Abstract
Since the 1980s, angler interest in recreational fisheries has increased the demand for pond production of North American and European percids for stocking to enhance wild populations or to establish new populations. In this paper, we analyze the fish and plankton ecology in the production ponds to provide a better understanding of the ecological and biological factors involved in optimal pond production of percid fingerlings for stocking. Much of the literature uses a “black-box method” for optimizing pond management, reporting on the survival, growth, and size at harvest of fish from ponds as a function of various fertilization and pond stocking regimens. In contrast, our research since 1987 has focused on the seasonal variation in the ecological interactions among fertilizers, algae, zooplankton, benthos, and larval fish in ponds. Accordingly, in this paper we examine management of large-scale production of percid fingerlings from an ecological perspective, concentrating primarily on our research through 2012 in three Ohio state fish hatcheries, incorporating other information from the literature as appropriate. We find that despite differences in walleye, saugeye, and yellow perch growth and development, rearing ponds can be managed similarly to produce desired size and harvest yields of fingerling fish by providing adequate food resources. Management protocol for fertilization, stocking schedules, and stocking density should be site specific considering the source water quality. Further, sequential culture of ponds may boost overall hatchery production, but we show reduced springtime percid yield due to carryover effects of chemicals added during summertime catfish culture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the 1980s, angler interest in recreational fisheries has increased the demand for stocking cultured walleye (Sander vitreum) and yellow perch (Perca flavescens), as well as saugeye (walleye ♀ X sauger S. canadense ♂), whether to enhance wild populations experiencing declining natural production from variable reproductive success and diminished habitat quality, or to establish new populations (Ellison and Franzin 1992; Fox 1989; Hushak et al. 1988; Mitzner 2002). Stocking fry into rivers, lakes and reservoirs can occasionally produce large year-classes, but in many instances fry stocking contributes little to recruitment (Mitzner 2002; Mathias et al. 1992; Fielder 1992; McWilliams and Larscheid 1992; Paragamian and Kingery 1992). Accordingly, managers favor stocking fingerlings (30–50 mm) or even larger fish, requiring effective methods for growing fry into stockable-sized fish. Producing really large fish (>5.0 g) typically requires feeding with manufactured feeds or providing minnows as forage, but this paper focuses on the techniques required to raise large numbers of 0.3–0.5 g percids in drainable earthen ponds, relying solely on the production of living invertebrate organisms in the ponds as prey for the fish.
Much of the literature uses a “black-box method” for optimizing pond management, reporting on the survival, growth, and size at harvest of fish from ponds as a function of various fertilization and pond stocking regimens. In contrast, our research since 1987 has focused on the seasonal variation in the ecological interactions among fertilizers, algae, zooplankton, benthos, and larval fish in ponds. Accordingly, in this paper we examine management of large-scale production of percid fingerlings from an ecological perspective, concentrating primarily on our research through 2012 in three Ohio state fish hatcheries, incorporating other information from the literature as appropriate.
During this research, we first studied existing techniques used by state fish hatchery managers for raising walleye, saugeye, and yellow perch fingerlings in a series of 0.4–2.8 ha ponds, 1–2 m in depth, with volumes of 1300–26,400 m3. They filled the ponds from adjacent surface water sources and fertilized them with granular inorganic and organic fertilizers a month prior to stocking with percid fry. Additional fertilization continued through the production season (30–90 days), and the ponds were drained to harvest fingerlings when they stopped growing 1 mm in length per week. Because walleye and sauger spawn earlier than yellow perch (Hokanson 1977) or striped bass (Morone saxatilis) or white bass (M. chrysops), which in turn spawn before channel catfish (Ictalurus punctatus), pond managers often arranged to produce some combination of walleye or saugeye, hybrid striped bass and yellow perch, and channel catfish in the same ponds, sequentially filling ponds, culturing fish, and draining the ponds to harvest the fish.
However, percid fingerling production varied widely, and some ponds contained no fish upon draining, whereas others produced 12,000–20,000 percid fingerlings/ha. Accordingly, we performed a series of experiments on walleye, saugeye, and yellow perch production to decrease the variability in culture success, maximize the number of fingerlings produced at a size suitable for stocking, and minimize mortality during pond culture, while decreasing costs of labor and supplies. Our approach was to optimize planktonic production by improving the pond fertilization procedures and to adjust fry stocking density and timing to coordinate fish dietary demands with plankton dynamics in the ponds.
These studies generated data on biweekly variation in nutrients and dissolved oxygen in the ponds; seasonal variation in phytoplankton and zooplankton taxonomic composition, abundance, biomass, and productivity; how fish diets and their preferences for different zooplankton taxa varied through time; and fish growth through the production season, their survival, and numbers and biomass at harvest. New management methods for pond fertilization and fry stocking and timing based on the results of these experiments were adopted in 1990–1991, and are reflected in increases in the total number of fingerlings produced and stocked in Ohio reservoirs from the three hatcheries since that time (Fig. 18.1). In this paper, we analyze the fish and plankton ecology reflected in these increased yields to provide a better understanding of the ecological and biological factors involved in optimal pond production of percids.
Total walleye, saugeye, and yellow perch fingerlings produced at Ohio’s St. Marys, Hebron, and Senecaville state fish hatcheries and stocked into lakes between 1970 and 2012. Variation reflects improved fertilization methods beginning in 1990 and the number of ponds placed in production for the three taxa
2 Pond Management and Stocking
2.1 Fertilization
Managing ponds to promote zooplankton availability for planktivorous larval percids is centered on providing an abundance of edible phytoplankton. A common practice is to increase primary productivity by adding nutrients (especially nitrogen and phosphorus) to the system (Culver 1991; Soderberg et al. 1997, 2000; Tew et al. 2006; Jacob and Culver 2010; Hartleb et al. 2012). Nutrient sources can be of either inorganic or organic origin, and their relative efficacy for percid culture has been compared (see review in Tew 2003). Organic fertilizers, such as animal manures and alfalfa meal, stimulate zooplankton production by encouraging growth of heterotrophic bacteria, but they also can reduce fish production because heterotrophic intermediates consume dissolved oxygen (Qin and Culver 1992; Qin et al. 1995; Tice et al. 1996), compromising water quality. Conversely, inorganic fertilizers stimulate photosynthetic algae which produce oxygen and these fertilizers can be precisely controlled to prevent over-fertilization (Jacob and Culver 2010).
As described in Tew et al. (2006), Ohio state hatchery ponds were filled from nearby reservoirs (mesotrophic Senecaville Lake for Senecaville hatchery, eutrophic Buckeye Lake for Hebron Hatchery, and hypereutrophic Grand Lake for St. Marys hatchery (Filbrun et al. 2008), although ponds in the last are now filled from two wells) approximately 3 days before stocking fry into the ponds. Each pond has a separate filling system with a 0.5 mm mesh screen or drum filter to prevent the introduction of undesired larval fish from the lake while most phytoplankton and zooplankton taxa passed through easily.
From 1977 to 1984, ponds for percid culture were enriched to stimulate plankton growth using both inorganic and organic agricultural fertilizers, applying 6-10-4 (N – P2O5 – K2O) granular fertilizer at the rate of 168.1 kg·ha−1 and alfalfa meal at the rate of 112 kg·ha−1 every week. This method produced an extremely low N:P ratio (1.5–1 by mass) and a weekly addition of >750 μg PO4-P·L−1 which promoted the proliferation of cyanobacterial (blue-green algal) and filamentous green (Chlorophyta) phytoplankton species, and a low abundance of edible smaller algae (Helal and Culver 1991). Newly-hatched larval fish consume zooplankton for their survival and growth, and decreasing algae edible by zooplankton leads to crashes in the zooplankton population a few weeks after ponds are filled. Other water quality concerns with high abundances of cyanobacteria and filamentous green algae include low dissolved oxygen (DO) concentrations and toxic ammonia concentrations due to high pH. Identifying the appropriate amounts of nitrogen and phosphorus to apply as inorganic fertilizers is thus essential (Culver 1991; Soderberg et al. 2000; Tew et al. 2006; Jacob and Culver 2010).
