Abstract
Changes in digestive enzyme activity and histology were studied in Atractosteus tropicus embryos, larvae and juvenile periods. Alkaline protease, chymotrypsin, carboxypeptidase A, lipase and α-amylase were detected in all periods and gradually increased until reaching the maximum peak in juveniles; meanwhile, acid protease was first detected at 5 days after hatching (dah) when first feeding started and trypsin and leucine aminopeptidase activities were detected from 19 dah, their values being increased gradually until reaching a maximum value at 31 dah. Acid and alkaline phosphatase activities increased from yolk-sac absorption (3 dah) until day 31 after hatching. Zymogram for acid protease showed two bands in active forms (0.4 and 0.5 Rfs) from day 5 after hatching and a third protease form (0.3 Rf) that appears at 31 dah. Two active forms (26.3 and 24.9 kDa) were detected using SDS-PAGE alkaline proteases zymogram at 5 dah, and an additional active form (44.1 kDa) was detected at 7 dah. Regarding the histological development of the digestive system, the exocrine pancreas containing zymogen granules was already visible at 3 dah, whereas at 5 dah first gastric glands were already detected in the stomach. Between 7 and 9 dah, the digestive tract of A. tropicus resembled that of a juvenile specimen with a well-developed and short oesophagus, stomach divided into a glandular and non-glandular (pyloric) stomach, folded intestine with pyloric caeca and a well-developed spiral valve (posterior intestine). Considering this, larvae of A. tropicus are capable of digesting several foods from yolk absorption (3 dah), maximizing its activities at 15 dah, age at which the organisms maximize its capability to absorb nutrients from diets provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical gar Atractosteus tropicus is a highly appreciated species in the south-eastern region of Mexico. As such, there is progressive and dramatic decrease in wild populations due to overfishing and habitat loss. Consequently, this species has received a lot of attention during the last 20 years, achieving important goals in its captive culture, such as reproduction, larviculture using live preys and balanced feeds. Additionally, studies on establishment of the nutritional requirements in protein, energy and lipids for juveniles have been conducted (Márquez-Couturier et al. 2006). The important research effort focused on this species has allowed creating commercial farms for seed production and their grow-out in diverse aquacultural systems (Alvarez-González et al. 2007). Nevertheless, during larviculture mortalities have been observed which might be attributed to two main factors: firstly, when larvae are fed with live preys (Artemia nauplii) and secondly, when they are weaned on to compound diets (commercial trout feed, Silver Cup, Pedregal, Edo. Mex.). In addition, it has been considered that the feeds not necessarily possess the adequate nutrients for this species, which can cause an increase in the aggression between them and consequently the appearance of cannibalism. In this way, if we want to improve the survival during the larval stage, it is necessary to conduct specific studies, based on in digestive physiology of species, that allow for evaluation of the digestive enzymatic capacity of the larvae. Also this information let us to understand the basic aspects of the digestive physiology to improve their feeding, to develop suitable balanced feed and to determine the moment to carry out the substitution of the live preys with artificial diet (Moyano et al. 2005). In this aspect, some of the most important studies in relation to the development of different digestive enzymes during early ontogeny are separated in freshwater and marine studies such as Senegalese sole (Solea senegalensis), blue disc (Symphysodon aequifasciatus), white seabream (Diplodus sargus), Nile tilapia (Oreochromis niloticus), European perch (Perca fluviatilis), Japanese eel (Anguilla japonica), grass carp (Hypophthalmichthys molitrix), Atlantic salmon (Salmo salar) (Sciaenops ocellatus), spotted sand bass (Paralabrax maculatofasciatus) and red drum (Atractoscion nobilis) (Fernández-Díaz et al. 2001; Chong et al. 2002; Tengjaroenkul et al. 2002; Cuvier-Pérez and Kestemont 2002; Cara et al. 2003; Pedersen et al. 2003; Rina et al. 2005; Rungruangsak-Torrissen et al. 2006; Lazo et al. 2007; Álvarez-González et al. 2008, 2010; Galaviz et al. 2011). Most of these studies have shown the dependency of the digestive activity with the digestive tract maturation (relation between cytosolic and parental digestive enzymes) using diverse techniques that allow the identification of several isoforms which are related to the feeding habits, also to define the adequate weaning when larvae change to juvenile stage (Moyano et al. 1996). For Lepisosteidae family, these kinds of studies have been performed by Aguilera (1999), with alligator gar Atractosteus spatula, and Comabella et al. (2006), with Cuban gar Atractosteus tristoechus. The former authors determined that these species were capable of digesting many varieties of feed items from mouth opening (endo–exotrophic stage) when the digestive enzyme activities were completed. In this sense, the objective of the present study was to determine the digestive enzymatic activity development and morphological changes in the digestive system during the larval period of A. tropicus.
Materials and methods
Larviculture and sampling
For this study, a total of 450 embryos of A. tropicus were obtained from one induced spawn of a broodstock (one female of 3.5 kg and three males of 1.5 kg mean weight) using LHRHa (35 μg kg of fish−1), which were maintained in a 2000-L circular plastic tank (2 m diameter) in the Laboratorio de Acuicultura Tropical (DACBIOL-UJAT). After spawn (16 h post-hormonal induction), fish were retired from tank, maintaining the adhesive eggs inside the tank until their hatching (3 days post-fertilization at 29 °C; 0 days after spawning). Yolk-sac larvae were transferred into three 70-L plastic tanks (150 larvae per tank) connected to a recirculating system which has a 1500-L reservoir using as biological filter, also 3/4 HP centrifuge pump (Jacuzzi, JWPA5D-230A, Delavan WI, USA), one sand filter (STA-RITE, S166T, Delavan WI, USA) and two titanium heathers (PSA, R9CE371, Delavan WI, USA). System’s water quality was monitored daily during the 31 days of larviculture registering the temperature (29.0 ± 1.0 °C), dissolved oxygen (6.4 ± 0.5 mg L−1) and pH (6.7 ± 0.2) with an oxymeter (YSI 85, Ohio, USA) and a pH meter, respectively (HANNA HI 991001, Romania, Europe). After yolk absorption (2 days after hatching, dah), the larvae were transferred to a recirculating system to initiate the feeding phase, which was made five times per day (8:00, 11:00, 13:00, 15:00 and 18:00 h) initiating with Artemia nauplii (AN, 2–5 nauplii mL−1) from mouth opening until 17 dah, afterwards with trout feed (TD, Silver Cup, 45 % protein and 15 % lipid) until 31 dah. Food particle size was provided at apparent satiation according to larval growth (250–500, 500–750 and >750 μm).
During the larviculture, sampling was conducted at different days after hatching (dah) 0, 3 and 5 dah with 48 organisms (16 per tank) and at 7, 9, 15, 20, 25 and 31 dah with 30 larvae (10 per tank). Larvae were obtained before the first feeding and were killed with an overdose of tricaine metasulphonate (MS-222, Argent, Redmond, WA, USA), freezed with liquid nitrogen and stored at −80 °C until biochemical analysis. For the histological description of the development of the digestive tract, 10 larvae per tank were sampled at 0, 1, 2, 3, 4, 5, 7, 9 11, 13 and 15 dah and fixed in Bouin for 24 h. Larvae were then washed in fresh water (four times), dehydrated in a graded series of ethanol (30, 40 and 50 %) to eliminate any remainder of fixing liquids and finally preserved in ethanol 70 % until their use for histological studies.
