counter-ion (Fig. 2d, entry 3). Under neutral conditions, the aryltrifluoroborate anion in B can be generated from the BF4‒ counter-anion originating from the thianthrenium salt (Supplementary Figs. 3–7), yet triflamide (NTf2‒) cannot form a similar structure (Fig. 2d, entry 5). Furthermore, arylpinacolboronate is less effective than arylboronic acid (Fig. 2d, entry 6). When aryltriflate is used as the coupling partner with organoboron reagents, the yield is less than 1% (Supplementary Table 3). Methanol is the best solvent for the reaction (Supplementary Table 1), consistent with the observed fast B‒X metathesis in alcohol solvents32. All observed results are consistent with the relevance of ion pair B under both neutral and acidic conditions for productive coupling.
Mechanistic study
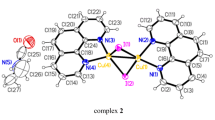
The reactions between phenylboronic acid, and 1-BF4 and HBF4·Et2O, respectively, were investigated by nuclear magnetic resonance (NMR) spectroscopy to establish a fast metathesis equilibrium to phenyltrifluoroborate under both acidic and neutral conditions (Fig. 3a and Supplementary Figs. 3–9). Oxidative addition of the arylthianthrenium salt 1-BF4 to the palladium catalyst proceeds quickly in the presence of HBF4 to afford palladium(II) intermediate A (specifically 3-BF4), which we could observe spectroscopically, but was unstable in the attempts to isolate its pure form (Fig. 3b and Supplementary Fig. 10). Our data suggest a weak interaction between the BF4‒ counter-ion and the palladium cation in 3-BF4 because we can observe a heteronuclear Overhauser effect between the two ions (Supplementary Fig. 6) yet no J-coupling between the 31P of the ligand and the 19F nuclei of the anion. Following addition of phenyltrifluoroborate, the spectroscopic data are consistent with the formation of an ion pair B that also evaded more detailed characterization; however, it did afford cross-coupling product 2 in 90% yield, as determined by NMR spectroscopy with an internal standard in the presence of HBF4 (Fig. 3b and Supplementary Fig. 16). Density-functional theory (DFT) calculations predict that the anion exchange between [H]+[Ph-BF3]‒ and [Ph-PdII]+BF4‒ (A)26 to form the ion pair B and HBF4 is exergonic by 6.3 kcal mol–1 (Fig. 3c), and has a Pd–C(ipso) distance in B of 2.42 Å, consistent with η1 coordination37. The key transmetallation from B has an activation energy of 22.5 kcal mol–1 and is turnover limiting for subsequent facile reductive elimination. Given the mechanism, we also probed the SMC reaction for aryldiazonium salts because a similar ion pairing should be accessible; however, the need for elevated temperatures to overcome the activation barrier to transmetallation leads to much lower yields due to the thermal instability of aryldiazonium salts (Supplementary Table 4). In contrast to aryldiazonium salts23,39, arylthianthrenium salts are thermally stable and, given their accessibility at a late-stage, seem to occupy a sweet spot for accessing ion pairs; that is, they are (1) sufficiently stable to form and resist acid, and (2) are reactive enough to proceed in the catalytic cycle of the SMC.
a, Observed B‒X bond metathesis equilibrium in methanol. b, Preparation of the cationic palladium intermediate 3-BF4 and its reaction with phenyltrifluoroborate. c, Proposed catalytic cycle and DFT calculation results. The Gibbs free energy (ΔG) is calculated at the PBE0-D3(BJ)/def2-TZVPP//PBE0-D3(BJ)/def2-SVP level in methanol. The carbon-bound hydrogen atoms have been omitted from the DFT-calculated structures of A and B for clarity. ΔG‡, difference in G between the transition state and reactant. d, Reactions of 2-pyridylboronic acid under basic, neutral and acidic conditions. We also show the X-ray crystal structure of [H]+[Py-BF3]‒ (4) with 50% probability ellipsoids; the hydrogen atoms were refined isotropically and carbon-bound hydrogen atoms are omitted for clarity. Pink, boron; yellow, fluorine; blue, nitrogen; grey, carbon; white, hydrogen. The SMC was between arylthianthrenium salt and 2-pyridylboronic acid. aYield determined by 19F NMR spectroscopy with fluorobenzene as an internal standard. HOE, heteronuclear Overhauser effect.