Culver (1996) suggested that the percid ponds should be treated to maintain nitrogen and phosphorus at an approximate mass ratio of 20:1 by spraying sufficient liquid inorganic fertilizers (liquid 28-0-0 inorganic fertilizer (NH4NO3 + Urea) and phosphoric acid (H3PO4 or 0-54-0) both diluted with pond water) weekly to restore the pond nutrient-concentrations to 600 μg NO3+NH4-N·L−1 and 30 μg PO4-P·L−1, based on weekly analyses of dissolved inorganic nitrogen and phosphorus in each pond. We diluted samples of fertilizer stocks by one part to one million to allow careful measurement of their phosphate, ammonia and nitrate concentrations, whereas urea content was measured by local hospital analytical labs. Not only are the actual N and P concentrations in liquid fertilizers delivered by agricultural fertilizer providers variable, but nitrogen fertilizers are often contaminated with large amounts of phosphate fertilizer. When typical amounts of 28-0-0 added are 5 l for a 5000 m3 pond, whereas the amounts of 0-54-0 to be added are only 0.1–0.3 l, accurately measuring variation in fertilizer N and P content is required.
We estimated pond volumes for the 1990–2000 production years from area and the estimated average depth of each pond to calculate the amount of fertilizer needed to raise N and P to the targeted concentrations each week. While the ponds were empty in winter 2001, however, we performed a series of measurements on each pond using global positioning (GPS) equipment (Trimble Pathfinder Pro XR) and its depth dimensions to allow a more accurate estimation of pond volume as a function of water level measured as distance below the fill structure. We then filled the ponds and used GPS to trace the outlines of the water’s edge at various water levels and determined the depths of the water column along a series of transects in each pond. The pond volume models are geometric/trigonometric constructs from the resultant areas, slopes, and depths (Details in J. Mion, Ohio Dept. Natural Resources, ms in prep.). After 2001, we used the new pond volume estimates as a function of water level to calculate the amounts of fertilizer needed each week in each pond.
This fertilization regimen improved percid survival and yield at Hebron SFH between 1991 and 1995, but in more recent years survival has both declined and become more variable, so the possibility of over-fertilization was examined (Tew et al. 2006). They found that lowering the phosphorus target concentration to 20 μg PO4-P·L−1 still supported adequate prey production for percids. Importantly, however, the source water for Hebron SFH is eutrophic to hyper-eutrophic. While these methods have succeeded in Ohio, percids are reared to the fingerling stage in hatcheries throughout the northeastern US under a wide variety of source water nutrient concentrations, including those with oligotrophic water supplies. Nevertheless, Jacob and Culver (2010) recommended further lowering the phosphorus fertilization rates to 10 μg PO4-P·L−1, as this concentration still supported adequate primary production and zooplankton prey for fish growth while minimizing the potential risks of poor water quality (e.g., low DO and high pH).
Briland (2010) recently tested whether a phosphate fertilization rate as low as 10 μg PO4-P·L−1, would be effective in a less productive system, Senecaville SFH, where the water source (Seneca Lake) is mesotrophic. Similar to Jacob and Culver’s (2010) results in the more eutrophic Hebron SFH, Briland found that fish yield, harvest and survival in saugeye ponds at Senecaville SFH did not differ among ponds fertilized with 30, 20, or 10 μg PO4-P·L−1 (N:P ratios of 20, 30, or 60 to 1 by mass), and fish growth during the culture period was not adversely affected by reducing the phosphorus fertilization rate. In fact, no evidence of resource limitation or deteriorated water quality (from excessive nutrients) was evident from this study. Ultimately, we recommended first fertilizing ponds at Senecaville at the traditional rate, i.e., raising ponds to a target 30 μg PO4-P·L−1 each week, and repeating the reduced fertilization rate experiment in future years if and when evidence of over-fertilization (i.e., low dissolved oxygen, high pH, and abundant cyanobacteria populations) occurs. This management decision was based on site-specific conditions, namely that fish sizes at Hebron SFH are consistently larger than at Senecaville and that whereas Hebron percid ponds were typically plagued with cyanobacterial dominance of the phytoplankton (Jacob and Culver 2010), Senecaville ponds predominantly produced algae types which are edible to zooplankton (Briland 2010).
2.2 Fry Production Methods
2.2.1 Walleye and Saugeye
Walleye eggs collected from nearby Ohio reservoirs were artificially inseminated with walleye sperm (for walleye fry) or sauger from the Ohio River (for saugeye fry). The fertilized eggs were treated with tannic acid to prevent clumping and Iodophor to prevent infection with Viral Hemorrhagic Septicemia (VHS), and then incubated at all three hatcheries in McDonald jars until they hatched. Fry were counted by volumetric displacement and stocked in ponds at 3–4 days posthatch in early mid-April at densities of 100,000–600,000 fry ha−1.
2.2.2 Yellow Perch
Spawning yellow perch females extrude eggs in ribbons of a gelatinous matrix (egg skeins) and then attach them to submerged aquatic vegetation, rocks, or woody debris, such as fallen trees (Thorpe 1977; Craig 1987; Kestemont and Mélard 2000). Yellow perch females’ extrusion of eggs in skeins requires different techniques for producing fry for stocking in ponds than those used for walleye and saugeye. Eggs for extensive pond production can be obtained either by natural spawning in ponds or by manual spawning in which eggs are manually stripped from ripe females, mixed with milt stripped from males, and “dry” fertilized in a plastic bowl, similar to techniques used for walleye and saugeye. Managers at Ohio’s St. Marys SFH used natural spawning through 2005, and manual spawning since then, permitting us to compare the two methods’ effectiveness in this paper.
For natural spawning, different densities of even numbers of males and females were stocked in ponds at either a low density (<60 parental pairs/ha), a medium density (60–120 pairs/ha), or a high density (>120 pairs/ha). Ponds were filled 1 day before stocking parents, adding suitable spawning substrates (such as dried conifer trees torched to burn off needles) before spawning begins in March through April, and the fish spawn naturally, suspending egg skeins from the branches. Unfortunately, there is little control of when the fish spawn, or the timing of hatch, and females vary in fecundity, so that there is potential for large variation in the number of fry produced per pond.
For manual spawning, spawning is synchronized by injection of females (214 fish in 2007) with human chorionic gonadotropin (hCG), 150–660 IU/kg wet weight. Spawning usually occurs a few days after injection, increasing fertility and leading to a larger percentage of fry hatching at the same time (Dabrowski et al. 1996; Hart et al. 2006). The eggs are incubated indoors on coated wire mesh in troughs with a constant water flow (Hart et al. 2006), or more recently on Heath vertical incubation trays, until they hatch, and then the fry are stocked into ponds at 740,000 ha−1. Because of the gelatinous matrix surrounding the eggs, maintaining appropriate oxygen delivery to developing eggs during incubation (and removal of contaminants and waste) can be more difficult than for walleye and saugeye egg incubation. Any degradation of the matrix or excessive touching or clumping will decrease the hatch rate (Hart et al. 2006). While manual spawning is more complex than natural spawning, it is possible to assure fertilization of the eggs, control the genetic pairings of males and females, measure the numbers of fry stocked to each pond, and assure that all fry in a pond begin culture at the same size, all of which help maximize the number of fingerlings produced.
2.3 Timing: Pond Filling and Fry Stocking
Another ecological principle relevant to percid pond management is the match-mismatch hypothesis (Cushing 1969, 1990; Mertz and Myers 1994), which proposes that, even if both consumer (stocking rate) and producer (nutrients) controls are optimized, if fish consumptive demand and production of their prey are temporally misaligned, fish production will be reduced. In a pond aquaculture setting, managers can manipulate the relative timing between filling ponds with source water and stocking with fry, with the intention of increasing the match between prey production and fry consumptive demand. However, few studies have considered this as a management strategy (Culver et al. 1993). Under the presumption that first-feeding larval fish needed abundant zooplankton prey to survive (critical period hypothesis), percid ponds have been filled as much as 1 month prior to stocking with fish in hopes that the zooplankton forage base would build (Geiger et al. 1985). Ideally, this schedule would match the increase in zooplankton prey with the increasing consumptive demand of the growing percid juveniles. Instead of increasing zooplankton forage, zooplankton biomass likely peaked within the 4 weeks period, and zooplankton likely overgrazed the phytoplankton, and forced a low algae “clear-water phase,” followed by a period of low zooplankton abundance even before fish were stocked (Munch et al. 1984; Culver 1988). Culver et al. (1993) tested the hypothesis that a short lag time (<1 week) aligns plankton dynamics with larval fish consumptive demand for a sustained period (up to 6 weeks). They found that fish predation controlled or removed large, efficient algal grazers (e.g. Daphnia), thus reducing grazing on algae and releasing small-bodied zooplankton (e.g., Bosmina) from competition, thus supplying larval fish with abundant prey. However, because the initial inocula of zooplankton from source waters likely vary with trophic status, we also tested whether a slightly longer (2 weeks) lag time for Senecaville SFH, whose source water is mesotrophic (Filbrun et al. 2008), would result in improved fish growth and production by better matching the peak in zooplankton abundance with larval percid consumption (Briland 2010).
