Abstract
The gut wastes of Sardinella longiceps were used as substrate for protease production. The gut waste has 61.6% proteins, 21.8% lipids, 8.5% carbohydrates on dry weight basis and trace elements. The significant factors of protease fermentation were screened by Plackett-Burman design. A protease activity of 68.56 U/ml was predicted at 46.31 °C, incubation time 71.11 h, inoculum 4.86% (v/v) and substrate concentration 2.66% (w/v), using response surface methodology. However, the validation experiment showed 73.52 U/ml activity. The artificial neural network was found as a better tool to predict the experimental results. The partially purified protease showed higher activity at pH 9 and 10 and retained 90% activity after 120 h at pH 9. It showed maximum activity at 50 °C and retained 88% residual activity until 90 min at 50 °C. Zn++ enhanced the protease activity by 40%. The protease retained an activity of 93, 103, 90 and 98% against urea, β-mercaptoethanol, SDS and tween 80 respectively. The alkaline protease was compatible with all the commercial detergents tested with the residual activity above 90%. The alkaline protease exhibited 22% higher activity on the tryptone soya substrate. The gut waste of S. longiceps is a worthy low cost substrate for the production of industrially important alkaline protease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Proteases are extracellular enzymes capable of hydrolysing large protein molecules to smaller peptides or amino acids that can be utilized by microorganisms. Microbes are ideal sources to produce protease enzymes because of their broad biochemical diversity, less complexity in the production process, easy maintenance of organisms, rapid growth, requirement of less space for cultivation and flexibility for manipulation at the genetic level1. Bacterial proteases are produced in large quantity due to their specificity, stability and activity in a broad range of physical conditions. Over 60% of the global production of industrial enzymes is proteolytic enzymes and among the proteolytic enzymes, 35% consists of alkaline proteases. These proteases are widely used in different industries such as brewing, baking, cheese-making, cosmetics, detergent, food, pharmaceutical, meat tenderization and leather2. In recent years, much interest has been shown in utilizing waste materials as a substrate for various fermentation processes3,4. Large amounts of solid and liquid wastes are generated during fish processing and its complex nature makes its disposal complicated and more expensive. Being a rich source of protein, these fish wastes could be utilized as a low cost substrate for the production of microbial proteases5.
The protease production is greatly affected by the nutritional and environmental conditions. Hence, it is possible to increment the protease production by manipulating the culture conditions. The required nutrient resources govern the cost of any microbial enzyme synthesis processes. Therefore, finding out a low cost medium and optimization strategies would economically benefit the production process. To accomplish this objective, the Plackett-Burman design (PB) and response surface methodology (RSM) were used in this study to evaluate the positive factors and select optimum conditions of variables for a maximum response. RSM can be used to design experiments, search optimum factors of the responses and evaluate the relative significance of selected factors even in the occurrence of complex interactions among the factors6. Further, the artificial neural network (ANN) model is built to test and predict the observed experimental results.
The microorganism, the nature of the substrate and the biochemical features of the enzyme produced are necessary factors to evaluate its biotechnological potential and its possible application in various industrial processes. The biochemical characterization of an enzyme indicates its performance and can forecast its utilization in specific applications7. In this study, protease has been produced by a native Bacillus licheniformis NK isolated from Nakhl hot spring, Oman using the gut waste of S. longiceps as an inexpensive substrate. The produced enzyme has also been characterized for its possible industrial applications.
Results and Discussion
Physical and chemical factors of sampling site
Totally 101 colonies were isolated from four different sites in Nakhl hot spring, Oman. Physical and chemical parameters recorded at the time of sampling in the four sites, N1, N2, N3 and N4, are given in Table 1. The temperature at the source of the hot spring was 38.8 °C. The pH and dissolved oxygen level of the water increased from the source towards downstream. Calcium and its salts are present in ionic form in hot water and they precipitate out when the temperature decreases. This could be a possible reason for the increase in pH and the dissolved oxygen is never limited in flowing water due to absorption of oxygen from air facilitated by the flow, photosynthetic production of benthic algae and decrease in temperature. The conductivity decreases with the reduction in water temperature because of the reduction in the ionic composition of water. The majority of the microbes was isolated from the soil collected from the sites N3 and N4, which were approximately 50 and 80 meters away from the source. Microorganisms surviving in hot springs have the adaptability to survive in harsh environmental conditions. This ability may be due to their molecular modifications at cellular and sub-cellular levels8. Both Bacillus spp. and Brevibacillus spp. isolated from various hot springs across the world were reported to produce thermostable proteases9,10.
Composition of gut waste substrate
The composition of gut waste of S. longiceps is given in Table 2. The gut waste was rich in proteins (61.6%) followed by lipids (21.8%) and carbohydrates (8.5%) on dry weight basis. The essential elements were in the magnitude order of K+ > Na+ > Ca2+ > Mg2+ > Fe2+ > Zn2+ > PO43−. Among salts, PO43- dominated the composition followed by Cl2−, SO42− and NO3−. Hence, the gut waste that has rich nutrients and essential elements required for bacterial growth has been considered as a substrate for protease production. It has been previously reported that fish waste comprises of 58% protein, 19% fat and trace amounts of minerals, mainly copper, phosphorus, magnesium, sodium, potassium, calcium, iron, zinc and manganese11. Bioconversion of these wastes will help in decreasing the production cost of useful microbial enzymes12,13.