Biochemical analyses
Enzymatic extracts were obtained by homogenization of the visceral bulk (dissecting tail, head, and the dorsal part of the body on ice) in Tris–HCl 50 mmol L−1, CaCl2 20 mmol L−1, pH 8 buffer solution (30 mg mL−1) using a tissue homogenizer (Ultra Turrax® IKA T18 Basic, Staufen, Germany) and centrifuged (16,000g for 5 min at 5 °C). The supernatant was collected from three pooled samples of larvae for each sampling day, placed in 500 µL samples and frozen at −80 °C (Thermo Fisher Scientific® 990, USA) for the enzymatic analysis. The concentration of soluble protein was evaluated using the Bradford (1976) technique and a standard curve of bovine serum albumin. Additionally, 30 larvae were collected and 5 days for biometry data was recorded from each species to evaluate growth as individual wet weight using an analytical balance (Sartorius AG, Gottingen, Germany; precision 1 × 10−4 g) and total length using a digital calliper (Neiko-HKMUND473, Homewood, IL, USA; precision 1 × 10−2 mm).
Total alkaline protease activity was measured using casein (0.5 %) in 50 mmol L−1 Tris–HCl buffer, pH 9.0, following the Kunitz method (1947), modified by Walter (1984). Acid protease activity was evaluated according to Anson (1938) using 0.5 % haemoglobin in 0.1 mmol L−1 glycine–HCl, pH 2.0. One unit of enzyme activity was defined as 1 μg tyrosine released per minute using a coefficient of molar extinction of 0.005 at 280 nm. Trypsin activity was assayed using BAPNA (N-a-benzoyl-dl-arginine 4-nitroanilide hydrochloride) as substrate according to Erlanger et al. (1961). Chymotrypsin activity in extracts was determined using SAAPNA (N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide) according to Del Mar et al. (1979). Leucine aminopeptidase was determined using leucine p-nitroanilide (0.1 mmol L−1 in DMSO) as substrate, according to Maraux et al. (1973). For trypsin, chymotrypsin and leucine aminopeptidase activities, one unit of enzyme activity was defined as 1 μmol L−1 p-nitroanilide released per minute using coefficients of molar extinction of 8.8 at 410 nm. Carboxypeptidase A activity was measured using the protocol of Folk and Schirmer (1963) using HPA (hippuryl-l-phenylalanine) as substrate dissolved in 25 mmol L−1 Tris–HCl, 10 mmol L−1 CaCl2 buffer, pH 7.8. One unit of enzyme activity was defined as 1 μmol L−1 of hippuryl hydrolysed per minute using a coefficient of molar extinction of 0.36 at 254 nm.
Determination of α-amylase activity was carried out following the Somogyi–Nelson procedure described by Robyt and Whelan (1968). One unit of activity was defined as the amount of enzyme to produce 1 μg maltose per minute at 600 nm. Lipase activity was quantified using β-naphthyl caprylate as substrate according to Versaw et al. (1989). One unit of activity was defined as 1 μg naphthol released per minute using a coefficient of molar extinction of 0.02 at 540 nm. Acid and alkaline phosphatases were assayed using 4-nitrophenyl phosphate in acid citrate buffer (pH 5.5) or glycine–NaOH buffer (pH 10.1) according to Bergmeyer (1974). One unit was defined as 1 μg nitrophenyl released per minute using a coefficient of molar extinction of 18.5 at 405 nm. All assays were performed by triplicate at 37 °C. Digestive enzyme activities were expressed as U mg protein−1 and U larvae−1 using the number of individuals in each pooled sample.
Electrophoretic analyses
The analysis of the alkaline protease isoforms was done using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; 10 % polyacrylamide) for each larval enzyme preparation in a Mini Protean II chamber (Bio-Rad) according to Laemmli (1970) using 8 × 10 × 0.075 cm gels. The samples were prepared and the zymograms of alkaline protease activities were obtained as described García-Carreño et al. (1993). The electrophoresis was carried out for 60 min at a constant voltage of 100 V per gel at 5 °C. After electrophoresis, the gels were washed and incubated for 30 min at 5 °C in a 0.5 % casein Hammerstein (Research Organics) solution at pH 9.0. Gels were then incubated for 90 min in the same solution at 25 °C without agitation. Finally, the gels were washed and fixed in 12 % trichloroacetic acid (Sigma-Aldrich) prior to staining with 0.1 % Coomassie brilliant blue R-250 (Research Organics) in a solution of methanol–acetic acid (Sigma-Aldrich)–water (50:20:50). Distaining was carried out in a solution of methanol–acetic acid–water (35:10:55). Clear zones, which indicated activity of alkaline proteases, were visible after 24 h.
To analyse acid protease activity in larval extracts, neutral native polyacrylamide electrophoresis was performed according to Williams and Reisfeld (1964). All electrophoresis procedures were performed at a constant voltage and amperage (100 V and 64 mA) per gel. After electrophoresis, gels were treated to reveal acid protease isoforms according to the procedure of Díaz et al. (1998). Same quantity of protein (30 μg per well) was applied to carry out each electrophoresis. Gels were removed from the cell and soaked in 100 mmol L−1 HCl to lower the pH to 2.0 so that the enzymes would become active. After 15 min, the gel was soaked in solution containing 0.25 % haemoglobin in 100 mmol L−1 glycine–HCl, pH 2.0, for 30 min at 4 °C and then transferred for 90 min in a fresh haemoglobin solution at 37 °C. Gels were washed in distilled water and fixed for 15 min in a 12 % trichloroacetic acid solution. When the areas of enzyme activity appeared, gels were stained using Coomassie brilliant blue R-250 solution. Distaining was carried out as mentioned earlier. Clear zones revealed the activity of acid proteases within a few minutes, although well-defined zones were obtained only after 2–4 h of staining. A low-range molecular weight marker (5 μL per well) containing phosphorylase b (97 kDa), bovine serum albumin (66 kDa), egg albumin (45 kDa), carbonic anhydrase (29 kDa), trypsinogen (24 kDa) and soybean trypsin inhibitor (20 kDa) was applied to each SDS-PAGE. The relative electromobility (Rf) was calculated for all zymograms (Igbokwe and Downe 1978), and the molecular weight (MW) of each band in the SDS-zymograms (alkaline protease) was calculated by a linearly adjusted model between the Rf and the decimal logarithm of MW proteins in the marker using the software program Quality One version 4.6.5 (Hercules, CA).
Histological analyses
For histological purposes, larvae per sampling date were dehydrated with graded series of ethanol and embedded in paraffin with an automatic tissue processor Histolab ZX-60 Myr. Paraffin blocks were then prepared in AP280-2 Myr station and cut into serial sagittal sections (3 µm thick) with an automatic microtome Microm HM (Leica Microsystems Nussloch GmbH, Germany). Paraffin larvae cuts were kept at 40 °C overnight. Samples were then deparaffined with graded series of xylene and stained by means of haematoxylin and eosin (H&E) for general histomorphological observations and periodic acid–Schiff (PAS) for detecting neutral mucosubstances. Histological preparations were observed in a microscopy Leica DMLB equipped with a digital camera Olympus DP70 (Leica Microsystems Nussloch GmbH). Measurements on histological slides were taken with an image analysis software package (Image Pro Plus).