The time-lag experiment revealed no improvement for walleye and saugeye production or growth, and both filling treatments provided similar and adequate amounts of preferred zooplankton prey (i.e., Daphnia spp. and adult stage copepods) during the early weeks of culture (Fig. 18.2; Briland 2010). In fact the zooplankton progression in both filling treatments followed the pattern theorized by Culver et al. (1993), whereby Daphnia spp. are replaced by abundant Bosmina due to predation. Thus, we concluded that while the normal (<7 days) filling and stocking schedule is appropriate for this hatchery, pond-filling schedules remain an effective pond management tool to manipulate zooplankton population dynamics, and may be particularly appropriate for ponds filled from wells or those located at higher latitudes, where management protocols call for a 2 weeks lag time before stocking with walleye fry (e.g., Flowers 1996). Further study should address the impact of initial zooplankton composition and the temperature and filling time effect on diapausing egg development to further study the role of abiotic variables in controlling the zooplankton production that is so important to planktivorous fish production.
Comparison of seasonal variation of crustacean zooplankton biomass (dry mass; μg·L−1) between early and normal pond filling and stocking schedules (early fill; 2 weeks prior to stocking with percid fry; normal fill less than 1 week prior to stocking) at Senecaville State Fish Hatchery, Ohio, 2007. Values for each date represent the mean for three replicate ponds with the same fish species (saugeye or walleye)
2.4 Fry Stocking Densities: Trade-Off of Fingerling Size Versus Numbers at Harvest
Hatchery managers can manipulate the size and number of fish harvested by altering initial stocking density in culture ponds (Fig. 18.3). Previous studies have shown that a trade-off often exists between size of fish at harvest and the number of fish produced (Fig. 18.4; Fox and Flowers 1990; Qin et al. 1994). This interaction may occur due to competition for limited food resources; however, it also has been attributed to heightened activity levels of larvae stocked at high density relative to those in low density ponds (Fox and Flowers 1990). Although fish growth can be density-dependent, a threshold density appears to exist at which fish mass at harvest no longer declines (Tew 2003), such that the overall yield of fish biomass increases with increasing stocking density (Qin et al. 1994; Culver and Wu 1997). This density-dependent growth and pond yield relationship is likely mediated by the productivity and forage base of the system. Nutrient-poor environments, where prey abundance is low, appear more sensitive to predation pressure (Dettmers and Stein 1992), and competition for resources can lead to density-dependent growth (Jenkins et al. 1991). In other studies, increased fish biomass was found to increase turnover rates of zooplankton biomass (Culver et al. 1992). For example, in ponds with high fish densities, intense predation pressure on large, filter-feeding Cladocera (e.g., Daphnia) reduced competition with small-bodied zooplankton (e.g., Bosmina). This competitive release allowed an increase in the secondary productivity of Bosmina (DeMott 1989), ultimately resulting in abundant small-bodied prey for percid consumption (Qin et al. 1994).
Briland (2010) tested how altering the saugeye fry stocking rate from a traditional rate of about 35 fry·m−3 (data not shown) to a lower (20 fry·m−3) or higher (50 fry·m−3) rate influences the fish predation pressure on zooplankton prey resources. In her study, percid growth and size at harvest was highest at the low stocking rate (20 fry·m−3), and lower at the higher stocking rates due to insufficient prey resources (Fig. 18.5). In fact, fish in ponds stocked with the highest rate of saugeye fry (50 fry·m−3) reduced zooplankton prey resources during the second week of culture and induced a premature diet shift to chironomid larvae prey, which were in turn exhausted, resulting in a switch back to zooplankton (primarily immature cyclopoids) during the final weeks of culture (Fig. 18.6). Further, the lower stocking density treatments allowed fish to achieve high growth toward the end of the culture period when incorporating chironomid prey in their diets; conversely, the high density fish had lower growth when they began eating chironomid prey. Likely, young fish lacked the requisite digestive enzymes to handle chironomids, or the cost of digestion (due to relative size ratio of prey to predator) was higher than for larger fish found later in the culture period, accounting for the poorer growth in the high density fish.
Comparison of saugeye predation rates on zooplankton and chironomid prey across three fry stocking rates (20, 35, and 50 fry·m−3) at Senecaville State Fish Hatchery during 2008, simulated with a bioenergetics model calibrated for larval walleye. Values on each date represent the mean from two replicate ponds for each treatment. Long dash and dot lines (─·─) indicate boundaries of the periods used for statistical analysis
Between 1977–1984 and 1996–2005, it was not possible to determine yellow perch fingerling survival because allowing the adults to spawn on trees in the ponds prevented our knowing the initial number of fry in the ponds. However, between 2006 and 2009, survival was variable between ponds and between years. Individual pond survival rates ranged from 7 % to 67 %, while average yearly survival was between 20 % and 46 %. Despite this variable survival in the yellow perch ponds, we have seen a higher density of fingerlings at harvest (Figs. 18.7 and 18.8) with the current production methods, albeit at a smaller size, comparable to walleye and saugeye production.
A comparison of numbers of yellow perch harvested and their mean individual weights at harvest for the periods (1977–1984, natural spawning, both inorganic and organic pond fertilization, and incubation periods >90 days) and current production methods (1996–2009, natural spawning from 1996 to 2005 and manual spawning from 2006 to 2009, and liquid inorganic pond fertilization to target N and P concentrations, and incubation periods <90 days)
A comparison of yellow perch yield vs. harvest density from St. Mary’s hatchery for 1977–1984 (filled triangles; natural spawning, both inorganic and organic pond fertilization, and incubation periods >90 days) with those from 1996 to 2009 (open circles; natural spawning from 1996 to 2005 and manual spawning from 2006 to 2009, and liquid inorganic pond fertilization to target N and P concentrations, and incubation periods <90 days). Dashed lines represent the breakpoint relationship between fish harvest densities (fish·m−2) and individual fish biomass
We (Doyle and Culver, ms in prep) analyzed historic yellow perch production from St. Marys SFH to determine how the change to the new fertilization methods and the duration that fingerlings spent in the ponds affected production. As previously mentioned, between 1977 and 1985, the hatchery fertilized ponds with both inorganic and organic fertilizer and broodstock were stocked in ponds in March to spawn naturally, and the fish were harvested in September/October (duration >90 days). From 1995 to 2009, the new fertilization regimen was adopted and fish were harvested in May/June (duration <90 days). For fish reared for >90 days, there was a significant breakpoint in the relationship between fish harvest m−2 and individual fish size. At harvest densities of >10 fish m−2, fish were always less than 2 g (Garvey et al. 1998; 2DKS test, DBKS = 0.25, P < 0.0001). However, for fish reared in ponds <90 days, at harvest densities >20 fish m−2, fish were less than 0.4 g (DBKS = 0.12, P < 0.0001) (Fig. 18.8). Starting in 2006, fry were produced by manual spawning and known numbers of fry were stocked into ponds. We found no differences between natural spawning vs. manual spawning from 1996 to 2009 for the number of fingerlings harvested or the density at harvest (fish m−2), but stocking fry produced larger fish (average total length and average weight) and greater yield (kg ha−1).