Screening for protease producers
In primary screening, out of 101 bacterial isolates, 53 colonies produced clearance zones in casein agar. Fish gut waste was used as the substrate for secondary screening. Twenty seven isolates out of 53 were found as best protease producers. In secondary screening, maximum growth was shown by bacterium 17 at 72 h and the least growth was shown by bacterium 5 (Fig. 1a). For the majority of the isolates, growth was maximum at 72 h, after which it enters into stationary phase.
Highest mean protease activity (48.42 units/ml) was exhibited by bacterium 7 at 72 h of incubation (Fig. 1b). One way ANOVA followed by Tukey’s Post Hoc analysis showed that the protease activity of bacterium 7 was significantly higher than all the other bacterial isolates (P < 0.05). This is in agreement with a study that reported on maximum protease production from B. licheniformis after 72 h in potassium nitrate containing basal medium14. It has also been previously reported that alkaline protease from Bacillus subtilis Y-108 has exhibited maximum activity at 72 h of incubation in medium containing shrimp and crab shell powder15. The extracellular protease production during stationary phase is a characteristic feature of many bacterial species. Three independent experiments were conducted with bacterium 7 using gut waste substrate and it produced maximum activity at 72 h of incubation, consistently. Thus, this bacterium was chosen for optimization and production of protease enzyme. Regression analysis was carried out for growth and protease activity of all the 27 bacteria. A positive relationship between growth and protease activity was observed (R2 = 0.73, R2 = 0.67, R2 = 0.63 R2 = 0.74 for 24, 48, 72 and 96 h respectively). This indicates that the bacteria utilized fish gut waste substrate as the primary nutrient for its growth by producing protease enzyme. Positive relationship between growth of bacteria and protease activity has been previously reported16.
Identification of bacteria
All the protease producing bacteria isolated in the secondary screening were identified using MALDI biotyper. The score values greater than 2 and 1.7 indicates a reliable identification at the species and genus levels respectively, whereas the score value less than 1.7 indicates unreliable identification. Among the identified, only one bacterium was identified as Brevibacillus spp. and all the other isolates were Bacillus spp. The best producer, bacterium 7 was identified as B. licheniformis with a score of 2.115. Molecular identification of the bacterium 7 was also carried out by 16 S rRNA sequencing and the bacterium was identified as B. licheniformis NK with the identity similarity of 99% and the error value of zero, thus, confirming the biotyper result. The sequence was submitted to NCBI (accession number: MG 808382). The evolutionary history was inferred using the UPGMA method. The optimal tree with the sum of branch length = 0.18940404 is shown in Fig. 2. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site. The analysis involved 15 nucleotide sequences and all the ambiguous positions were removed for each sequence pair. Totally 1574 positions were in the final data set and the evolutionary analyses were carried out using MEGA7.
Screening of significant factors by PB design
The significant variables necessary for the enhanced protease production were screened using the PB design. Five variables, including temperature, pH, time, inoculum and substrate concentration were analyzed (Table 3) for their effects on growth and protease production. The runs generated in PB are shown in Table 4 along with the response variable. The effect of variables on protease activity could be explained with the help of effect plot developed using Minitab version 17. Points above the hatch line have a positive effect and below have a negative effect. The effect of factors in PB design showed that the growth was maximum at 40 °C (Fig. 3a), but the increase in temperature inhibited the bacterial growth. Most favourable pH for growth was 7, and increasing pH decreased the growth. Growth was the maximum at 24 h of incubation and slightly deceased at 72 h. Growth of B. licheniformis NK was increased with the increasing concentration of inoculum and was maximum at 5% inoculum size. Increase in substrate concentration had a negative effect on growth. Protease activity was maximum at 40 °C (Fig. 3b) and decreased after that. Hence, a range between 40 and 50 °C was selected for RSM design. The levels of pH did not affect the protease production much. Although pH had a slight negative effect on the protease production, in all the runs the final pH obtained was 9 irrespective of the initial pH. Hence, pH 9 was maintained for all the runs in RSM design. Protease activity increased with increasing time and was maximum at 72 h. The protease activity increased linearly with increasing inoculum size and substrate concentration. Among the five factors, three of them, including time, substrate concentration and inoculum size showed a positive effect, but the temperature and pH showed a negative effect.
Optimization by RSM
The range of variables used in response surface design is presented in Table 5. The RSM was performed in 31 runs with four factors (temperature, time, inoculum size and substrate concentration) using the central composite design. Though temperature had a negative effect, it was included for RSM to find out the exact temperature at which the bacterium could produce maximum protease activity. All the runs were conducted at pH 9. The application of the response surface methodology for the obtained data yielded the following quadratic equation in coded units.