Substrate scope
Acid is not required for the catalytic mechanism shown in Fig. 3c and is not expected to influence the formation of the ion pairs. Yet, acidic conditions allow substrates that would otherwise not be tolerated to participate. For example, protodeboronation of 2-pyridylboronic acid proceeds too quickly under both basic and neutral reaction conditions (Fig. 3d). Previous protocols to address the 2-pyridyl problem under basic conditions require an additional step to prepare base-tolerant organoboron reagents15,16 or stoichiometric copper additives17. Under traditional basic SMC conditions, Lewis acids can coordinate to the pyridine ring and form a zwitterionic intermediate [M]+[Py-B(OH)3]‒, whereas under neutral reaction conditions, the related zwitterion [H]+[Py-B(OH)3]‒ is formed. Both readily hydrolyse, and the transition state of protodeboronation proceeds via heterolytic C–B bond fragmentation with protonation of the carbanionic carbon as boric acid (B(OH)3) is expelled4. We rationalize that a similar heterolytic fragmentation in 4 would proceed at a much slower rate because BF3 is more Lewis-acidic than B(OH)3, and is therefore more stable towards hydrolysis because C–B bond heterolysis does not proceed at an appreciable rate (Supplementary Fig. 23). Zwitterion [H]+[Py-BF3]‒ (4) is formed via a reaction between 2-pyridylboronic acid and HBF4·Et2O in methanol. The related structure potassium 2-pyridyltrifluoroborate was reported but decomposes quickly in non-acidic SMC reaction conditions33. By contrast, 4 is stable towards hydrolysis in acid (Supplementary Fig. 24)40. The reaction between 2-pyridylboronic acid and arylthianthrenium salt only produces coupling product 5 in 5% yield under neutral conditions, yet, in the presence of external HBF4·Et2O furnished 5 in 88% yield, consistent with the claims made in this manuscript about the relevance of stoichiometric strong acid.
Due to favourable ion pair formation, a large variety of substrates that are incompatible with conventional SMC reaction conditions can now participate directly when using boronic acids as starting materials (Fig. 4 and Supplementary Table 2). Tolerance towards acid extends the substrate scope to compounds that cannot be converted through other reported SMC reactions directly from boronic acids without additional independent protection steps. For example, cross-couplings featuring basic heterocycles or amines (6–17) participate well in acid—a previous SMC to synthesize the nematicidal active compound41 14 resulted in a yield of 17% due to the presence of coordinative nitrogen atoms, but now can be obtained in 98% yield under acidic conditions through ion pair formation. Electron-donating or -withdrawing groups are well-tolerated on boronic acids (Supplementary Table 5). The scope of the ion-pair-based cross-coupling reaction also includes all those compound classes that are accessible with other modern SMC reactions, for example for heteroarylboronic acids that are sensitive to base, such as 2-thiophenyl (18, 20), 2-furanyl (19), 2-benzofuranyl (21), 5-pyrimidyl (22), 3-pyrazolyl (23), 2-pyrrolyl (24) and 4-isoxazolyl (25). Likewise, base-sensitive pentafluorophenylboronic acid5,42 can be coupled with the etofenprox-derived thianthrenium salt to produce 34 in 30 s at ambient temperature, which positions the SMC as a general solution to all compound classes for SMC. The reaction is robust and can be executed in ambient atmosphere and wet solvent; for example, SMC to produce 2 in 95% yield tolerates up to 50 vol% water (Supplementary Table 1).
aArylthianthrenium salt or aryl(tetrafluoro)thianthrenium salt (0.1 or 0.2 mmol), arylboronic acid (1.1‒2.0 equiv.), Pd(tBu3P)2 (5 mol%), MeOH (0.1 M). bHBF4·Et2O (1.0‒2.0 equiv.). c110 °C, 12 h. d90 °C, 10 min to 12 h. e60 °C, 10 min to 12 h. fPd(tBu3P)2 (1 mol%). gPerformed on 4 mmol scale; 1.02 g product. h25 °C, 30 s. Ac, acetyl; Boc, tert-butyloxycarbonyl.
Conclusion
Our study reveals an unusual mechanism that can facilitate a general transmetallation pathway for C–C bond formation reactions under acidic conditions that has not been illustrated for other cross-coupling reactions. We anticipate that the ion-pair-interaction-promoted transmetallation can serve as a fundamentally different mechanism that is also suitable for other cross-coupling reactions such as carbon–heteroatom bond formations, which is under investigation in our laboratory.
Methods
General procedure for the SMC under neutral conditions
Under an ambient atmosphere, arylthianthrenium salt (0.200 mmol, 1.00 equiv.), arylboronic acid (0.220 mmol, 1.10 equiv.) and Pd(tBu3P)2 (5.0 mg, 10 μmol, 5.0 mol%) were added to a 4 ml vial containing a magnetic stir bar, followed by MeOH (2 ml, 0.1 M). The vial was sealed with a septum cap, and the reaction mixture was stirred vigorously at 60 °C on a heating block. After the indicated time, the reaction vessel was opened to air, and the resulting mixture was concentrated by rotary evaporation. The residue was purified by chromatography on silica gel to obtain the pure product.