3 Relevant Biology and Ecology
3.1 Larval Through Fingerling Development
Young percids experience rapid development during approximately 4 weeks of culture in hatchery ponds. The early stages of walleye development are described by Mathias and Li (1982) and are differentiated by notable changes in body structure relative to body length and days post hatch (dph). Walleye fry total length (TL) at hatch has a relatively broad range of 4.8–9.0 mm, but this may be an artifact of inconsistent methods across studies (Summerfelt et al. 2011). Upon hatching, walleye fry are considered in the prolarval stage while the yolk sac is still present, lasting through approximately 5 dph and about 10.0 mm TL (Li and Mathias 1982). Mouth opening and even exogenous feeding can occur during the prolarval stage at around 3 dph, although the young fry is not yet nutritionally dependent on capturing prey (Summerfelt et al. 2011). Walleye and saugeye fry are typically stocked into culture ponds during the prolarval stage at 3 or 4 dph. Within a few days of pond culture young percids advance to postlarva I, after the yolk sac is absorbed, but an oil globule is still present (10–13.2 mm TL and 5–10 dph) (Li and Mathias 1982). Walleye and saugeye achieved approximately 10 mm TL after only 1 week of culture in hatchery ponds (Fig. 18.9). Diet analysis of larval saugeye captured during the postlarval I stage, after only 4–7 days in a culture pond, show active feeding on large zooplankton species including Daphnia (Briland 2010), although the fish are not yet dependent on external food sources. Tank-studies of walleye show the highest mortality occurs during this stage and is associated with exogenous feeding, leading the authors to suggest an optimum food density of 100 daphnids per liter during postlarva I stage (Li and Mathias 1982). However, percid culture ponds do not achieve this density ranging 1–3 daphnids per liter (density is consistent with biomass shown in Fig. 18.2), and still high survival (83–95 %) was realized (Briland 2010), indicating that access to prey items during initial feeding (i.e., critical period) does not determine survival or production success.
By the second week of culture, percids advance to postlarva II stage once the oil globule is absorbed and fish are completely dependent on exogenous feeding (Summerfelt et al. 2011). The juvenile stage follows at around 14 dph and when fish reach 16 mm TL and substantial external and internal development occurs, including fin and ray growth, scales and pigmentation, as well as gastrointestinal development (Summerfelt et al. 2011). Nunn et al. (2012) reviewed the foraging ecology of larval and juvenile fishes, and noted that improvements in vision and swimming ability, and increases in mouth gape and the digestive capability of the alimentary tract often lead to changes in diet, requiring changes in pond management.
Herein, we extend larval stages designated for walleye to saugeye, without accounting for differences in growth and/or development between the parent and hybrid species; however, we note that saugeye growth (length and weight) exceeded that of walleye after only 10 days of culture in ponds (Fig. 18.9), indicating their growth and development may differ. Compared with walleye and saugeye culture of 4–6 weeks, yellow-perch culture is extended for several months, and the highest growth rate occurs during the first week of culture (Fig. 18.10). Yellow perch require more time, 8–10 weeks, to achieve 30 mm TL (Fig. 18.10), which saugeye reach at only 4 weeks (Fig. 18.9). Due to the higher trophic status of walleye and saugeye as piscivores compared with the planktivorous-benthivorous yellow perch, yellow perch are sustained by natural prey in culture ponds and achieve positive growth for the majority of their 5 month culture period. In contrast, saugeye and walleye growth diminishes rapidly after the first 2 weeks of culture and in order to maintain positive growth abundant prey resources are required later in the culture period (Briland 2010). Thus, walleye and saugeye hatchery ponds must be managed to support fish growth for the duration of the culture period rather than being maximized at the time of fry stocking.
Our study comparing stocking rates of saugeye fry shows that at a low density of saugeye (20 fish m−3), the larval fish population followed optimal consumption of prey: first and increasing consumption of zooplankton prey (days 1–8), followed by a stable rate of zooplankton consumption and supplementing it with chironomid prey from the middle to the end of culture (Fig. 18.6). However, at a high density of saugeye (50 fish m−3), fish consumption exceeded zooplankton production by the second week of culture, forcing the postlarva II stage saugeye to prematurely switch to chironomid prey, and once those were depleted fish returned to a predominantly zooplankton diet (Fig. 18.6). Further, saugeye in the high density ponds did not experience higher growth generally associated with ontogenetic diet shift to larger prey, such as chironomids (Briland 2010). The lack of growth may be attributed to the underdeveloped gastrointestinal tract of the young fish which lacks digestive enzymes required to break down chironomids (Dabrowski and Culver 1991) or to other digestive inefficiency, such as a high energetic cost considering the large prey size relative to that of the predator. Although the mode of action needs further investigation, it is clear that adequate zooplankton prey is prerequisite to desired growth and development for walleye, saugeye, and yellow perch culture.
3.2 Seasonal Variation in Zooplankton Species, Size, and Prey Preferences
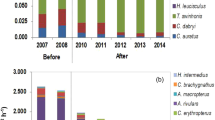
A typical pattern of seasonal zooplankton dynamics in ponds fertilized to maintain 30 μg PO4·P·L−1 shows a peak of cladocerans and copepods in the first 2 weeks of culture followed by their decline and an increase in rotifers, nauplii, and benthic cladocerans from then on. This pattern was observed at Hebron hatchery in 2001 in which the major zooplankton taxa present in saugeye ponds were cladocerans (Bosmina spp., Chydorus sp.), copepods (Acanthocyclops vernalis, Diacyclops thomasi, nauplii), and rotifers (Asplanchna sp., Brachionus sp., Keratella spp.) (Tew et al. 2006) (Fig. 18.11). The early decline in zooplankton may be the result of the early collapse of phytoplankton from high densities of filter-feeding zooplankton (Munch et al. 1984; Culver 1988). This effectively ensures large-bodied cladocerans are not as abundant as smaller cladocerans and copepods in the ponds before larval saugeye increase their consumption of prey (Qin and Culver 1992).
In 2001, we found that larval saugeye avoided rotifers and nauplii throughout the culture season (Fig. 18.12). Saugeye consumed primarily cyclopoid copepods selected during the first 2 weeks of the culture period, then their consumption declined in favor of Bosmina and benthic cladocerans such as chydorids and Simocephalus spp. in the last 2 weeks of culture. Although Bosmina was the most abundant zooplankter comprising the zooplankton biomass, saugeye exhibited a negative selection for them throughout the culture period (Fig. 18.12). Larval saugeye first consumed chironomids on the first date we collected fish (17 April) and they were present in their diets throughout the production season, although saugeye incorporated more benthic taxa in May (e.g., chironomid pupae, harpacticoids, ostracods, nematodes, etc.).
Seasonal variation in saugeye dietary preferences for eight zooplankton taxa based on stomach analyses and net samples (Fig. 18.9) from 6 ponds at the Hebron SFH, 2001. 30 μgP/L fertilizer regimen (17 April–11 May) Rotifers were primarily Keratella. Cyclopoids include primarily Acanthocyclops vernalis, plus Diacyclops thomasi, Mesocyclops edax, Macrocyclops albidus, and unidentified spp. Calanoids were primarily Skistodiaptomus oregonensis. Daphnia included mostly D. galeata mendotae and D. retrocurva. Other cladocerans include Alona spp., Simocephalus spp., Ceriodaphnia, Scapholeberis, and Diaphanosoma. Saugeye consumed chironomids on the first date we collected fish (17 April) and were present in the diets throughout the production season
Tandem production of walleye and saugeye reveals that the two species exhibit similar preferences for food items (Fig. 18.13). Within only 4 weeks of culture, both percid species transition from feeding on a variety of planktonic and benthic prey items. Initially, both fish show a strong preference for large zooplankters, Daphnia spp. and copepods (stage V and VI copepodites). Small prey items were avoided entirely by walleye and saugeye with occurrences in fish diets of 0 % for copepod nauplii and 1 % for rotifers (Briland 2010). As the large, preferred prey decline in abundance during weeks 2–3, smaller zooplankters come to comprise the bulk of the diet (Briland 2010) but are not selected for disproportionate to their abundance in the pond. Near the end of the culture period (weeks 3–4), benthic cladocerans (i.e., Chydorus spp., Simocephalus spp., and Alona spp.) are favored, but a variety of zooplankton (Bosmina and copepods) and an increasing proportion of chironomids comprise walleye and saugeye diets. The strong preference by walleye and saugeye for large zooplankton prey during their initial week in the culture ponds indicates their gape size provides no limitation to the types of prey they consume.