Protease activity (U/ml) = −2583 + 43.6 a + 39.5 b + 66.9 c + 59.1 d − 0.4883 a2−0.2789 b2−5.74 c2 − 15.01 d2 + 0.006 a*b − 0.107 a*c + 0.625 a*d − 0.018 b*c + 0.021 b*d − 1.90 c*d,
Where, a-temperature, b-time, c-inoculum size and d-substrate concentration.
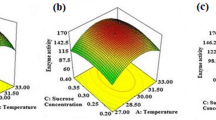
The runs and the corresponding observed and predicted response variables are given in Table 6. The adequacy of the quadratic model fitting of data was tested by ANOVA and the low P value (0.001) indicates a high significance in the regression model. The R2 value of 82.92% confirmed a satisfactory adjustment of the quadratic model to the experimental data and suggested that there is no significant difference between the predicted and experimental values, thus, making the model acceptable to optimize the conditions for protease17. Contour plots were plotted to show the effect of two factors on the protease activity, keeping other factors at constant central values (Fig. 4a–f). The best conditions for protease production observed from contour plots were temperature 45 °C, incubation time of 70 h, inoculum size 5% and substrate concentration 2.3%. Using these experimental results, the optimum conditions for protease production were predicted using Minitab as temperature 46.31 °C, incubation time of 71.11 h, inoculum size 4.86% and substrate concentration of 2.66%. The predicted maximum protease activity was 68.56 U/ml. The validation experiment of the model optimization was carried out. There was no significant difference between the observed (73.52 U/ml) and predicted protease activity using a t-test at the 5% level (t-test; P > 0.05). Thus, the model was accepted and the same conditions were used for the final protease production. The experiment under optimized conditions showed a 1.5-fold increase in protease production. A study reported by Beg et al.18 showed that alkaline protease production by Bacillus mojavensis was increased up to 4.2 fold in a 14 L bioreactor using RSM. In another study, there was an overall increase of 2.3 fold in protease production by Bacillus sp. RKY3 after optimization19. Cost-effective media formulation and statistical optimization are very useful for maximum enzyme production in laboratory and industrial-scale enzyme production. The optimum temperature 46.31 °C for the protease indicates that the produced alkaline protease is thermotolerant in nature.
Contour plot of protease activity with interaction between (a) temperature and time, (b) inoculum size and substrate concentration, (c) temperature and inoculum size, (d) temperature and substrate concentration, (e) time and inoculum size and (f) time and substrate concentration. Hold values: Inoculum size 5%, substract concentration 2.25%, time 70 h, and temperature 45 °C.
ANN
The ANN is a black box concept based model which involves training, validation and testing structure and functionalities of biological neural networks. Artificial neuron (in terms of black box based simple artificial function) is a fundamental concept of ANN20. Such a model has three simple set of rules: multiplication, summation and activation. Inputs are weighted at the entrance of artificial neurons, sum function that sums all weighted inputs and bias performed in the middle section of artificial neuron. At the exit of artificial neuron, the sum of formerly weighted inputs and bias is passing through an activation function called the transfer function (Fig. 5a). The overall coefficient of determination (R2) value of 82.2% using RSM method encouraged us to make use of ANN to obtain a better model with higher accuracy in R2. ANN has been proven to be a successful tool in black-box based studies by a large number of researchers20 and their improved results motivated us to gain further understanding of protease activity with respect to its associated independent decision variables. The detailed working mechanism of ANN along with information on its control parameters, number of hidden layers, transfer function, algorithm used for training, etc. is summarized below along with improved results.
In the existing problem, initially the data consisting of 31 data points having four variables data (namely, temperature, time, inoculum and substrate concentration) and the corresponding overall activity were considered. The data were filtered and one data point corresponding to the lowest extreme value of overall activity of 0.48 was discarded from ANN analysis. Thus, in total 30 numbers of data points were considered, Among the 30 points, 20 randomly chosen points were used for training purpose, 5 points were used for validation and the remaining 5 individual solutions were used for testing the neural network. ‘Nntraintool’ toolbox from Matlab 2011 library was used. The number of hidden layers was varied and several trial runs were carried out. Finally, it was observed that with 12 numbers of hidden layers, the best possible predictions were obtained. Levenberg-Marquardt algorithm was used for training purpose. The mean squared error (MSE) criteria were used to test performance of predicted data. Random algorithm was used for data division. The mean squared error (MSE) for testing was observed to 0.29 (for 20 sets of data points), whereas for validation and testing, the corresponding MSE was 32.62 and 43.46 respectively (for each 5 sets of data points). The Table 6 and Fig. 5b shows the predicted values of protease activity obtained using ANN. Figure, 5b also shows the comparative plot between the observed and predicted activity as obtained using RSM and ANN methods. The figure clearly shows that using the black box based ANN method using Levenberg-Marquardt algorithm for training purpose resulted in solutions such that the majority of the solutions complement each other in terms of observed and predicted activity when ANN was used. The overall coefficient of determination (R2) value using ANN is 96.67%, which is higher than the 82.2% obtained by RSM method. Thus, ANN is observed to be an improved tool to predict the experimental results.