Statistical analyses
Growth of larvae was determined with an exponential model Y = aebX, with previous data transformation using logarithm to the base 10, and the parameters of the model were calculated by the use of the least squares technique. As enzymatic activity values were not normally distributed, data were compared with the nonparametric Kruskal–Wallis test, and a nonparametric Nemenyi test was used when significant differences were detected between treatments, with a significance level of 0.05. All tests were performed with Statistica software version 7.0. (StatSoft, Tulsa, OK, USA).
Results
Larval growth
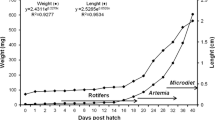
Individual weight growth rate during larviculture followed an exponential model (R 2 = 0.92, P < 0.05) with a mean daily growth value of 0.152 g day−1 larvae−1, while the individual total length rate had a model adjust (R 2 = 0.86, P < 0.05) showing a value of 0.078 mm day−1 larvae−1 (Fig. 1a, b).
Histological development of the digestive system
At hatching, the larval digestive system of A. tropicus was represented by two rudiments, a large endodermal yolk sac and a primordial hindgut (Fig. 2a). The endodermal yolk sac was filled with eosinophilic yolk platelets and lined with a squamous epithelium (Fig. 2b). The hindgut, containing a small amount of yolk within enterocytes, as a resultant of the meroblastic cleavage of eggs, appeared as an undifferentiated straight and narrow tube, which would differentiate into the hindgut and rectum (Fig. 2c). The liver rudiment was already present at hatching (Fig. 2d). The histological differentiation of the digestive system after hatching is described below.
Histological images of the digestive system in A. tropicus larvae aged 1 day after hatching. Note the large yolk sac with eosinophilic yolk platelets and the oesophagus in differentiation (a), the undifferentiated rectilinear intestine (b) and details of the oesophagus with its lumen filled with remnants of yolk platelets (c), and differentiating enterocytes containing yolk inclusions. I intestine, N notochord, OE oesophagus, Yp yolk platelets, YS yolk sac
The differentiation of the intestine started at 1 dah, progressing in posteroanterior direction. The differentiation of the intestinal mucosa was concomitant with the disappearance of eosinophilic yolk in the supranuclear vacuoles from enterocytes (Fig. 3a). The intestine was lined by a simple columnar epithelium with basal nuclei, slightly basophilic cytoplasm and prominent eosinophilic microvilli. The mucosa had generally similar histological structure along the length of intestine, with the exception of number and size of intestinal folds which were less abundant and smaller in the posterior region (Fig. 3b). Regarding the oesophagus, its differentiation proceeded from the posterior region to buccopharynx. Between 1 and 3 dah, the oesophagus started to develop and an incipient folding of the oesophageal mucosa at a longitudinal level appeared. At 3 dah, the oesophagus wall consisted of a pseudostratified columnar epithelium with numerous cells containing yolk inclusions, which disappeared at later stages of development between 4 and 6 dah. Between hatching and 3 dah, the liver greatly developed and occupied most part of the abdominal cavity (Fig. 3c). Hepatic tissue was arranged along sinusoids and consisted of polyhedral hepatocytes with centrally located nuclei, reduced eosinophilic cytoplasm and few and small lipid inclusions. Biliary ducts were already visible at this age and were lined by short and ciliated columnar epithelium with basal nuclei occupying most part of the cytoplasm. At this age, the exocrine pancreas was already differentiated and contained zymogen acidophilic granules. This accessory digestive gland was organized in polyhedral basophilic cells arranged in acini grouped in rosette patterns, containing round-shaped eosinophilic and PAS-positive zymogen granules (Fig. 3d). During this period, the intestine grew in length and small mucosal folds were visible in the posterior intestinal region, the future spiral valve, close to the anal opening.
Histological images of different regions of the digestive system in A. tropicus larvae aged 4 days after hatching. Note the pseudostratified columnar epithelium of the oesophagus in differentiation (a), the development of intestinal villi with large goblet cells and prominent eosinophilic microvilli (b) and the liver with round-shaped hepatocytes with lipid deposits (asterisk) in the hepatic parenchyma (c) and the exocrine pancreas (d). EP exocrine pancreas, GC goblet cell, I intestine, L liver, OE oesophagus
At 4 dah, first gastric glands were observed between the posterior oesophagus and anterior intestine, whereas between 5 and 7 dah, the cardiac and fundic regions of the stomach greatly developed and a large number of gastric glands were visible, as the multicellular tubular glands composed of a single-type secretory cells with eosinophilic apical borders and the secretory products containing neutral (PAS-positive) mucosubstances (Fig. 4a). These glands were surrounded by compact layers of connective tissue, smooth circular musculature and a thin serosa. In addition, the pyloric (non-glandular) stomach developed and was histologically characterized by several mucosal folds surrounded by a prominent tunica muscularis (Fig. 4b). The epithelium lining the lumen of this region of the stomach consisted of ciliated columnar cells with supranuclear vacuoles containing eosinophilic and neutral (PAS-positive) mucosubstances (Fig. 4c). The wall of the pyloric stomach was composed of s submucosa with connective fibres, some scattered blood vessels, circular muscle fibres and a thin serosa with basophilic squamous cells (Fig. 4d).
General view of the digestive system in A. tropicus larvae aged 5 days after hatching showing the large development of the liver, the formation of the gastric stomach (cardiac and fundic regions) (a), as well as the intestine differentiated into anterior, middle and posterior regions (b). Stomach formation (c) and detail of two multicellular tubular gastric glands surrounded by connective tissue and composed of a single-type secretory cell with an eosinophilic apical border (d). EP exocrine pancreas, GB gall bladder, I intestine, L liver, PS pyloric (non-glandular) stomach, S stomach, Y yolk
Between 7 and 9 dah, the digestive tract of A. tropicus resembled that of a juvenile specimen (11–15 dah). In this sense, the oesophagus was composed of simple columnar epithelium with numerous goblet cells, a thin mucosa with a lamina propria (loose connective tissue and a layer of musculature), a submucosa (connective tissue fibres with some blood vessels) and a serosa lined by a thin layer of squamous epithelium. No remarkable changes in the histological organization of the oesophagus were observed after 9 dah other than its increase in length and folding with age (Fig. 5a). Regarding the stomach, the gastric and non-gastric regions greatly developed, especially the number and size of gastric glands that occupied most part of the anterior part of the abdominal cavity, as well as the size and thickness of the muscular layer that surrounded the pyloric region of the stomach (non-gastric part). The most relevant histological features at this stage were the presence of pyloric caeca with a mucosa similar to that of its intestinal counterpart, which were lined by a simple columnar epithelium covered by prominent eosinophilic microvilli with scattered goblet cells, and the complete development of the spiral valve (posterior intestine) (Fig. 5b).