Percid electivity of zooplankton prey items cultured at Senecaville SFH during 2007. Walleye are indicated by open square icons, and saugeye are indicated by closed circles. Data points represent mean from six ponds per fish species, and error bars indicate pooled standard error; approximately 10 fish per pond were analyzed on each sampling data (Total fish diets analyzed: Walleye = 200, Saugeye = 196)
The zooplankton biomass was dominated by small cladocerans from the start of production at Hebron SFH, with source water from a highly eutrophic reservoir dominated by filamentous blue-green algae (Oscillatoria spp.). Large cladocerans, such as Daphnia spp., do not thrive in systems with a high abundance of Oscillatoria spp. (Infante and Abella 1985). Senecaville SFH, with a mesotrophic water source, on the other hand, had Daphnia spp. present in the forage base in the beginning of the culture period, allowing for the strong selection for Daphnia spp. in the diets. Yet, saugeye selected benthic cladocerans the last 2 weeks of the culture period in both studies and also incorporated chironomids in increasing proportions. Their diets are similar, and if the source water and forage base were more similar, then the diets for both hatcheries would probably mirror one another.
It is not exclusively the fish taxonomic and size preferences which determine the dietary ontogeny of percids. For example, percid habitat use and foraging behavior are related to the structure and development of the eye (Ali and Anctil 1977). Ali et al. (1977) described how differences in photoreceptor structure and organization between Perca spp. and Sander spp. correspond to differences in their feeding habits: (1) yellow perch have relatively large and evenly distributed rods, but moderate-sized cones with smooth ellipsoids, whereas walleye and sauger have rods that are extremely small and are “distributed in groups between the processes of the pigment epithelium” and bulky cones with granular ellipsoids; (2) walleye and sauger retinomotor responses involve only the rods and pigment epithelium, but for yellow perch, the cones also contribute; and (3) yellow perch do not have a tapetum lucidum in the pigment epithelium as walleye and sauger have, but instead have a large concentration of melanin. The adaptations to the retina of walleye and sauger allow for increased scotopic sensitivity, or the ability to function in low light conditions (such as increased activity at night, feeding at dusk and dawn, and a preference for turbid waters). Braekevelt et al. (1989) determined that the tapetum lucidum did not begin to appear in the retina of walleye until 30 mm TL and it was fully developed by the time they reached 125–140 mm TL. Initially when no tapetum lucidum is present, larval walleye are positively phototactic, but as the tapetum becomes fully formed, walleye become negatively phototactic. Yellow perch, on the other hand, have a retina specialized for photopic conditions (Richmond et al. 2004), preferring clear waters and diurnal activity. In contrast, Guma’a (1982) found that larval Eurasian perch (<10 mm TL) retina consisted of pure cones and mostly of the twin type. Rods developed at 100 mm TL, when the fish became demersal. Visual acuity was dependent on the focal length of the lens, rather than the number of cones in the retina.
Loew and Wahl (1991) determined that the small, single cones at the corner of the square retinal cone mosaic are present in juvenile yellow perch up to 40 mm standard length (SL), and have a short wavelength absorbance maximum in the 400 nm (UV) region. Loew and Wahl propose these cones could be advantageous for juvenile yellow perch feeding in surface waters where the cones enhance the contrast between their planktonic prey and its background. The cones disappear when the juveniles become demersal (30–40 mm SL) and are completely absent in adults (>100 mm SL). Loew et al. (1993) determined that juvenile yellow perch obtain enough information from the near-UV receptors to identify and attack prey, whereas walleye do not have them (Wahl 1989, cited in Loew and Wahl 1991).
Wahl et al. (1993) found that juvenile yellow perch in Oneida Lake were eating smaller prey items than what was typically available in the lake and that as yellow perch grew, the average size of prey increased as well, although their preferred prey choice was not constrained by their gape. Prey detection distance increased as visual acuity increased, from <20 cm for fish <30 mm SL to 45 cm for 50 mm fish. They also found that cone morphology changed and lens diameter increased as the body length increased, corresponding to an increase in visual acuity and a movement from pelagic to demersal habitat around 24–31 mm SL, when their visual acuity is approaching adult values. Mills et al. (1984) found from field and laboratory experiments that yellow perch did not select Daphnia until the fish moved to the demersal habitat at about 25–30 mm TL, and that smaller prey items were preferred. Reaction distance was greater for Daphnia than calanoid copepods and increased as the size of the Daphnia increased. Miller et al. (1993) found a strong size-dependent relationship for visual acuity and that behavioral estimates underestimate the ability of fish to spot prey.
3.3 Gape Limitation
On the other hand, yellow perch are initially gape limited (Bremigan et al. 2003) and feed primarily on rotifers and nauplii the first few weeks in the pond (Fig. 18.14). We found that when ponds typically exhibit the “clear water” phase and the crustacean zooplankton crashes, yellow perch are in the process of incorporating larger zooplankters, such as Acanthocyclops vernalis, Skistodiaptomus oregonensis, and smaller cladocerans (Bosmina longirostris, Alona spp., Diaphanosoma birgei, and Daphnia parvula) into their diets (Fig. 18.14). Unlike walleye and saugeye, yellow perch did not incorporate benthic organisms, such as chironomids, into their diet until the end of May, and chironomids were never a major component of their diet. We also found that yellow perch exhibited slow growth during the period of low crustacean biomass in the ponds (Figs. 18.10 and 18.14) and many fish consumed primarily rotifers in the first few weeks of June. The growth pattern we observed is similar to that of yellow perch produced in Wisconsin (Hartleb 2003) and the Eurasian perch (Cuvier-Péres and Kestemont 2002). When crustacean zooplankton densities started to recover by the end of July and into August, cladocerans became the preferred prey (Fig. 18.14), which seems to correspond to increases in fish total length and weight (Fig. 18.10).
A comparison of zooplankton biomass (dry mg L−1) in two yellow perch ponds from April to September 1991 (note the different y-axis scales) and the electivity (Chesson’s ε) for prey found in yellow perch diets. Crustacean biomass declined the second week of May, corresponding to a low algae “clear water” phase about 4 weeks after egg skeins were seen in the ponds. Cladocerans include Bosmina longirostris, Alona spp., Diaphanosoma birgei, Daphnia parvula, and unidentified cladocerans. Copepods included cyclopoid and calanoid nauplii, and Acanthocyclops vernalis and Skistodiaptomus oregonensis copepodites
Graeb et al. (2005) found that gape width increased more quickly with size in larval walleye than larval yellow perch which allows walleye to switch to larger prey, such as other fish, sooner than yellow perch. Walleye also exhibits higher growth rates when feeding on fish compared to yellow perch (Graeb et al. 2005). Although both walleye and yellow perch captured small prey (zooplankton) with high efficiency, walleye (20 mm) were more efficient at capturing large prey (Graeb et al. 2005), further illustrating differences between these fish. Yellow perch consumed more zooplankton, while consumption of benthic organisms and fish were similar at larger sizes (Graeb et al. 2005). It would not be a selective advantage for yellow perch to feed on their conspecifics unless there were no other prey items available. We have not seen any evidence of cannibalism in the diets of yellow perch, saugeye, or walleye collected from hatchery ponds.
3.4 Cannibalism
Much of the current information regarding cannibalism has come from studies involving cultured fish that are used to enhance populations of sportfish species by rearing and stocking their larvae and/or fingerlings into lakes (Smith and Reay 1991; Baras and Jobling 2002; Loadman et al. 1986; van Kooten et al. 2010). Smith and Reay (1991) list seven percid species that are cannibalistic., four of which, walleye, yellow perch, pikeperch (Sander lucioperca), and Eurasian perch (Perca fluviatilis) are important species for culture as either food fish and sportfish (Malison and Held 1992; Loadman et al. 1986). Studies of cannibalism in culture typically involve observations of behaviors under intensive culture conditions in tanks for fish that are eventually habituated to formulated feed under controlled conditions in which they cannot escape predation. This intracohort cannibalism occurs during larval and juvenile stages of development (Smith and Reay 1991; Baras and Jobling 2002; Mandiki et al. 2007; Cuff 1977, 1980; Loadman et al. 1986).