Partial purification and molecular weight determination
The stages of purification and the corresponding protease activities are given in Table 7. After purification, the activity increased by 6.5-fold. The purified enzyme produced two bands each corresponding to molecular weight 60 and 65 KDa (Fig. 6; Supplementary Fig. 6). Two band formation could be due to the presence of two types of proteases21 or single enzyme with two polypeptide chains22.
Characterization of the protease
Effect of pH
The optimum pH range for alkaline proteases generally ranges from 9–11. The purified protease from B. licheniformis NK exhibited a reasonable activity in a pH range of 5–11 with a significantly higher activity at pH 9 and 10 (Fig. 7a). It retained 98% activity at pH 10 and 82% at pH 11. Though it is alkaline, the protease retained 57% activity at pH 5. The stability of the enzyme was measured at pH 9 by incubating the enzyme for 150 min and measuring the activity at every 30 min interval. The enzyme was stable up to 90 min at pH 9 with a residual activity of 93% (Fig. 7b). The protease retained 90% activity, even at 120 h at pH 9. Thus, the activity of protease produced from B. licheniformis NK is comparable to the commercialized detergent enzymes such as Maxatase (Gist-brocades, The Netherlands), produced by B. subtilis having an optimum range between pH 9 and 1023. The broad pH range of activity, its optimum activity at higher pH values and the stability for a longer duration at alkaline pH suits this enzyme highly attractive for detergent industry, since laundry detergents generally operate at a pH of 7–11 and other industrial processes involving alkaline processes. Apart from detergent industries, alkaline proteases are also utilized in the formulation of household dishwashing and cleaning detergents, preparation of protein hydrolysates, dehairing and bating of skin and hides during leather processing24.
Effect of temperature
Alkaline protease from B. licheniformis NK showed a significantly higher activity at 50 °C and a reasonable activity was observed between 40 to 60 °C (Fig. 8a). Yang et al.15 reported that the protease from B. subtilis also showed maximum activity at 50 °C. The stability of the protease was studied at 50 °C. The protease maintained a residual activity of 94% at 30 min at 50 °C (Fig. 8b) and retained 70% residual activity, even at 150 min. The produced protease had a residual activity of 89% after one hour at 50 °C, which was much higher than 30% of residual activity reported by Banik et al.25. Proteases used in detergent industries should have high activity at alkaline pH ranges and should be thermostable in order to be effective during washing. The protease produced in this study is alkaline in nature and thermotolerant and therefore has the potential for detergent industry applications. In addition, thermostable enzymes can minimize the problem of contamination by running the processes not suitable for mesophilic microbes. This property would be very helpful when fish gut waste is used as a substrate for protease production. Further, the high temperature increases the substrate solubility and lower the viscosity of liquids which helps in mixing of medium components.
Effect of metal ions
The effect of different metal ions on protease activity was examined (Fig. 9a). The inducers Zn++ and Na+ increased the activity by 40% and 11% respectively. Binding sites of Zn++ and Na+ may stabilize and activate the enzyme. However, Fe++, Cu++, K+, Ca++, and Mg++ inhibited the activity. Abou-Elela et al.26 reported that protease produced from Bacillus cereus MCM B-326 was completely inhibited by Mg2+ and Zn2+ and partially inhibited by Na+, whereas Ca2+ was not required for activity but was essential for growth22. In contrast, it was reported that Mn2+, Ca2+ and Mg2+ ions have increased the relative protease activity of Bacillus megaterium isolated from Thai fish sauce27. Following the addition of MnCl2 and CaCl2, the activity of alkaline protease from Trametes cingulata strain CTM10101 was enhanced by 170 and 219%, respectively28. This shows that there is no standard cofactor for enhancement of protease activity. The enzyme produced by different strains of bacteria of diverse locations may require different metal ions to trigger their action.
Influence of (a) metal ions (10 mM), (b) surfactants (1%) and inhibitors (2.5 mM) on protease activity at pH 9 and temperature 50 °C. Bars with same alphabets are not significantly different from each other (Turkey’s test; P < 0.05). Residual activity (%) compared to the activity of pure enzyme is given within the box above each bar.
Effect of surfactants and inhibitors
Inhibition studies give an idea about the nature of the enzyme, its active center and its cofactor requirements. The alkaline protease was stable against SDS and Tween 80 by showing residual activity of 90 and 98% respectively (Fig. 9b). The stability towards SDS is important in detergent industry as SDS is a very common additive in commercial detergents and Bacillus spp. are known to be the most common producers of such stable enzymes. The protease enzyme of this study showed a residual activity of 93 and 103% against urea and βME. Urea is a common surfactant used in detergents. βME is an aspartic protease inhibitor29, and it did not have any effect on the enzyme, indicating that this enzyme may not be an aspartic protease.