General view of the digestive system in A. tropicus larvae aged 7 days after hatching showing the large liver, the gastric stomach with numerous gastric glands, the exocrine pancreas and gall bladder and remnants of yolk (a). The pyloric stomach (non-glandular region) surrounded by a thick tunica muscularis is also shown (b). AI anterior intestine, MI mid intestine, GG gastric gland, L liver, M trunk musculature, PI posterior intestine, S stomach, Y yolk
Activity of digestive enzymes
Changes in digestive enzyme activities were observed during ontogeny (Figs. 6, 7; P < 0.05). The specific activity (U mg protein−1) of acid protease was detectable from 5 dah (12.4 ± 0.8 mm), remaining constant since then until 25 dah (32.4 ± 2.6 mm), reaching its maximum activity at 31 dah (48.8 ± 3.9 mm) (Fig. 6a). The acid protease individual activity displayed two main increases, the first at 5 and the second at 31 dah, maintaining their oscillating until the end of larval period (Fig. 6b). Specific activity of alkaline protease followed the same pattern, but pronounced from 0 dah (embryos, 1.0 ± 0.0 mm), fluctuating slightly and increasing its activity at 15 dah, reaching its maximum activity at 31 dah, appearing the same pattern in individual activity (Fig. 6a, b). Specific and individual trypsin activities were detected in minimum levels after hatching during the early larval development, increasing gradually from 19 dah (26.6 ± 2.4 mm), to reach their maximum activities at 31 dah (Fig. 6c, d). Specific chymotrypsin activity was detected from embryo (0 dah) and maintained until 15 dah; afterwards, individual activity increases gradually until reaching the maximum peak at 31 dah (Fig. 6e); nevertheless, the individual activity of this enzyme presented an inversed pattern, since the maximum peak of activity was detected at 0 dah (embryos), falling rapidly until 9 dah (17.6 ± 0.5 mm) and, fluctuating from this day, showing a slight increase at 31 dah (Fig. 6f). The specific and individual activities of leucine aminopeptidase were detected in very low levels from 3 dah, increasing gradually until the end of the study at 31 dah (Fig. 6g, h). The specific activity of carboxypeptidase A was early detected, presenting a progressive increase during the larval development reaching its maximum activity at 31 dah (Fig. 6i). The individual activity of carboxypeptidase A was detected from the embryo (0 dah), with fluctuating during larvae development reaching maximum peak at 31 dah (Fig. 6j).
Digestive enzyme activity during A. tropicus larviculture (mean ± SD, n = 30 pooled larvae). a Specific alkaline protease and b individual alkaline protease activities, c specific acid protease and d individual acid protease activities, e specific trypsin and f individual trypsin activities, g specific chymotrypsin and h individual chymotrypsin activities, i specific carboxypeptidase A and j individual carboxypeptidase A activities, k specific leucine aminopeptidase and l individual leucine aminopeptidase activities. AN Artemia nauplii, TD trout diet
Digestive enzyme activity during A. tropicus larviculture (mean ± SD, n = 30 pooled larvae). a Specific and b individual lipase activities, c specific and d individual α-amylase activities, e specific and f individual acid phosphatase activities, g specific and h individual alkaline phosphatase activities. AN Artemia nauplii, TD trout diet
Specific lipase activity was detected in embryos (0 dah) showing a peak of activity at 3 dah (6.8 ± 0.4216 mm), while during the larval development it showed some fluctuations with a maximum of activity at 31 dah (Fig. 7a). On the other hand, individual lipase activity presented a maximum value at 3 dah and subsequently showed a constant decrement until the end of the larval period (Fig. 7b). The specific activity of α-amylase was detected from the embryo in low levels, increasing quickly at 3 dah, being fluctuating during larval development, although at days 3, 7 and 19 after hatching we detected some peaks in activity (Fig. 7c). Nevertheless, the individual α-amylase activity had the maximum value at 3 dah, presenting a successive decrement until the end of the larval period (Fig. 7d). The specific activities of acid and alkaline phosphatase presented a similar pattern throughout the larval development, detecting two maximum peaks at 7 (16.3 ± 0.8 mm) and 31 dah, although in the case of alkaline phosphatase the maximum activity was detected at 31 dah, whereas for acid phosphatase was detected at 7 dah (Fig. 7e). Finally, individual acid phosphatase activity was detected with a maximum value at 5 dah, decreasing gradually until day 31 after hatching, whereas individual alkaline phosphatase activity displayed fluctuations a long of the early ontogeny, where two peaks with high activities were detected for the days 3 and 7 after hatching (Fig. 7f).
PAGE zymogram revealed two active forms (0.3 and 0.4 Rf) of acid proteases from day five after hatching being constant until the end of the larval period. An additional active form (0.5 Rf) was detected at 31 dah (Fig. 8a). On the other hand, zymogram for the detection of alkaline proteases using SDS-PAGE allowed identifying two active bands with 26.34 and 24.92 kDa at 5 dah being constant until the end of the larval period. In addition, from day seven after hatching, the appearance of a third band was detected (44.12 kDa calculated MW), which also remains constant until day 31 after hatching (Fig. 8b).
Zymograms of acid (a) and alkaline digestive proteases (b) during larval development of A. tropicus. Numbers at the top of the gel indicate the mean of days after hatching. The first well indicates LWMM (kDa): 97 phosphorylase, 66 bovine serum albumin, 45 ovalbumin, 29 carbonic anhydrase, 24 trypsinogen, 20 trypsin soybean inhibitor
Discussion
The general pattern regarding the histological development of the digestive tract in A. tropicus resembles that of other fish species with meroblastic egg cleavage like acipenserids (Gisbert et al. 1998, 1999; Gisbert and Doroshov 2003; Ostaszewska and Dabrowski 2009; Wegner et al. 2009; Babaei et al. 2011; Asgari et al. 2014). However, there were important differences in relation to the timing of development of different digestive organs, which were due to different reproductive guilds between A. tropicus and acipenserids, as well as environmental rearing conditions, especially water temperature that resulted in a much faster organogenesis of the digestive system in this species. Consequently, authors have decided to focus this section to the ontogenic changes in the histological organization of the digestive tract and relate them to changes in digestive enzyme activities from hatching to the juvenile stage.
Regarding the activity of digestive enzymes in A. tropicus, our results showed two groups of digestive enzymes with different profiles during larval development. Firstly, the specific activity of acidic proteases, trypsin, chymotrypsin, carboxypeptidase A and leucine aminopeptidase had the same profile appearing early only a few days after hatching or yolk absorption; meanwhile, lipase, amylase, and acid and alkaline phosphatase present another behaviour that explained the maturation of the digestive system in larvae. In this context, the activities of alkaline proteases in A. tropicus were detected from the embryonic period (egg), increasing gradually from day 15 after hatching, which was in agreement with our histological findings regarding the morphogenesis of the digestive organs, as well as with other freshwater and marine species, such as African catfish, Clarias gariepinus (García-Ortega et al. 2000), P. fluviatilis (Cuvier-Péres and Kestemont 2002), amberjack, Seriola lalandi (Chen et al. 2006), California halibut, Paralichthys californicus (Alvarez-González et al. 2006), S. ocellatus (Lazo et al. 2007) and P. maculatofasciatus (Alvarez-González et al. 2008). This increase in the alkaline proteolytic activity is mainly associated with the trypsin, which has been considered as an initial endoprotease for the activation of other digestive enzymes, catalysing the conversion of zymogens to active enzymes. In addition, both trypsin and chymotrypsin are responsible to nutrient process (hydrolyse proteins) until the complete development of a functional stomach (Zambonino-Infante and Cahu 2001; Kvåle et al. 2007).