Baras and Jobling (2002) suggest that Type I cannibalism (where the size of the prey is large relative to the size of the predator) occurs in early larval development. The predator ingests the prey tail first up to its head, which is usually larger than its body, and the head is eventually discarded (Cuff 1980). Type II cannibalism (where the predator is larger than the prey) occurs more often among cohorts in which a few individuals grow faster than others enabling them to completely swallow a smaller individual, and is well documented in intensive culture of walleye (Loadman et al. 1986; Li and Mathias 1982; Cuff 1977, 1980), yellow perch (Malison and Held 1992), and Eurasian perch (Mandiki et al. 2007; Kestemont et al. 2003; Baras et al. 2003). The major causes of intracohort cannibalism in culture have been linked to size at hatching (or differential/asynchronous egg hatching), low stocking densities (creating size heterogeneity), limiting food resources/or food availability, lack of refuges in order to escape predation, and limited lighting conditions above or in tanks (Smith and Reay 1991; Baras et al. 2003; Loadman et al. 1986; Cuff 1977, 1980; Colesante 1989; van Kooten et al. 2010).
Many of the studies describing observed cannibalism use a high density of fish (ranging from 1 to 100 fish L−1) in relatively small glass aquaria (from 10 to 200 L) and fish are deprived of food (Loadman et al. 1986; Li and Mathias 1982; Cuff 1977, 1980; Malison and Held 1992; Kestemont et al. 2003). For example, Li and Mathias (1982) examined the effects of fish density (1, 10, and 100 fish L−1 in 30 L aquaria) on survival and found that the rate of cannibalistic attacks increased as fish density increased and also observed delayed feeding increased cannibalistic attacks, yet once the fish developed better avoidance capabilities, cannibalism ceased. Kestemont et al. (2003) also found that perch (Perca fluviatilis) post-larvae stage was impacted by cannibalism the greatest at densities of 10 fish L−1, yet cannibalism was inversely proportional to stocking density in the larval stage (greater impact at 10 fish L−1 than 100 fish L−1). We have found no incidences of cannibalism in over 5700 percid stomach analyses (4774 saugeye, 782 walleye, and 208 yellow perch). This lack of verifiable cannibalism in pond culture may be due to the low density of fish in ponds (0.001–0.006 fish L−1) and the inability to observe fish behavior in the hatchery ponds.
3.5 Trade-Offs for Sequential Culture in Earthen Ponds
Survival of larval percids can vary from year to year and pond to pond within years for a variety of reasons such as poor water quality (low DO), inadequate forage base, fish density, or the possibility of cannibalism. An additional area of concern, however, specifically for percid culture in Ohio, is the history of pond use in the sequential culture of catfish with saugeye and walleye (Tew et al. 2010). Fertilization history may affect the accumulation in pond sediments of nitrogen and phosphorus, and detritus from decomposing algae, as well as chemicals from algaecides such as copper sulfate (CuSO4) (Knud-Hansen 1992; McNevin and Boyd 2004; Tew et al. 2010).
Fertilization of ponds is a useful management strategy to optimize and manage edible algae leading to increased percid production (Qin et al. 1995). Knud-Hansen (1992) found that nutrients applied to ponds remained available for use 2 years after initial application. Phosphorus was particularly enhanced by oxygen concentration (low) and pH in the adjacent water layer. However, Boyd and Musig (1981) suggest that using a liquid fertilizer applied in small amounts more frequently would result in greater absorption by the phytoplankton community rather than a large addition of granules which would most likely be absorbed by the sediments, decreasing the ability of sediments to remove inorganic phosphorus from the water column. In addition, catfish are supplementarily fed a dry feed pellet with a high phosphorus content (TP ≈ 300 μg P/g dry food) (Tew 2003). According to Edwards (1993), exposing the sediments to the air mineralizes the sediments and releases the nutrients tied up in the soils, whereby they become available for use when ponds are re-filled.
Copper sulfate is an algaecide used to reduce off-flavor in channel catfish produced in ponds as well as to reduce blue-green algae and the treatment of Icthyophthirus multifiliis infestations during the culture period (Schlenk et al. 1998; McNevin and Boyd 2004). Copper sulfate readily absorbs into the sediment at the bottom of ponds and is concentrated in the top 6 cm of the soil, creating the potential for resuspension at the sediment-water interface (McNevin and Boyd 2004).
Tew et al. (2010) tested whether percid production was affected by the accumulation of phosphorus and copper in the sediments of ponds used for culturing catfish by comparing survival, growth and yield of percids in ponds that were single-cropped (SS ponds) with only saugeye produced in the spring for the previous two successive years with percids that were double-cropped (DD ponds) with channel catfish produced in the summer (Fig. 18.15). They found that a significant amount of copper remained in the sediment after the ponds were drained (on average 2.5–5.6 times higher) in DD ponds, while average phosphorus was consistently higher in the SS ponds for both soluble reactive phosphorus (SRP) and total phosphorus (TP), possibly due to the removal of fertile organic matter with an additional draining per year of the DD ponds. Although sediment SRP and TP concentrations were higher in SS ponds, there were no differences in total phytoplankton biomass, possibly due to the weekly addition of fertilizer as well as the growth of benthic filamentous algae and vascular plants in the catfish ponds. However, there was a significantly higher total zooplankton biomass in SS ponds than DD ponds throughout the season (Fig. 18.16). This difference was not related to the phytoplankton biomass, suggesting there could be a negative correlation with copper concentration, as other studies have shown that waterborne copper can reduce zooplankton abundance and fecundity.
A comparison of double cropped (DD) and single cropped (SS) ponds in a 3-year sequence at Hebron State Fish Hatchery, OH. SS ponds are used for growing percids (P) only, and are left dry in between growing seasons; while DD ponds are used for growing percids between April and mid-May, drained, and then refilled in mid-July for growing channel catfish (CCF) until late September. The open space represents the period in which the pond is empty
These findings are consistent with the fish production results, in which there was a negative correlation between growth (TL), survival (%), and total yield (kg) in DD ponds relative to SS ponds (Tew et al. 2010). They also found that saugeye total length (TL) and wet weight were significantly higher in SS ponds throughout the 2002 growing season (P < 0.05, one-way repeated-measures ANOVA) with an average TL at harvest of 29.7 mm in SS ponds compared with 22.7 mm in DD ponds, and average wet weights of 173.4 mg and 81.4 mg in SS and DD ponds, respectively. Analyzing 22 ponds treated with copper sulfate from 1994 to 2001 revealed a negative correlation between survival (%) and yield (kg/ha) of percids harvested in 2001 with the cumulative amount of copper applied to the ponds over the previous 7 years (Fig. 18.17).
Thus, the accumulation of sediment copper through repeated use of copper sulfate applications during catfish production has a negative effect on percid survival/production the following spring in the same pond indirectly through food web dynamics or directly on fish via chronic exposure. Using hatchery ponds for sequential culture of fish may increase the total number of species produced during the production year, but it may not maximize the production of all species, especially double-cropping percids with catfish. Single-cropped ponds may maximize percid production while mitigating the carryover effects of double-cropping.
4 Conclusion/Additional Considerations (Non-manageable)
Percid production in hatchery ponds is dictated by external forces beyond hatchery management such as traditional abiotic factors (i.e., temperature and weather) or land management practices that eutrophicate the hatchery’s source water. Herein, we show that zooplankton composition differs between hatcheries due to the trophic status of source water, with highly eutrophic waters favoring small bodied zooplankton due to the predominance of cyanobacteria species. Further, we recommend that pond management practices be specific to the hatchery and source water’s trophic status. Specifically, a hatchery with eutrophic source water should be fertilized with the minimum necessary phosphate-phosphorus to promote adequate algal abundance (i.e., 10 μg PO4-P·L−1) while minimizing the risk of poor water quality. Alternatively, given a mesotrophic water source with phytoplankton dominated by non-cyanobacterial algae and an inoculum of low zooplankton abundance, we recommend that the hatchery ponds may benefit from a higher fertilization rate of 30 μg PO4-P·L−1 without risking a negative effect from reduced water quality.