General procedure for the SMC under acidic conditions
Under an ambient atmosphere, arylthianthrenium salt (0.200 mmol, 1.00 equiv.), arylboronic acid (0.220 mmol, 1.10 equiv.), Pd(tBu3P)2 (5.0 mg, 10 μmol, 5.0 mol%) and MeOH (2 ml, 0.1 M) were added to a 4 ml vial containing a magnetic stir bar, followed by HBF4∙OEt2 (28 μl, 33 mg, 0.20 mmol, 1.0 equiv.). The vial was sealed with a septum cap, and the reaction mixture was stirred vigorously at 60 °C on a heating block. After the indicated time, the reaction vessel was opened to air, and the resulting mixture was concentrated by rotary evaporation. The residue was purified by chromatography on silica gel to obtain the pure product.
Data availability
Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition no. CCDC 2280716. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The data reported in this Article are available in the main text or Supplementary Information.
References
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Schneider, N. et al. Big data from pharmaceutical patents: a computational analysis of medicinal chemists’ bread and butter. J. Med. Chem. 59, 4385–4402 (2016).
Hall, D. G. (ed.) Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials (Wiley, 2011).
Cox, P. A., Leach, A. G., Campbell, A. D. & Lloyd-Jones, G. C. Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH–rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016).
Cox, P. A. et al. Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Carrow, B. P. & Hartwig, J. F. Distinguishing between pathways for transmetalation in Suzuki−Miyaura reactions. J. Am. Chem. Soc. 133, 2116–2119 (2011).
Amatore, C., Jutand, A. & Le Duc, G. The triple role of fluoride ions in palladium-catalyzed Suzuki–Miyaura reactions: unprecedented transmetalation from [ArPdFL2] complexes. Angew. Chem. Int. Ed. 51, 1379–1382 (2012).
Lennox, A. J. & Lloyd-Jones, G. C. Transmetalation in the Suzuki–Miyaura coupling: the fork in the trail. Angew. Chem. Int. Ed. 52, 7362–7370 (2013).
Thomas, A. A. & Denmark, S. E. Pre-transmetalation intermediates in the Suzuki–Miyaura reaction revealed: the missing link. Science 352, 329–332 (2016).
Brown, D. G. & Bostrom, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? Miniperspective. J. Med. Chem. 59, 4443–4458 (2016).
Crawley, M. L. & Trost, B. M. Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective (John Wiley & Sons, 2012).
Hilton, M. C. et al. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science 362, 799–804 (2018).
Cook, X. A. et al. The 2-pyridyl problem: challenging nucleophiles in cross-coupling arylations. Angew. Chem. Int. Ed. 60, 11068–11091 (2021).
Blakemore, D. C., Doyle, P. M. & Fobian, Y. M. (eds) Synthetic Methods in Drug Discovery: Volume 1 (Royal Society of Chemistry, 2016).
Yamamoto, Y., Takizawa, M., Yu, X.-Q. & Miyaura, N. Cyclic triolborates: air- and water-stable ate complexes of organoboronic acids. Angew. Chem. Int. Ed. 47, 928–931 (2008).
Knapp, D. M., Gillis, E. P. & Burke, M. D. A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates. J. Am. Chem. Soc. 131, 6961–6963 (2009).
Deng, J. Z. et al. Copper-facilitated Suzuki reactions: application to 2-heterocyclic boronates. Org. Lett. 11, 345–347 (2009).
Kinzel, T., Zhang, Y. & Buchwald, S. L. A new palladium precatalyst allows for the fast Suzuki−Miyaura coupling reactions of unstable polyfluorophenyl and 2-heteroaryl boronic acids. J. Am. Chem. Soc. 132, 14073–14075 (2010).
Ge, S. & Hartwig, J. F. Highly reactive, single-component nickel catalyst precursor for Suzuki−Miyuara cross-coupling of heteroaryl boronic acids with heteroaryl halides. Angew. Chem. Int. Ed. 51, 13009–13013 (2012).
Düfert, M. A., Billingsley, K. L. & Buchwald, S. L. Suzuki−Miyaura cross-coupling of unprotected, nitrogen-rich heterocycles: substrate scope and mechanistic investigation. J. Am. Chem. Soc. 135, 12877–12885 (2013).
Malapit, C. A., Bour, J. R., Brigham, C. E. & Sanford, M. S. Base-free nickel-catalyzed decarbonylative Suzuki–Miyaura coupling of acid fluorides. Nature 563, 100–104 (2018).