On the other hand, the acid proteolytic activity was detected before yolk absorption (5 dah), coinciding with the first detection of gastric glands and increased gradually through A. tropicus development. Pepsin activity indicates the beginning of stomach functionality and is considered the most important protease, which initiates protein digestion in an acidic pH. Thus, we can consider that A. tropicus is a precocious larvae, being similar to the other lepisosteids such as A. spatula and A. tristoechus (Aguilera 1999; Comabella et al. 2006). These results are different from those detected for marine fish larvae (Moyano et al. 1996). The former authors reported that during the development of the digestive system the activity of alkaline proteases is detected early before the opening of the mouth, whereas the activity of the pepsin is detected later during the development, being activated several weeks with the stomach development (around 25 dah) that has been reported for P. californicus and P. maculatofasciatus (Álvarez-González et al. 2006, 2008). Moreover, for tropical freshwater species such as monkeyface prickleback (Cebidichthys violaceus), rock prickleback (Xiphister mucosus), black prickleback (Xiphister atropurpureus), high cockscomb (Anoplarchus purpurescens), red tilapia (Oreochromis mossambicus) (Chan et al. 2004; Ming-Ji and Ching-Feng 2006) and Mayan cichlid (Cichlasoma urophthalmus) (López-Ramírez et al. 2010), acid protease activities appeared in low levels around the 30 dah, while for the Japanese flounder (Paralichthys olivaceus) the stomach secretory glands were detected until 45 dah (Kurokawa and Suzuki 1996). The early presence of the acid protease activity in A. tropicus is indicative of the presence of a functional stomach and well-developed gastric glands, which is characteristic of a monogastric fish, that has been observed in some species such as rainbow trout (Oncorinchus mykiss), lake sturgeon (Acipenser fulvencens) (Buddington 1985; Buddington et al. 1997), Atlantic halibut (Hippoglossus hippoglossus) (Gawlicka et al. 2000), Surubí (Pseudoplatystoma corruscans) (Lundstedt et al. 2004), A. spatula (Mendoza et al. 2002) and A. tristoechus (Comabella et al. 2006).
In the zymogram for acid proteases of A. tropicus, two active bands were detected at 5 dah and third band appeared at day 31 after hatching, which is in agreement with those results observed by Comabella et al. (2006) in A. tristoechus, although the above-mentioned authors only detected a single active band at 5 dah and a second active band at 7 dah. In addition, Mendoza and Aguilera (2001) found that larvae of A. spatula presented a complete functional stomach at 5 dah. Considering the former studies and data from this work, it appears that the Lepisosteidae family has a high capacity to digest any kind of food in the endo–exotrophic stage. For alkaline protease zymogram characterization, our results showed two active bands (24.92 and 26.34 kDa apparent MW, respectively) at 5 dah and an additional active band (44 kDa apparent MW) at 7 dah, which agree with trypsin- and chymotrypsin-like enzymes, which demonstrates that larvae at hatching already have the capacity to digest exogenous food even if yolk remains. The presence of these enzymes is genetically programmed and the precursors for the activation of other digestive enzymes (Zambonino-Infante and Cahu 1994). This way the presence of this same type of active forms has been observed in diverse marine fish such as P. maculatofasciatus with four isoforms of 21.8, 23.8, 56.5 and 52.0 kDa (Álvarez-González et al. 2008) and in fresh water fish such as C. urophthalmus with two active forms of 22 and 26 kDa (López-Ramírez et al. 2010). Other studies showed the presence of trypsin and chymotrypsin with similar molecular weight as found in S. aequifasciatus (Chong et al. 2002) with two active forms 73.3–76.5 and 19.2–21.8 kDa, respectively; also, Rivera (2003) reports the presence of a trypsin with molecular weight of 26.1 kDa in silk snapper (Lutjanus vivanus), whereas Rodriguez (2004) and Souza et al. (2007) found trypsin-like enzymes with molecular weights of 24 kDa in the grunt (Haemulon plumierii) and 21 kDa in spotted goatfish (Pseudupeneus maculatus).
Contrary to our findings, trypsin is one of the first enzymes detected during larval development in many marine species (Gawlicka et al. 2000; Cuvier-Péres and Kestemont 2002; Álvarez-González et al. 2006; Kvåle et al. 2007) and freshwater (Mendoza et al. 2002; Comabella et al. 2006; López-Ramírez et al. 2010); however, high activity of chymotrypsin was detected during the embryonic development of A. tropicus as was reported by Kolkovski (2001) who indicates that this digestive alkaline protease compensates the low or null acid protease activity during the first days of life of the larvae. Trypsin is the sensitive key protease under condition favouring growth and genetically and environmentally affected. Chymotrypsin, on the other hand, plays a major role when growth is limited or depressed (Rungruangsak-Torrissen et al. 2006).
The early presence of alkaline luminal proteases is an indicative of a functional pancreas before the yolk absorption as was detected in carnivorous fish larvae, such as A. purpurescens and pike-perch (Sander lucioperca) (Chan et al. 2004; Hamza et al. 2007). The maximum trypsin activity was observed in these fish between the 22 and 30 dah, respectively. Furthermore, the secretion of these endopeptidases (intestinal trypsin/chymotrypsin activity ratio) has been used as indicator of nutrients assimilation capacity, when the chymotrypsin activity is constant and the trypsin activity increased, coinciding with the maturation of the microvilli and the improvement of the final hydrolysis of the nutrients and absorption (Ming-Ji and Ching-Feng 2006; Lazo et al. 2007).
The enzymatic activity of the leucine aminopeptidase was low from the embryonic period and increased at 15 dah, whereas the activity of carboxypeptidase A increases from 5 dah. In this sense, when both activities appear in microvilli of the enterocytes (parietal digestion), indicates an active protein hydrolysis at carboxyl and amino terminal bonds levels, also carboxypeptidase A could be measured in pancreatic tissue, that should be considered because total body extract where done. Aminopeptidase N and/or alkaline phosphatase have also been used as indicators of the nutritional quality, while leucine–alanine peptidase activity decreases. This pattern also indicates the maturation of the microvilli of the enterocytes that has been detected in larvae of European sea bass (Dicentrarcus labrax) (4–5 dah) and O. niloticus (3 dah), being observed the presence of leucine aminopeptidase enzyme in the intestine before the first feeding of larvae, increasing its activity after 3 dah, when the opening of the mouth occurs (Zambonino-Infante and Cahu 1994, 2001; Gawlicka et al. 1995; Tengjaroenkul et al. 2002; Hakim et al. 2007). Also, it has been reported in marine fish larvae such as yellowtail flounder (Pleuronectes ferruginea), winter flounder (Pseudopleuronectes americanus) (Baglole et al. 1998), common snook (Centropomus undecimalis) (Jiménez-Martínez et al. 2012) and some freshwater fish such as C. urophthalmus (López-Ramírez et al. 2010) and bay snook (Petenia splendida) (Uscanga et al. 2011).