Beyond manipulatable variables, temperature and weather set the stage for good growth of cultured fish and ultimate successful fish production. During successive years of studying saugeye and walleye growth, we have seen a vast difference in temperature regime between years (Fig. 18.18) and that springtime temperatures generally fall below the optimal range for larval walleye growth in Ohio (Madon and Culver 1993). We estimated the effect of sub-optimal temperature on fish growth via a bioenergetics model for larval walleye (Madon and Culver 1993), and found that the annual temperature regime that yielded the highest growth occurred in 2007 and fish growth was reduced by 8 % when substituting temperatures from 2008, but growth was drastically reduced, by 43 %, with the cold temperature regime of 2005 (Fig. 18.18). However, temperature also reduces zooplankton production during the cooler years by increasing development time of cladoceran eggs and instar stages as well as copepod nauplii and copepodite stages. Cooler springs are generally accompanied by adverse weather such that heavy, prolonged cloud cover limits phytoplankton production, causing further limitation to zooplankton and, ultimately, fish production. Thus, despite the best efforts of hatchery managers, ponds are still subject to annual variability. However, given the ecological tools at hand (i.e., bioenergetics models, zooplankton production, and stocking records), we suggest that hatchery managers can quantify the impact of temperature relative to production goals, and allow for long-term management of the ponds and overall production while testing appropriate management action to improve our understanding of these systems and adjust management practices to maintain consistent production.
References
Ali MA, Anctil M (1977) Retinal structure and function in the walleye (Stizostedion vitreum vitreum) and Sauger (S. canadense). J Fish Res Board Can 34:1467–1474
Ali MA, Ryder RA, Anctil M (1977) Photoreceptors and visual pigments as related to behavioral responses and preferred habitats of Perches (Perca spp.) and Pikeperches (Stizostedion spp.). J Fish Res Board Can 34:1475–1480
Baras E, Jobling M (2002) Dynamics of intracohort cannibalism in cultured fish. Aquac Res 33:461–479
Baras E, Kestemont P, Mélard C (2003) Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 219:241–255
Boyd CE, Musig Y (1981) Orthophosphate uptake by phytoplankton and sediment. Aquaculture 22:165–173
Braekevelt CR, McIntyre DB, Ward FJ (1989) Development of the retinal tapetum lucidum of the walleye (Stizostedion vitreum vitreum). Histol Histopathol 4:63–70
Bremigan MT, Dettmers JM, Mahan AL (2003) Zooplankton selectivity by larval yellow perch in Green Bay, Lake Michigan. J Great Lakes Res 29:501–510
Briland RD (2010) Optimizing larval fish survival and growth through an analysis of consumer and resource interactions in percid culture ponds. M.S. thesis, The Ohio State University
Colesante RT (1989) Improved survival of walleye fry during the first 30 days of intensive rearing on brine shrimp and zooplankton. Prog Fish Cult 51:109–111
Craig JF (1987) The biology of perch and related fish. Timber Press, Portland
Cuff WR (1977) Initiation and control of cannibalism in larval walleye. Prog Fish Cult 39:29–32
Cuff WV (1980) Behavioral aspects of cannibalism in larval walleye, Stizostedion vitreum. Can J Zool 58:1504–1507
Culver DA (1988) Plankton ecology in fish hatchery ponds in Narrandera, NSW, Australia. Verh Int Ver Theor Angew Limnol 23:1085–1089
Culver DA (1991) Effects of the N:P ratio in fertilizer for fish hatchery ponds. Verh Int Ver Theor Angew Limnol 24:1503–1507
Culver DA (1996) Fertilization procedures for pond culture of walleye and saugeye. In: Summerfelt RC (ed) Walleye culture manual. North Central Regional Aquaculture Center Publications Office, Iowa State University, Ames, p. 115–122
Culver DA, Wu L (1997) Relative importance of predation and competition in the seasonal dynamics of zooplankton: results from larval fish rearing ponds. Arch Hydrobiol Spec Iss Adv Limnol 49:27–35
Culver DA, Qin J, Madon SP, Helal HA (1992) Daphnia production techniques for rearing fingerling walleye and saugeye. Federal aid in sport fish restoration, Project F-57-R, Final report. Ohio Department of Natural Resources, Columbus
Culver DA, Madon SP, Qin J (1993) Percid pond production techniques; timing, enrichment, and stocking density manipulation. J Appl Aquac 2:9–31
Cushing DH (1969) The regularity of the spawning season of some fishes. J Cons Int l’Explor de la Mer 33:81–92
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293
Cuvier-Péres A, Kestemont P (2002) Development of digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Dabrowski K, Ciereszko RE, Ciereszko A, Toth GP, Christ SA, El-Saidy D, Ottobre JS (1996) Reproductive physiology of yellow perch (Perca flavescens): environmental and endocrinological cues. J Appl Ichth 12:139–148
Dabrowski K, Culver DA, Brooks C, Voss A, Binkowski F, Yeo A, Balogun A (1992) Biochemical aspects of the early life history of yellow perch (Perca flavescens). In: Kaushik S, Luquet P (eds) Fish nutrition in practice, pp 531–539
DeMott WR (1989) The role of competition in zooplankton succession. In: Sommer U (ed) Plankton ecology: succession in plankton communities. Springer, New York
Dettmers JM, Stein RA (1992) Food consumption by larval gizzard shad: zooplankton effects and implications for reservoir communities. Trans Am Fish Soc 121:494–507
Edwards P (1993) Environmental issues in integrated agriculture-aquaculture wastewater-fed fish systems. In: Pullin RSV, Rosenthal H, Maclean JL (eds) Environmental and aquaculture in developing countries, vol 31, Proceedings of international center for living aquatic resources management conference. ICLARM, Manila, p 359
Ellison DG, Franzin WG (1992) Overview of the symposium on walleye stocks and stocking. N Am J Fish Manag 12:271–275
Fielder DG (1992) Evaluation of stocking walleye fry and fingerlings and factors affecting their success in lower Lake Oahe, South Dakota. N Am J Fish Manag 12:336–345
Filbrun JE, Culver DA, Briland RD, Doyle CM (2008) The quality of Ohio state fish hatcheries’ water supplies, 2005–2008. Federal aid in fish restoration project F-69-P, Interim report. Ohio Department of Natural Resources, Columbus
Flowers DD (1996) Fingerling production in drainable ponds at White Lake Fish Culture Station. In: Summerfelt RC (ed) Walleye culture manual. North Central Regional Aquaculture Center Publications Office, Iowa State University, Ames, p. 123–128
Fox MG (1989) Effect of prey density and size on growth and survival of juvenile walleye (Stizostedion vitreum vitreum). Can J Fish Aquat Sci 46:1323–1328
Fox MG, Flowers DD (1990) Effect of fish density on growth, survival, and food consumption by juvenile walleyes in rearing ponds. Trans Am Fish Soc 119:112–121
Garvey JE, Marschall EA, Wright RA (1998) From star charts to stoneflies: detecting relationships in continuous bivariate data. Ecology 79:442–447
Geiger JG, Turner CJ, Fitzmayer K, Nicols WC (1985) Feeding habits of larval and fingerling striped bass and zooplankton dynamics in fertilized rearing ponds. Prog Fish Cult 47:213–223
Graeb BDS, Galarowicz T, Wahl DH, JM D, Simpson MJ (2005) Foraging behavior, morphology, and life history variation determine the ontogeny of piscivory in two closely related predators. Can J Fish Aquat Sci 62:2010–2020
Guma’a SA (1982) Retinal development and retinomotor responses in perch, Perca fluviatilis L. J Fish Biol 20:611–618
Hart SD, Garling DL, Malison JA (2006) Yellow perch (Perca flavescens) culture guide. Iowa State University, Ames
Hartleb CF (2003) Food chain dynamics and diets of larval and post-larval yellow perch in culture ponds. In: Barry TP, Malison JA (eds) Proceedings of Percis III: the third international percid fish symposium, University of Wisconsin Sea Grant Institute, Madison