Niwa, T. et al. Lewis acid-mediated Suzuki–Miyaura cross-coupling reaction. Nat. Catal. 4, 1080–1088 (2021).
Chen, L., Sanchez, D. R., Zhang, B. & Carrow, B. P. ‘Cationic’ Suzuki–Miyaura coupling with acutely base-sensitive boronic acids. J. Am. Chem. Soc. 139, 12418–12421 (2017).
Li, Z. et al. Pd-catalyzed Suzuki coupling reactions of aryl halides containing basic nitrogen centers with arylboronic acids in water in the absence of added base. New J. Chem. 41, 15420–15432 (2017).
Li, Z. et al. Aqueous Suzuki coupling reactions of basic nitrogen-containing substrates in the absence of added base and ligand: observation of high yields under acidic conditions. J. Org. Chem. 81, 8520–8529 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Kassel, V. M., Hanneman, C. M., Delaney, C. P. & Denmark, S. E. Heteroaryl–heteroaryl, Suzuki–Miyaura, anhydrous cross-coupling reactions enabled by trimethyl borate. J. Am. Chem. Soc. 143, 13845–13853 (2021).
Sather, A. C. & Buchwald, S. L. The evolution of Pd0/PdII-catalyzed aromatic fluorination. Acc. Chem. Res. 49, 2146–2157 (2016).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Zhao, D. et al. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 600, 444–449 (2021).
Molander, G. A. & Ellis, N. Organotrifluoroborates: protected boronic acids that expand the versatility of the Suzuki coupling reaction. Acc. Chem. Res. 40, 275–286 (2007).
Molander, G. A. & Elia, M. D. Suzuki−Miyaura cross-coupling reactions of benzyl halides with potassium aryltrifluoroborates. J. Org. Chem. 71, 9198–9202 (2006).
Lozada, J. et al. Salt metathesis: tetrafluoroborate anion rapidly fluoridates organoboronic acids to give organotrifluoroborates. Angew. Chem. Int. Ed. 62, e202215371 (2023).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Mahmudov, K. T., Gurbanov, A. V., Guseinov, F. I. & da Silva, M. F. C. G. Non-covalent interactions in metal complex catalysis. Coord. Chem. Rev. 387, 32–46 (2019).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki−Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Kawatsura, M. & Hartwig, J. F. Simple, highly active palladium catalysts for ketone and malonate arylation: dissecting the importance of chelation and steric hindrance. J. Am. Chem. Soc. 121, 1473–1478 (1999).
Sanhueza, I. A. et al. Base-free cross-coupling of aryl diazonium salts in methanol: PdII-alkoxy as reactivity-controlling intermediate. Angew. Chem. Int. Ed. 60, 7007–7012 (2021).
Chansaenpak, K. et al. [18F]–NHC–BF3 adducts as water stable radio-prosthetic groups for PET imaging. Chem. Commun. 51, 12439–12442 (2015).
Slomczynska, U. J. & Haakenson, W. P. N-, C-Disubstituted azoles and compositions and methods for controlling nematode pests. US Patent US 2014/0274689 A1 (2014).
Gillis, E. P. et al. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Acknowledgements
We thank B. Lansbergen, C. Li and S. Lin (all at MPI für Kohlenforschung) for helpful discussions. We thank J. Yan for providing arylthianthrenium salts. We thank J. Rust for X-ray analysis; M. Leutzsch for NMR spectroscopy analysis; D. Kampen, F. Kohler, N. Haupt and D. Margold for mass spectrometry analysis; K. Bohdan for DFT analysis; and F. Marlow and U. Petrat for conductivity measurements (all at MPI für Kohlenforschung). We thank the MPI für Kohlenforschung for funding. Z.B. acknowledges the Alexander von Humboldt Foundation for a Humboldt Research Fellowship.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
L.Z. developed the SMC. L.Z. and Y.X. investigated the mechanism. L.Z., Y.X. and Z.B. explored the substrate scope. L.Z. and T.R. wrote the paper. T.R. directed the project.
Corresponding author
Ethics declarations
Competing interests
T.R. may benefit from royalty payments related to sales from thianthrene-based compounds. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Louis-Charles Campeau for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, experimental procedures, product characterization and mechanistic studies.
Supplementary Data 1
Crystallographic data for compound 4 (CCDC reference no. 2280716).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Xie, Y., Bai, Z. et al. Suzuki–Miyaura coupling of arylthianthrenium tetrafluoroborate salts under acidic conditions. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00631-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00631-4
- Springer Nature Limited