The specific activity of lipase was detected during the embryonic phase, displaying a maximum value at 30 dah and presenting variations along the larval development. This activity pattern is in agreement with other species, such as O. niloticus (Tengjaroenkul et al. 2002), Haddock (Melanogrammus aeglefinus) (Pérez-Casanova et al. 2006), orange-spotted grouper (Epinephelus coloides) (Eusebio et al. 2004), H. molitrix (Chakrabarti et al. 2006), A. tristoechus (Comabella et al. 2006) and P. maculatofasciatus (Alvarez-González et al. 2008), unlike to other species such as turbot (Scophthalmus maximus) (Cousin et al. 1987), sixfinger threadfin (Poliydactylus sexfilis) (Bong et al. 2001), P. californicus (Alvarez-González et al. 2006) and C. urophthalmus, where this activity was detected until 40 dah (López-Ramírez et al. 2010). However, the lipolytic activity has not been detected during the first days after hatching, and its action begins after several days after the mouth opening, which has been reported for halibut, S. maximus (Cousin et al. 1987), P. sexfilis (Bong et al. 2001), P. californicus (Alvarez-González et al. 2006), even with species as C. urophthalmus where this activity was detected until 40 dah (López-Ramírez et al. 2010). In the same sense, Oozeki and Bailey (1995) suggest the existence of two types of lipase, one related to the absorption of yolk sac and the other in which the activity is developed after several days and is related to the digestion of exogenous lipids. According to Alvarez-González et al. (2008), the lipid catabolism is possible at the beginning of the larval period by esterases and later by bile salt-dependent lipases, when the digestive system has been completed. Considering our study, the lipolytic activity increased during the first days and some variations were detected during larval development, which reflects the importance of this pancreatic enzyme in the hydrolysis of lipids, which seems to magnify at 15 dah, in agreement with results from A. tristoechus (Comabella et al. 2006). The former authors reported that the increase in this activity between 12 and 14 dah in A. tristoechus could be related to the development of the exocrine pancreas and also is also correlated with the change in diet, because of the mobilization of accumulated lipid and the increase in the capacity to digest lipids contained in foods.
Amylase presented the same pattern of activity than lipase, showing low activity levels at hatching and progressively increasing after the opening of the mouth. Similar results have been reported in different fish larvae, such as perch, P. fluvialitis (Cuvier-Péres and Kestemont 2002), H. molitrix (Chakrabarti et al. 2006), P. californicus (Alvarez-González et al. 2006), S. lucioperca (Hamza et al. 2007) and P. maculatofasciatus (Alvarez-González et al. 2008), where these variations throughout the larval development were related to the species-specific feeding habits (Horn et al. 1986). Thus, the amylase activity in A. tropicus was low during the larval development, being similar to A. spatula (Mendoza et al. 2002) and A. tristoechus (Comabella et al. 2006), which was in agreement with fish with strictly carnivorous habits, where the expression of amylase tends to decrease during the larviculture. Cahu et al. (2004) mentioned that this enzyme indicates not only the maturation of the digestive system, but also the possibility of include certain amount of carbohydrates such as starch and glycogen from vegetable and animal sources, which could contribute with energy during the feeding of the larvae, although for carnivorous fish this capacity is limited.
The main functions of phosphatases are to promote inorganic phosphate hydrolysis, used to produce energy and the transport of the nutrients through the membrane towards the interior of the cells, being facilitated the enzymatic action and the absorption of Ca2+ among other functions (Alvarez-González et al. 2006). The decrease in the acid phosphatase activity and the increase in the activity alkaline phosphatase during the first stages of development have been observed by Alvarez-González et al. (2008) in P. maculatofasciatus and Aguilera (1999) in A. spatula. In studies with other fish larvae, a previous low activity during the first days of life has been detected with a maximum increase around the 20 dah that has been reported in P. sexfilis (Bong et al. 2001), P. fluvialitis (Cuvier-Péres and Kestemont 2002), S. lucioperca (Hamza et al. 2007) and C. urophthalmus (López-Ramírez et al. 2010). In this aspect, Ribeiro et al. (2002), and Zambonino-Infante and Cahu (2007) have determined that an abrupt increase in the alkaline phosphatase activity during the larval period is a consequence of the maturation of the enterocytes, reason why the crossing in relation to exopeptidases (as aminopeptidase N) is one of the best indicators that marks the change in the larval to juvenile period. Nevertheless, this pattern not always has been observed in other species such as P. californicus (Alvarez-González et al. 2006), P. maculatofasciatus (Peña-Martínez et al. 2003; Alvarez-González et al. 2008) and C. undecimalis (Jiménez-Martínez et al. 2012), who reported the maximum alkaline phosphatase activity in the day 12 dah with later decline, suggesting for these species that the enterocytes already have a high capacity to absorb the nutrients from the diet. Also, Martinez et al. (1999) reported the maximum activity in the first days of life of S. senegalensis, explaining the possibility of the existence of other pancreatic enzymes such as the trypsin, which participate in higher levels during the luminal digestion, reason why the parietal digestion is not high (Moyano et al. 2005). In this manner, the presence of the activities of phosphatases agrees with those reported by Comabella et al. (2006) in A. tristoechus, who mentioned an increase with variations of both activities throughout ontogeny. This way, both activities, in action with other pancreatic enzymes during the first stages of the larval period allows the organism to digest and absorb the nutrients from yolk absorption and at the moment of the first feeding, which compensate the lack of stomach as it is the case of marine fish larvae (Gawlicka et al. 2000; Alvarez-González et al. 2008), or the low activity that appears in fresh water fish such as A. spatula, A. tristoechus and P. splendida (Mendoza et al. 2002; Comabella et al. 2006; Uscanga et al. 2011).
In general, the activities of digestive enzymes in A. tropicus larvae were detected from the embryonic period, increasing throughout the larval development, whereas the activities of acid and alkaline proteases, chymotrypsin, trypsin, carboxypeptidase A, and acid and alkaline phosphatase presented an increment starting from 5 dah, when the mouth opening and first feeding occur, even with an endogenous feeding (yolk sac) remains several days after hatching. Also, the amylase and lipase activities were detected at 3 dah, whereas the trypsin and leucine aminopeptidase activities presented an increase from the day 15 after hatching, indicating the beginning of the juvenile period and the maturation of the digestive system, in agreement with the morphophysiological changes during the larviculture, which allows the larvae to digest and to absorb the nutrients contained in the reserves of the yolk sac and to adapt rapidly to the exogenous feeding.
According to these results, we can conclude that the adequate moment to initiate the weaning period (co-feeding process) should start at 9 dah, when an increase in most of the activities has been observed, mainly acid and alkaline proteases and lipases; considering the above-mentioned results, larva has the capacity to digest proteins and lipids of the provided food (live or artificial). In addition, at 15 dah a total replace of live food with the artificial diet is satisfactory taking into account the type of digestive enzymes present; nevertheless, it is necessary to conduct studies in relation to the nutritional requirements of the larvae in order to improve the larviculture of this species, which allows decreasing the mortality caused during the actual weaning process (using trout diet), and the development of an appropriate diet that allows to improve the growth and seed production.