Hartleb CF, Johnson, JA, Held, JA (2012) Walleye and yellow perch pond fertilization. In: Mischke CC (ed) Aquaculture pond fertilization: impacts of nutrient input on production. John Wiley & Sons, Ames, Iowa. doi:10.1002/9781118329443.ch11
Helal H, Culver DA (1991) N:P ratio and plankton production in fish ponds. Verh Int Ver Theor Angew Limnol 24:1508–1511
Hokanson KEF (1977) Temperature requirements of some percids and adaptations to the seasonal temperature cycle. J Fish Res Board Can 34:1524–1550
Hushak LJ, Winslow JM, Dutta N (1988) Economic value of Great Lakes sportfishing: the case of private-boat fishing in Ohio’s Lake Erie. Trans Am Fish Soc 117:363–373
Infante A, Abella SEB (1985) Inhibition of Daphnia by Oscillatoria in Lake Washington. Limnol Oceanogr 30:1046–1052
Jacob AP, Culver DA (2010) Experimental evaluation of the impacts of reduced inorganic phosphorus fertilization rates on juvenile saugeye production. Aquaculture 304:22–33
Jenkins GP, Young W, Davis TLO (1991) Density-dependence of larval growth of a marine fish, the southern bluefin tuna, Thunnus maccoyi. Can J Fish Aquat Sci 48:1358–1363
Kestemont P, Mélard C (2000) Aquaculture. In: Craig JF (ed) Percid fishes: systematics, ecology, and exploitation. Blackwell Science, Oxford
Kestemont P, Jourdan S, Houbart M, Mélard C, Paspatis M, Fontaine P, Cuvier A, Kentouri M, Baras E (2003) Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: biotic and abiotic influences. Aquaculture 227:333–356
Knud-Hansen CF (1992) Pond history as a source of error in fish culture experiments: a quantitative assessment using covariate analysis. Aquaculture 105:21–36
Li S, Mathias JA (1982) Causes of high mortality among cultured larval walleyes. Trans Am Fish Soc 111:710–721
Loadman NL, Moodie GEE, Mathias JA (1986) Significance of cannibalism in larval walleye (Stizostedion vitreum). Can J Fish Aquat Sci 43:613–618
Loew ER, Wahl CM (1991) A short-wavelength sensitive cone mechanism in juvenile yellow perch, Perca flavescens. Vis Res 31:353–360
Loew ER, McFarland WN, Mills EL, Hunter D (1993) A chromatic action spectrum for planktonic predation by juvenile yellow perch, Perca flavescens. Can J Zool 71:384–386
Madon SP, Culver DA (1993) Bioenergetics model for larval and juvenile walleye: an in situ approach using experimental ponds. Trans Am Fish Soc 122:797–813
Malison JA, Held JA (1992) Effects of fish size at harvest, initial stocking density and tank lighting conditions on the habituation of pond-reared yellow perch (Perca flavescens) to intensive culture conditions. Aquaculture 104:67–78
Mandiki SNM, Babiak I, Krol J, Rasolo JFR, Kestemont P (2007) How initial predator-prey ratio affects intra-cohort cannibalism and growth in Eurasian perch Perca fluviatilis L larvae and juveniles under controlled conditions. Aquaculture 268:149–155
Mathias JA, Li S (1982) Feeding habits of walleye larvae and juveniles: comparative laboratory and field studies. Trans Am Fish Soc 111:722–735
Mathias JA, Franzin WG, Craig JF, Bablauk JA, Flannagan JF (1992) Evaluation of stocking walleye fry to enhance a commercial fishery in a large, Canadian prairie lake. N Am J Fish Manag 12:299–306
McNevin AA, Boyd CB (2004) Copper concentrations in channel catfish Ictalurus punctatus ponds treated with copper sulfate. J World Aquac Soc 35:16–24
McWilliams RH, Larscheid JG (1992) Assessment of walleye fry and fingerling stocking in the Okoboji Lake, Iowa. N Am J Fish Manag 12:329–335
Mertz G, Myers RA (1994) Match/mismatch predictions of spawning duration versus recruitment variability. Fish Oceanogr 3:236–245
Miller TJ, Crowder LB, Rice JA (1993) Ontogenetic changes in behavioural and histological measures of visual acuity in three species of fish. Environ Biol Fish 37:1–8
Mills EL, Confer JL, Ready RC (1984) Prey selection by young yellow perch: the influence of capture success, visual acuity, and prey choice. Trans Am Fish Soc 113:579–587
Mitzner L (2002) Effectiveness of walleye fry and fingerling stocking in Rathbun Lake, Iowa, 1990–1999. N Am J Fish Manag 22:1007–1013
Munch CS, Vaga RM, Culver DA (1984) Evidence for size-selective grazing of phytoplankton species by zooplankton in fish hatchery ponds. Verh Int Ver Theor Angew Limnol 22:1640–1644
Nunn AD, Tewson LH, Cowx IG (2012) The foraging ecology of larval and juvenile fishes. Rev Fish Biol Fish 22:377–408
Paragamian VL, Kingery R (1992) A comparison of walleye fry and fingerling stockings in three rivers in Iowa. N Am J Fish Manag 12:313–320
Qin J, Culver DA (1992) The survival and growth of larval walleye, Stizostedion vitreum, and trophic dynamics in fertilized ponds. Aquaculture 108:257–276
Qin J, Culver DA, Yu N (1994) Comparisons of larval walleye and saugeye (walleye x sauger hybrid) growth and impacts on zooplankton in experimental ponds. Prog Fish Cult 56:91–99
Qin J, Culver DA, Yu N (1995) Effect of organic fertilizer on heterotrophs and autotrophs: implications for water quality management. Aquac Res 26:911–920
Qin J, Madon SP, Culver DA (1995) Effects of larval walleye (Stizostedion vitreum) and fertilization on the plankton community: implications for larval fish culture. Aquaculture 130:51–65
Richmond HE, Hrabik TR, Mensinger AF (2004) Light intensity, prey detection and foraging mechanisms of age 0 year yellow perch. J Fish Biol 65:195–205
Schlenk D, Gollon JL, Griffin BR (1998) Efficacy of copper sulfate for the treatment of Ichthyophthiriasis in channel catfish. J Aquat Anim Health 10:390–396
Smith C, Reay P (1991) Cannibalism in teleost fish. Rev Fish Biol Fish 1:41–64
Soderberg RW, Kirby JM, Lunger D, Marcinko MT (1997) Comparison of organic and inorganic fertilizers for the pond production of walleye Stizostedion vitreum. J Appl Aquac 7:23–30
Soderberg RW, Kirby JM, Marcinko MT (2000) Lack of response of juvenile walleyes to increased levels of fertilization or liming in soft-water ponds. N Am J Aquac 62:26–32
Summerfelt RC, Johnson JA, Clouse CP (2011) Culture of walleye, sauger, and hybrid walleye. In: Barton BA (ed) Biology, management, and culture of walleye and sauger. American Fisheries Society, Maryland
Tew KS (2003) The impacts of algal control in catfish and percid aquaculture ponds. Dissertation, The Ohio State University
Tew KS, Conroy JD, Culver DA (2006) Effect of lowered phosphorus fertilization rates on pond production of percid fingerlings. Aquaculture 255:436–446
Tew KS, Conroy JD, Culver DA (2010) The importance of phosphorus and copper carryover in pond sediments for juvenile percid survival, growth, and yield. N Am J Aquac 72:22–33
Thorpe JE (1977) Morphology, physiology, behavior, and ecology of Perca fluviatilis and P flavescens Mitchill. J Fish Res Board Can 34:1504–1514
Tice BJ, Soderberg RJ, Kirby JM, Marcinko MT (1996) Growth and survival of walleyes reared in ponds fertilized with organic or inorganic materials. Prog Fish Cult 58:135–139
van Kooten T, Andersson J, Byström P, Persson L, de Roos AM (2010) Size at hatching determines population dynamics and response to harvesting in cannibalistic fish. Can J Fish Aquac Sci 67:401–416
Wahl CM, Mills EL, McFarland WN, DeGisi JS (1993) Ontogenetic changes in prey selection and visual acuity of the yellow perch, Perca flavescens. Can J Fish Aquac Sci 50:743–749
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Briland, R.D., Doyle, C.M., Culver, D.A. (2015). Large-Scale Production of Yellow Perch, Walleye, and Hybrid Walleye in Ponds. In: Kestemont, P., Dabrowski, K., Summerfelt, R. (eds) Biology and Culture of Percid Fishes. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7227-3_18
Download citation
DOI: https://doi.org/10.1007/978-94-017-7227-3_18
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7226-6
Online ISBN: 978-94-017-7227-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)