Compatibility with commercial detergents
The purified protease is compatible with all the commercial detergents used and the residual activity was above 90% in all the detergents (Fig. 10). Maximum residual activity was observed with Arial and Bahar (97%), followed by Tide (95%) and Bonux (92%). The small bars show the protease activity in the respective commercial detergents available in the market. It has been reported that the alkaline protease produced from B. licheniformis RP1 retained 95% of its initial activity with Arial followed by Axion (94%) and then Dixan (93.5%) when pre incubated with detergents at 40 °C for 60 min30. A serine alkaline protease from Bacillus sp. SSR1 exhibited almost 70–80% of activity in most of the detergents at 40 °C31. The detergent compatibility of the alkaline protease produced in this study is higher than the previously reported enzymes, which suits the enzyme as a best additive to the detergents.
Substrate specificity
Different substrates such as peptone, BSA, tryptone soya and gelatin (1%) were used to compare their influence on protease activity as opposed to that of the pure enzyme with casein. Results showed that tryptone soya, gelatin and peptone are suitable substrates having activity 122, 119 and 104% respectively, whereas with BSA the activity decreased to 73% (Fig. 11a). The results suggested that this protease is able to digest various protein substrates and could convert them into small peptides and amino acids, demonstrating that this alkaline protease has a broad range of substrate specificity. The protein hydrolysates obtained from casein, soy protein and whey protein are useful in the formulation of hypoallergenic infant food. Further, they can also be useful in fortification of fruit juices and in preparation of high protein therapeutic diets32. In addition to its stability at different pH, temperature, surfactants, inhibitors and detergents, this wide range of substrate utilization makes the enzyme as a suitable candidate for various industrial applications including food industries.
Effect of (a) Different substrates (1%) on protease activity. Protease activity in the presence of casein was considered 100%. Bars with same alphabets are not significantly different from each other (Tukey’s test; P < 0.05). Residual activity (%)compared to the activity of pure enzyme is given within the box above each bar.(b) Substrate concentration on protease activity using non-linear regression and estimation of enzyme kinetic parameters.
Enzyme kinetics
The kinetic parameters, Vmax, the maximum enzyme velocity and Km, the Michaelis- Menten constant of the protease enzyme were estimated by measuring the enzyme activity at different concentrations of the substrate casein (0.156–15 mg/ml). The equation describing the relationship is Y = Vmax*X/(Km + X). Figure 11b shows the results of the non-linear regression and the values of Vmax (88.57 U/ml) and Km (0.4384 mg/ml). The tested null hypothesis is the Michaelis-Menten as opposed the alternate hypothesis of allosteric sigmoidal. The calculated F = 0.6232< tabulated F0.05, 1,27 = 4.21 at P = 0.8048 and goodness of fit as R2 = 0.9872 accepts the null hypothesis and the best model fitting the present data is Michaelis-Menten. The increase in substrate concentration here increased the protease activity up to 10 mg/ml, after which it is saturated. The smaller Km value indicates the high affinity and efficient catalytic role of the enzyme towards the substrate33.
Conclusion
In this study, gut waste of S. longiceps has been used as a low cost substrate for protease production using B. licheniformis NK isolated from Nakhl hot spring, Oman. The best conditions for the protease production were optimized as 46.31 °C, incubation time 71.11 h, inoculum 4.86% and substrate concentration 2.66%. The predicted maximum protease activity was 68.56 U/ml. The validation experiment of the model optimization showed a protease activity of 73.52 U/ml. ANN is a better tool to predict the experimental results. The protease enzyme retains maximum activity and stability at pH 9 and 50 °C. The enzyme was stable against surfactants, inhibitors and compatible with the commercial detergents. This study concludes that fish gut waste is a worthy low cost substrate for protease production and the produced detergent compatible, thermotolerant, alkaline protease has the potential for industrial applications.
Materials and Methods
Sampling
Soil and water samples were collected from four different sites from Nakhl hot spring (23°22′32“N; 57°49′39″E) in Oman. Air and water temperatures, pH, conductivity and dissolved oxygen levels were recorded at each site during the time of sampling.
Preparation and analysis of fish gut waste substrate
Guts from S. longiceps were excised and washed with water to remove the blood and boiled for 20 min. After draining the excess water, the boiled waste was minced and dried at 50 °C for 72 h in an oven. The dried substance was finely ground to a powder and stored at room temperature. The gut waste was analyzed for its total protein concentration34, total lipids35 and total carbohydrates36 on dry weight basis. Chemical elements in the gut waste were analyzed by inductively coupled plasma mass spectrometry (ICP-MS, BRUKER Aurora M90, USA) and compound anions were estimated by Ion Chromatography (IC, 850 Professional, Metrohm, Switzerland).