References
Aguilera GC (1999) Physiological basis of gar larvae development (Atractosteus spatula) and prospects for cultivation. PhD thesis, University of Nuevo León, México
Álvarez-González CA, Cervantes-Trujano M, Tovar-Ramírez D, Conklin D, Nolasco H, Gisbert E, Piedahita R (2006) Development of digestive enzymes in California halibut Paralichthys californicus larvae. Fish Physiol Biochem 31:83–93
Álvarez-González CA, Márquez-Couturier G, Contreras-Sánchez WM, Rodríguez-Valencia W (2007) Strategy for the sustainable use of fisheries resources in Boca Chilapa, biosphere reserve Centla Swamp, Tabasco: establishment of a production plant native fish: alligator gar, Snook and Mayan cichlid. In: Halffter G, Guevara S, Melic A (eds) Towards a culture of conservation of biological diversity, vol VI. Monographs millennium, Zaragoza, pp 197–205
Álvarez-González CA, Moyano-López F, Civera-Cerecedo R, Carrasco-Chávez V, Ortíz-Galindo JL, Dumas S (2008) Development of digestive enzyme activity in larvae of spotted sand bass (Palabrax maculatofasciatus). Fish Physiol Biochem 34:373–384
Álvarez-González CA, Moyano-López F, Civera-Cercedo R, Carrasco-Chávez V, Ortiz-Galindo JL, Nolasco-Soria H, Tovar-Ramírez D, Dumas S (2010) Development of digestive enzyme activity in larvae of spotted sand bass (Palabrax maculatofasciatus) II: electrophoretic analysis. Fish Physiol Biochem 36:29–37
Anson ML (1938) The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Asgari R, Rafiee Gh, Eagdei S, Shahrooz R, Pourbagher H, Agh N, Gisbert E (2014) Ontogeny of the digestive system in hatchery produced Beluga (Huso huso Linnaeus, 1758); a comparative study between Beluga and genus Acipenser. Aquac Nutr 20:595–608
Babaei SS, Kenari AA, Nazari R, Gisbert E (2011) Developmental changes of digestive enzymes in Persian sturgeon (Acipenser persicus) during larval ontogeny. Aquaculture 318:138–144
Baglole CJ, Goff G, Wright G (1998) Distribution and ontogeny of digestive enzymes in larval yellowtail and winter flounder. J Fish Biol 53:767–784
Bergmeyer HU (1974) Phosphatases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 1196–1201
Bong G, Divakaran S, Brown C, Ostrowski A (2001) Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polidactylus sexfilis) and bluefin trevally (Canrax melampygus). Fish Physiol Biochem 24:225–241
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buddington R (1985) Digestive secretions of lake sturgeon, Acipenser fulvencens, during early development. J Fish Biol 26:715–723
Buddington R, Krogdahl A, Bakke-Mckellep A (1997) The intestine of carnivorous fish: structure and functions and the relation with diet. Acta Physiol Scan 161:67–80
Cahu C, Rønnestad I, Grangier V, Zambonino-Infante J (2004) Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolyzed dietary protein; involvement of cholecystokinin. Aquaculture 238:295–308
Cara JB, Moyano F, Cárdenas S, Fernández C, Yúfera M (2003) Assessment of digestive enzyme activities during larval development of white bream (Diplodus sargus). J Fish Biol 63:48–58
Chakrabarti R, Rathore R, Kumar S (2006) Study of digestive enzyme activities and partial characterization of digestive proteases in a freshwater teleost, Labeo rohita, during early ontogeny. Aquac Nutr 12:35–43
Chan AS, Horn M, Dickson K, Gawlicka A (2004) Digestive enzyme activities in carnivores and herbivores: comparisons among four closely related prickleback fishes (Teleostei: Stichaeidae) from a California rocky intertidal habitat. J Fish Biol 65:848–858
Chen BN, Jian G, Martin S, Wayne G, Steven M (2006) Ontogenic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256:489–501
Chong AS, Hashim R, Lee L, Ali A (2002) Characterization of protease activity in developing discus (Symphysodon aequifasciatus) larvae. Aquac Res 33:663–672
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García-Galano T (2006) Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiol Biochem 32:147–157
Cousin JC, Baudin-Laurencin F, Gabaudan J (1987) Ontogeny of enzymatic activities in fed and fasting turbot, Scophthalmus maximus L. J Fish Biol 30:15–33
Cuvier-Pérez A, Kestemont P (2002) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Del Mar EG, Largman C, Brodrick J, Geokas M (1979) A sensitive new substrate for chymotrypsin. Anal Biochem 99:316–320
Diaz M, Moyano F, Alarcón F, García-Carreño F, Navarrete del Toro M (1998) Characterization of fish acid proteases by substrate-gel electrophoresis. Comp Biochem Physiology 121B:369–377
Erlanger B, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Eusebio PS, Toledo J, Mamauag R, Bernas M (2004) Digestive enzyme activity in developing grouper (Epinephelus coioides) larvae. In: Rimmer MA, McBride S, Williams KC (eds) Advances in grouper aquaculture. Melbourne, CIAR Monograph Series, pp 35–40
Fernández-Díaz C, Yúfera M, Cañavete J, Moyano F, Alarcón F, Díaz M (2001) Growth and physiological changes during metamorphosis of (Senegal sole) reared in the laboratory. J Fish Biol 58:1086–1097
Folk J, Schirmer E (1963) The porcine pancreatic carboxypeptidase A System. I. Three forms of the active enzyme. J Biol Chem 238:38–84
Galaviz MA, García-Gasca A, Drawbridge M, Álvarez-González CA, López LM (2011) Ontogeny of the digestive tract and enzymatic activity in white seabass, Atractoscion nobilis, larvae. Aquaculture 318:162–168
García-Carreño FL, Dimes LE, Haard NF (1993) Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
García-Ortega A, Verrent J, Segner H (2000) Post-prandial protease activity in the digestive tract of African catfish Clarias gariepinus larvae fed decapsulated cysts of Artemia. Fish Physiol Biochem 22:237–244
Gawlicka A, Teh SJ, Hung S, Hinton D, De la Noue J (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol Biochem 14:357–371
Gawlicka AB, Parent MH, Horn N, Ross I, Torrinsen O (2000) Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture 184:303–314
Gisbert E, Doroshov SI (2003) Histology of the developing digestive system and the effect of food deprivation in larval green sturgeon (Acipenser medirostris). Aquat Living Res 16:77–89
Gisbert E, Rodríguez A, Williot P, Castelló-Orvay F (1998) A histological study of the development of the digestive tract of Siberian sturgeon (Acipenser baeri) during early ontogeny. Aquaculture 167:195–209
Gisbert E, Sarasquete MC, Williot P, Castello-Orvay F (1999) Histochemistry of the development of the digestive system of Siberian sturgeon (Acipenser baeri, Brandt) during early ontogeny. J Fish Biol 55:596–616
Hakim Y, Rowland S, Guy J, Mifsud C, Uni Z, Harpaz S (2007) Effects of genetic strain and holding facility on the characteristics of alkaline phosphatase and brush border enzymes in silver perch (Bidyanus bidyanus). Aquac Res 38:361–372
Hamza N, Mhetli M, Kestemont P (2007) Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol Biochem 33:121–133
Horn MH, Neighbors M, Murray S (1986) Herbivore responses to a seasonally fluctuating food supply: growth potential of two temperate intertidal fishes based on the protein and energy assimilated from their macroalgal diets. J Exp Mar Biol Ecol 103:217–234
Igbokwe EC, Downe AE (1978) Electrophoretic and histochemical comparison of three strains of Aedes aegypti. Comp Biochem Physiol 60B:131–136