Screening and identification of protease producing bacteria
Soil and water samples collected from Nakhl hot spring were inoculated into casein agar plates (g/l: casein 2, glucose 2, K2PO4 0.2, MgSO4 0.2, FeSO4.7H2O 0.01, and agar 20). Protease producing bacteria were selected based on the size of the clearance zone formed around the colonies. Secondary screening was carried out in 1% fish gut waste broth medium (without any additives), with 4% (v/v) inoculum and incubated at 50 °C in order to identify the best protease producing bacteria. The bacterial strains that showed positive results in secondary screening were identified by matrix-assisted laser desorption/ionization (MALDI) biotyper (BRUKER, Microflex). The best protease producing bacteria after secondary screening was identified by 16 S rRNA sequencing.
Protease assay
Protease activity was measured by the modified method of Puri et al.17 using casein as a substrate and tyrosine as a standard. To 0.25 ml of sample, 0.25 ml of 1% casein in 50 mM glycine NaOH buffer (pH 9) was added and incubated for 10 min at 50 °C. To stop the reaction 0.5 ml of 0.4 M TCA was added and the mixture was centrifuged at 10000 rpm for 10 min. From the supernatant 0.5 ml was taken and to this 2.5 ml of 0.4 M sodium carbonate and 0.25 ml of 2 N Folin’s reagent was added and incubated for 30 min at room temperature. Absorbance was read at 660 nm using a spectrophotometer (Thermo spectronic, USA). One unit of protease activity is defined as the amount of enzyme required to liberate 1 µmole of tyrosine under defined standard assay conditions.
Optimization and production
Screening of significant factors by PB design
The PB design is an effective way to find factors with positive effects37 on protease production among the number of variables used. The PB design is a two level fractional factorial screening design for studying N-1 variables using N runs, where N is a multiple of four. Each factor was studied at two levels, −1 for a low level and + 1 for a high level. The variables selected for this study were temperature, time, pH, percent of inoculum (v/v) and percent substrate concentration (w/v) (Table 3). Totally, 12 runs were generated in PB design.
Optimization by RSM
Based on the results obtained from PB runs, a response surface design was generated to determine the optimum levels of the selected variables in protease production37. In RSM, 31 experiments were conducted in triplicate using central composite design. The factors considered for RSM designs are given in Table 5. The protease activity, the response variable in the model generated, was statistically analyzed and conditions for maximum protease activity were predicted. A validation experiment was conducted in triplicate to confirm the prediction. Both PB and RSM were designed using the statistical software Minitab, Version 17.
ANN
Thirty-one data sets having four variables, temperature, time, inoculum size and substrate concentration and the corresponding overall activity were considered for ANN study. The data were filtered and the lowest activity of 0.48 was not included in ANN analysis. Out of 30 available data sets, 20 were used for training purpose, 5 used for validation and the remaining 5 to test the neural network. ‘Nntraintool’ toolbox from Matlab 2011 library was used.
Enzyme production and partial purification
After the fermentation, the cells were removed from the crude supernatant by centrifugation at 10000 rpm for 10 min. The protein in the supernatant was precipitated by 60% ammonium sulphate and dialyzed against Tris HCl buffer. The dialyzed sample was loaded onto a Sephadex G-100 column pre-equilibrated with 25 mM Tris HCl eluting buffer (pH 7) and was eluted at a flow rate of 0.2 ml/min through the column. The eluted fractions with the highest activity were pooled and used for characterization.
Characterization
Molecular weight determination
The molecular weight of the purified protease was determined using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% polyacrylamide gel. Kaleidoscopic prestained low molecular weight protein standard (6.2 KDa – 199.4 KDa) from BIORAD was used as the ladder.
Effect of pH on the activity and stability
To evaluate the effect of pH on protease, the activity of the purified protease was measured at different range of pH (3–11). The buffers used for different pH ranges were glycine HCl (pH 3–4), phosphate buffer (pH 5–6), Tris-HCl buffer (pH 7–8) and Glycine NaOH (pH 9–11). The pH at which the protease showed high activity was considered as optimum and the pH stability of the enzyme was determined by measuring the protease activity at every 30 min interval up to 2.5 h by incubating the enzyme at the optimum pH. Residual activity was measured and the activity measured without pre-incubation was considered as the control with 100% activity.
Effect of temperature on the activity and stability
The effect of temperature on the protease activity has been studied by incubating the protease at various temperatures (20–70 °C) for 30 min at optimum pH. Thermal stability was studied by incubating the protease for 150 min at optimum temperature. Aliquots were withdrawn at 30 min intervals to test the residual activity at optimum conditions. The activity measured without pre-incubation was considered as the control with 100% activity and the above optimum pH and temperature were used for the remaining characterization assays.
Effect of metal ions, surfactants, inhibitors, detergents and substrates
The effect of some metal ions (10 mM) such as Zn++, Na+, Fe++, Cu++, K+, Ca++ and Mg+, surfactants (0.1%) such as sodium dodecyl sulphate (SDS) and Tween 80 and 2.5 mM of inhibitors such as β-mercaptoethanol (βME) and urea were used to study their effect on protease activity. Effect of commercial detergents (0.1% w/v) such as Arial, Bahar, Bonux and Tide were used to study their effect on protease enzyme. The influence of various substrates (1%) like peptone, bovine serum albumin (BSA), tryptone soya and gelatin were used to study their effect on protease activity. For all experiments, the enzyme activity was studied by pre-incubating the protease enzyme with the respective component for 30 min at room temperature. Residual activity after 30 min was measured and activity without pre-incubation was considered as 100% activity.
Determination of Vmax and Km values
The enzyme kinetic parameters, Vmax and Km values of the protease enzyme were estimated by measuring the protease activity using different concentrations of substrate (0.156–15 mg/ml). Vmax and Km values were determined using the Michaelis-Menten equation through nonlinear regression analysis using the statistical software Graphpad PRISM version 6.
Statistical analysis
All the experiments were conducted in triplicate. Significant changes in the mean values were analyzed by one-way ANOVA followed by Tukey’s Post Hoc analysis using IBM SPSS statistics software version 21. Statistical difference at p < 0.05 were considered as significant.
Data availability
All data generated or analyzed during the study are included in this article.
References
Sivakumar, N., Remya, R. & Al Bahry, S. Partial characterization of proteases produced by three fungal isolates from the rhizosphere of wild yam Dioscorea wallichii. J. Appl. Biol. Sci. 3, 71–75 (2009).
Dias, D. R., Vilela, D. M., Silvestre, M. P. C. & Schwan, R. F. Alkaline protease from Bacillus sp. isolated from coffee bean grown on cheese whey. World J. Microb. Biot. 24, 2027–2034 (2008).
Karthikeyan, A. & Sivakumar, N. Citric acid production by Koji fermentation using banana peel as a novel substrate. Bioresour. Technol. 101, 5552–5556 (2010).
El-Bakry, M., Gea, T. & Sanchez, A. Inoculation effect of thermophilic microorganisms on protease production through solid-state fermentation under non-sterile conditions at lab and bench scale (SSF). Bioprocess Biosyst. Eng. 39, 585–592 (2016).
Ramkumar, A., Sivakumar, N. & Victor, R. Fish waste-potential low cost substrate for bacterial protease production: A brief review. Open Biot. J. 10, 335–341 (2016).
Andre, I. K. & Siuli, M. Response surface methodology. WIREs Comp. Stat. 2, 128–149 (2010).
Okino-Delgado, C. H. & Fleuri, L. F. Obtaining lipases from byproducts of orange juice processing. Food. Chem. 163, 103–107 (2014).
Panda, M. K., Sahu, M. K. & Tayung, K. Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. Iran. J. Microbiol. 5, 159–165 (2013).
Zilda, D. S. et al. Screening of thermostable protease producing microorganisms isolated from Indonesian hotspring. Squalen. 7, 105–114 (2013).
Ahmetoglu, N., Bekler, F. M., Acer, O., Guven, R. G. & Guven, K. Production, purification and characterisation of thermostable metallo-protease from newly isolated Bacillus sp. KG5. Eurasia. J. Biosci. 9, 1–11 (2015).
Ramakrishnan, V., Ghaly, A. E., Brooks, M. S. & Budge, S. M. Enzymatic extraction of amino acids from fish waste for possible use as a substrate for production of jadomycin. Enz. Eng. 2, 112 (2013).
Esakkiraj, P., Sankaralingam, S., Usha, R., Palavesam, A. & Immanuel, G. Solid-state protease production using anchovy waste meal by moderate halophile Serratia proteamaculans AP-CMST isolated from fish intestine. Ann. Microbiol. 61, 749–755 (2011).
Annamalai, N., Rajeswari, M. V. & Balasubramanian, T. Extraction, purification and application of thermostable and halostable alkaline protease from Bacillus alveayuensis CAS 5 using marine wastes. Food Bioprod. Process. 92, 335–342 (2014).
Lakshmi, B. K. M., Sri, P. R., Devi, K. A. & Hemalatha, K. P. J. Media optimization of protease production by Bacillus licheniformis and partial characterization of alkaline protease. Int. J. Curr. Microbiol. App. Sci. 3, 650–659 (2014).
Yang, J. K., Shih, I. L., Tzeng, Y. M. & Wang, S. L. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb. Technol. 26, 406–413 (2000).
Kumar, R. S., Ananthan, G. & Iyappan, K. Response surface method to optimize the low cost medium for protease production using anchovy meal from ascidian associated Bacillus sp. GA CAS10. Afr. J. Biotechnol. 13, 2741–2749 (2014).
Puri, S., Beg, Q. K. & Gupta, R. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr. Microbiol. 44, 286–290 (2002).
Beg, Q. K. & Gupta, R. Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb. Technol. 32, 294–304 (2003).
Reddy, L. V. A., Wee, Y. J., Yun, J. S. & Ryu, H. W. Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett–Burman and response surface methodological approaches. Bioresour. Technol. 99, 2242–2249 (2008).