Jiménez-Martínez LD, Alvarez-González CA, Tovar-Ramírez D, Gaxiola G, Sanchez-Zamora A, Moyano FJ, Alarcón FJ, Márquez-Couturier G, Gisbert E, Contreras-Sánchez WM, Perales-García N, Arias-Rodríguez L, Indy JR, Páramo-Delgadillo S, Palomino-Albarrán IG (2012) Digestive enzyme activities during early ontogeny in Common snook (Centropomus undecimalis). Fish Physiol Biochem 38:441–454
Kolkovski S (2001) Digestive enzymes in fish larvae and juveniles-implications and applications to formulated diets. Aquaculture 200:181–201
Kunitz M (1947) Crystalline soybean trypsin inhibitor II. General properties. J Gen Physiol 30:291–310
Kurokawa T, Suzuki T (1996) Formation of the diffuse pancreas and the development of digestive enzyme synthesis in larvae of the Japanese flounder Paralichthys olivaceus. Aquaculture 141:267–276
Kvåle A, Mangor-Jensen A, Moren M, Espe M, Hamre K (2007) Development and characterization of some intestinal enzymes in Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture 264:457–468
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lazo JP, Mendoza R, Holt G, Aguilera C, Arnold C (2007) Characterization of digestive enzymes during larval development of red drum (Sciaenops ocellatus). Aquaculture 265:194–205
López-Ramírez G, Cuenca-Soria CA, Alvarez-González CA, Tovar-Ramírez D, Ortiz-Galindo JL, Perales-García N, Márquez-Couturier G, Arias-Rodríguez L, Indy JR, Contreras-Sánchez WM, Gisbert E, Moyano FJ (2010) Development of digestive enzymes in larvae of Mayan cichlid Cichlasoma urophthalmus. Fish Physiol Biochem 37:197–208
Lundstedt LM, Bibiano J, Morales G (2004) Digestive enzymes and metabolic profile of Pseudoplatystoma corruscans (Teleostei: Siluriformes) in response to diet composition. Comp Biochem Physiol 137B:331–339
Maraux S, Louvard D, Baratti J (1973) The aminopeptidase from hog-intestinal brush border. Acta Biochim Biophys Sin 321:282–295
Márquez-Couturier G, Álvarez C, Contreras W, Hernández U, Hernández A, Mendoza R, Aguilera C, García T, Civera R, Goytortua E (2006) Advances in food and nutrition tropical gar Atractosteus tropicus. In: Cruz LE, Ricque D, Tapia M, Nieto M, Villarreal D, Puello A, García A (eds) Proceedings of the eighth international symposium on aquaculture nutrition. UANL, Monterrey pp 446–523
Martínez MI, Moyano F, Fernández-Díaz C, Yúfera M (1999) Digestive enzyme activity during larval development of the Senegal solea (Solea senegalensis). Fish Physiol Biochem 21:317–323
Mendoza R, Aguilera C (2001) Physiological basis of larval development Atractosteus spatula and prospects for cultivation. Sci UANL 4:161–166
Mendoza R, Aguilera C, Rodríguez G, González M, Castro R (2002) Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Fish Biol 12:133–142
Ming-Ji L, Chin-Feng W (2006) Developmental regulation of gastric pepsin and pancreatic serine protease in larvae of the euryhaline teleost Oreochromis mossambicus. Aquaculture 261:1403–1412
Moyano FJ, Díaz M, Alarcón F, Sarasquete M (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem 15:121–130
Moyano FJ, Barros A, Prieto A, Cañavate J, Cárdenas S (2005) Evaluation of the ontogeny of digestive enzymes in larvae steals, Pagrus auriga (Pisces: Sparidae). AquaTiC 22:39–47
Oozeki Y, Baley M (1995) Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogramma. Mar Biol 122:177–186
Ostaszewska T, Dabrowski K (2009) Early development of Acipenseriformes (Chondrostei: Actinopterygii). In: Kunz WY, Luer AC, Kapoor BG (eds) Development of non-teleost fishes. Science Publishers, Enfield, pp 170–229
Pedersen BH, Uberchär B, Kurokawa T (2003) Digestive response and rates of growth in pre-leptocephalus larvae of the Japanese eel (Anguilla japonica) reared on artificial diets. Aquaculture 215:321–338
Peña-Martínez R, Dumas S, Villalejo-Fuerte M, Ortíz-Galindo J (2003) Ontogenetic development of the digestive tract in reared spotted sand bass Paralabrax maculatofasciatus larvae. Aquaculture 219:633–644
Pérez-Casanova JC, Murray H, Gallant J, Ross N, Douglas S, Johnson S (2006) Development of the digestive capacity in larvae of haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua). Aquaculture 251:377–401
Ribeiro L, Zambonino-Infante J, Cahu C, Dinis M (2002) Digestive enzymes profile of Solea senegalensis post larvae fed Artemia and a compound diet. Fish Physiol Biochem 27:61–69
Rina C, Raja M, Prabhat M, Sunil K (2005) Functional changes in digestive enzymes and characterization of proteases of silver carp (♂) and bighead carp (♀) hybrid during early ontogeny. Aquaculture 253:694–702
Rivera SM (2003) Purification and characterization of trypsin from intestinal and pyloric caecal tissues of the silk snapper, Lutjanus vivanus (Cuvier, 1828), undergraduate thesis. University of Puerto Rico, Puerto Rico
Robyt JF, Whelan W (1968) Amylases. In: Radley JA (ed) Starch and its Derivates. Chapman and Hall, England, pp 430–476
Rodríguez MA (2004) Purification and kinetic characterization of trypsin from the intestine and pyloric caeca of the white grunt, Haemulon plumierii, (Lacepède, 1801). Undergraduate thesis. University of Puerto Rico, Puerto Rico
Rungruangsak-Torrissen K, Moss R, Andresen L, Berg A, Waagbo R (2006) Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L). Fish Physiol Biochem 32:7–23
Souza AA, Amaral I, Albérico R, Carvalho L, Bezerra R (2007) Trypsin-like enzyme from intestine and pyloric caeca of spotted goatfish (Pseudupeneus maculatus). Food Chem 100:1429–1434
Tengjaroenkul B, Smith B, Smith S, Chatreewongsin U (2002) Ontogenic development of intestinal enzymes of cultured Nile tilapia (Oreochromis niloticus L.). Aquaculture 211:241–251
Uscanga A, Perales-García N, Alvarez-González CA, Moyano FJ, Tovar-Ramírez D, Gisbert E, Márquez-Couturier G, Contreras-Sánchez WM, Arias-Rodríguez L, Indy JR (2011) Changes in digestive enzyme activity during initial ontogeny of bay snook Petenia splendida. Fish Physiol Biochem 37:667–680
Versaw WS, Cuppett D, Winters L (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:232–254
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyern HU (ed) Methods of enzymatic analysis, vol V. Chemic Weinham, Germany, pp 270–277
Wegner A, Ostaszewska T, Rozek W (2009) The ontogenetic development of the digestive tract and accessory glands of sterlet (Acipenser ruthenus L.) larvae during endogenous feeding. Rev Fish Biol 19:431–444
Williams DE, Reisfeld RA (1964) Disc electrophoresis in polyacrylamide gels: extension to new conditions of pH and buffers. Ann N Y Acad Sci 121:373–381
Zambonino-Infante JL, Cahu CL (1994) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408
Zambonino-Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol 130:477–487
Zambonino-Infante JL, Cahu CL (2007) Dietary modulation of some digestive enzymes and metabolic processes in developing marine fish: applications to diet formulation. Aquaculture 268:98–105
Acknowledgments
This work was made possible thanks to the Project “Estudios de la fisiología digestiva en larvas de pejelagarto Atractosteus tropicus para el diseño de alimentos artificiales” UJAT-2009-C05-09. Author thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Programa Institucional de Superación Académica y Fortalecimiento del Posgrado (UJAT) for the fellowship grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frías-Quintana, C.A., Márquez-Couturier, G., Alvarez-González, C.A. et al. Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus . Fish Physiol Biochem 41, 1075–1091 (2015). https://doi.org/10.1007/s10695-015-0070-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0070-9