Kuo-lin, H., Gupta, H. V. & Sorooshian, S. Artificial Neural Network Modeling of the Rainfall-Runoff Process. Water Resour. Res. 31, 2517–2530 (1995).
Sabtecha, B., Jayapriya, J. & Tamilselvi, A. Extraction and characterization of proteolytic enzymes from fish visceral waste: Potential applications as destainer and dehairing agent. Int. J. Chem. Tech. Res. 6, 4504–4510 (2014).
Nilegaonkar, S. S., Zambare, V. P., Kanekar, P. P., Dhakephalkar, P. K. & Sarnaik, S. S. Production and partial characterization of dehairing protease from Bacillus cereus MCM B-326. Bioresour. Technol. 98, 1238–1245 (2007).
Beg, Q. K., Sahai, V. & Gupta, R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 39, 203–209 (2003).
Neklyudov, A. D., Ivankin, A. N. & Berdutina, A. V. Properties and uses of protein hydrolysates (Review). Appl. Biochem. Microbiol. 36, 452–459 (2000).
Banik, R. M. & Prakash, M. Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Microbiol. Res. 159, 135–140 (2004).
Abou-Elela, G. M., Ibrahim, H. A., Hassan, S. W., Abd-Elnaby, H. & El-Toukhy, N. M. Alkaline protease production by alkaliphilic marine bacteria isolated from Marsa-Matrouh (Egypt) with special emphasis on Bacillus cereus purified protease. Afr. J. Biotechnol. 10, 4631–4642 (2011).
Yossan, S., Reungsang, A. & Yasuda, M. Purification and characterization of alkaline protease from Bacillus megaterium isolated from Thai fish sauce fermentation process. Science Asia. 32, 377–383 (2006).
Benmrad, M. O. et al. A novel organic solvent- and detergent-stable serine alkaline proteasefrom Trametes cingulata strain CTM10101. Int. J. Biol. Macromolec. 91, 961–972 (2016).
Kezia, D., Chandrakala, G., Prasanthi, V., Naidu, S. V. & Rao, M. N. Influence of different factors on production of purified protease by Bacillus subtilis DKMNR. Int. J. Pharm. Bio. Sci. 2, 73–85 (2011).
Sellami-Kamoun, A. et al. Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol. Res. 163, 299–306 (2008).
Singh, J., Batra, N. & Sobti, R. C. Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Process Biochem. 36, 781–785 (2001).
Adamson, N. J. & Reynolds, E. C. Characterization of casein phosphopeptides prepared using alcalase: Determination of enzyme specificity. Enzyme Microb. Technol. 19, 202–207 (1996).
Rao, C. S., Sathish, T., Ravichandra, P. & Prakasham, R. S. Characterization of thermo-and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem. 44, 262–268 (2009).
Bremner, J. M. Determination of nitrogen in soil by the Kjeldahl method. J. Agr. Sci. 55, 11–33 (1960).
Manirakiza, P., Covaci, A. & Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J. Food. Compos. Anal. 14, 93–100 (2001).
Dubois, M., Gilles, G. A., Hamilton, J. K., Rober, P. A. & Smith, F. Colorimetric estimation of carbohydrates by phenol sulphuric acid method. Anal. Chem. 28, 350–356 (1956).
Al Azkawi, A.S., Sivakumar, N. & Al Bahry, S. Bioprocessing of cardboard waste for cellulase production. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-018-0309-7 (2018)
Acknowledgements
The authors are grateful to the Sultan Qaboos University, Oman for the scholarship granted to carry out the project. The authors gratefully acknowledge the staff of Central Analytical and Applied Research Unit (CAARU), SQU, for their support.
Author information
Authors and Affiliations
Contributions
A.R. carried out the experiment and prepared the first draft of the manuscript, N.S. designed the experiment, supervised the work and revised the manuscript, A.M.G. performed the artificial neural network and R.V. co-supervised the work, analyzed the data and corrected the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramkumar, A., Sivakumar, N., Gujarathi, A.M. et al. Production of thermotolerant, detergent stable alkaline protease using the gut waste of Sardinella longiceps as a substrate: Optimization and characterization. Sci Rep 8, 12442 (2018). https://doi.org/10.1038/s41598-018-30155-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30155-9
- Springer Nature Limited
This article is cited by
-
Targeted Screening of Fiber Degrading Bacteria with Probiotic Function in Herbivore Feces
Probiotics and Antimicrobial Proteins (2024)
-
Characterization, whole-genome sequence analysis, and protease production of a new thermophilic Bacillus licheniformis strain isolated from Debagh hot spring, Algeria
International Microbiology (2024)
-
Proteomic perspectives on thermotolerant microbes: an updated review
Molecular Biology Reports (2022)
-
Evaluation of various mathematical models for cell growth and high bioconversion potent protease production of Microbacterium sp. in shake flask fermentations
Biomass Conversion and Biorefinery (2022)
-
Homoscleromorpha-derived Bacillus spp. as potential sources of biotechnologically-relevant hydrolases and biosurfactants
World Journal of Microbiology and Biotechnology (2022)