Abstract
The production of protease enzyme was evaluated through the solid state fermentation (SSF) of soy fibre, a waste product that acted as a sole substrate for the fermentation, at a laboratory and bench scale using a 500-mL (batch size 115 g) and 10-L (batch size 2300 g) bioreactors. The objective was to assess the effect of the inoculation of the thermophilic bacteria Thermus sp. on the production of the enzyme when working at laboratory and bench scale under non-sterile conditions, since scaling-up and the need of sterilization are the main challenges of SSF, preventing its industrial development. Results revealed that the inoculation led to a substantial increase in the protease obtained on both scales when compared to non-inoculated fermentation. The maximum protease activities increased as a result of the inoculation from 500 to 800 and from 350 to 670 U/g dry matter of soy fibre in the lab and bench scale bioreactors, respectively. Finally, a very good correlation was found between the protease activities obtained and the fermentation most relevant parameters: oxygen uptake rate (R 2 = 0.81) and temperature (R 2 = 0.82). In this work, we have demonstrated that inoculation is effective even under non-sterile conditions at the kg scale and that this strain is able to compete with autochthonous microbiota and increase the protease production to levels higher than those previously reported in literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-state fermentation (SSF) is the fermentation performed on non-soluble materials, namely the substrate, in the absence or near absence of free water [1]. The SSF process has been extensively studied for the production of high value added products, e.g., biofuels and enzymes [2]. In particular, there are several advantages of using SSF for the production of enzymes such as proteases and lipases. SSF allows for the production of enzymes with high activity and stability at low water and energy demands [2, 3]. From environmental and economical perspectives, there is a possibility of carrying out the process under non-sterile conditions as low or ‘no-cost’ waste products act as substrates for the microbial fermentation [4, 5]. Despite these advantages, the use of SSF in industrial processes is not widely applied due to challenges concerning monitoring, controlling and scaling-up [3, 6].
Proteases (EC 3.4.21-24), which are hydrolases that catalyse the cleavage of peptide bonds in proteins, are a highly complex group of enzymes that differ in their substrate specificity and catalytic mechanism and account for about 60 % of the world market of industrial enzymes [7]. Proteases can be produced by SSF using specific strains grown on wastes of animal or plant origin, such as cow dung and hair waste or potato peels and soy fibres [8]. Cow dung was used in the presence of an inoculum of Halomonas sp. leading to the production of proteases of a relatively high activity of 1351 U/g [9]. Mukherjee et al. [10] screened various agro-industrial and kitchen waste materials of plant origin, such as oil cake, wheat and rice bran, grass, banana leaves, potato peels and used tea leaves, for the use as substrate for protease production through SSF by Bacillus subtilis. It was found that the substrates of potato peel and grass led to the production of proteases with the highest protease activity of up to 2383 U/ml enzyme extract. The same microorganism was used for the production of protease using chicken feather as substrate under optimized culture conditions suitable for the bacteria [11]. Thanapimmetha et al. [12] showed that it is feasible to use Aspergillus oryzae as inoculum and deoiled Jatropha curcas, a major energy crop in Thailand, as substrate in the process of SSF for the production of protease after the optimisation of the conditions of moisture, inoculum and temperature.
However, most studies inoculated a specific strain for the production of protease and were carried out on a small scale using few grams of the waste (substrate) and media suitable for the microbial growth and often performed under sterile conditions [8]. Although inoculation leads to consistent fermentation processes and high enzyme yields, this process is usually associated with very high costs and a difficult scale-up mainly due to the sterilisation that is required for avoiding the growth of competing microorganisms.
Recently, Abraham et al. [4, 5] have shown protease production by autochthonous microorganisms already present in the wastes. Authors used 4.5-L bioreactors working near-adiabatic conditions where temperature profile evolved from mesophilic to thermophilic values as organic matter was degraded. Microbial population evolved together with temperature and biochemical changes attaining interesting protease yields: 395 U/g when using soy fibre and 469 U/g with hair waste from the tanning industry mixed with raw sludge from wastewater treatment. A potential strategy to produce thermotolerant proteases is the inoculation of thermophilic microorganisms in this SSF process [13].
In the current study, soy fibre was chosen to act as sole substrate in thermophilic SSF for the production of protease. The aim was to assess the effect of the inoculation of thermophilic bacteria on the production of protease under non-sterile conditions, at a laboratory as well as bench scale using 500-mL and 10-L bioreactors, respectively. In addition, the data of the protease activities obtained were correlated with the oxygen uptake rate and temperature measured during the fermentations. Apart from the protease production, another important objective was to propose an easily scalable, low-cost and reproducible process for the production of proteases from solid-state substrates.
Materials and methods
Materials
The material used as substrate in SSF was soy fibre, a waste obtained from a local food company (Barcelona, Spain). Wood chopsticks and wood chips acted as a bulking agent: the inert material that provides the proper porosity and maintains aerobic conditions during SSF in the process of fermentation [14]. The chopsticks were purchased from a local supermarket and wood chips were supplied by a composting plant in Barcelona (Spain). All chemical reagents used were of analytical grade and were purchased from Sigma-Aldrich (Barcelona, Spain).
Inoculum
The inoculum used in SSF was a thermophilic bacterial strain of Thermus sp. (ATCC® 3174) purchased from ATCC® (American Type Culture Collection). The medium used for the culture re-hydration and activation was the ATCC® Medium 461: Castenholz TYE. The inoculum was added to the substrate in SSF with a concentration of 5 % (v/w) in the form of a Castenholz broth containing 107 CFU/ml of thermophilic bacteria.
Analytical methods
The following was determined in the substrate used in SSF, according to the standard procedures of analyses recommended by the Test Methods for the Examination of Composting and Compost [15]. In soy fibre, water content was determined by gravimetric analyses after drying at a temperature of 105 °C until constant weight. The organic matter content was determined from mass loss after heating at 550 °C. The total nitrogen was determined after digestion of the samples according to the Kjeldahl method, and the total organic carbon was determined using an O.I. Analytical solid total organic carbon Analyser/Win TOC Solids v3.0. For both, the total nitrogen and total organic carbon determination, the samples were previously dried up and sieved at 0.5 cm. pH and electric conductivity (EC) were determined in an extract 1:5 (w:v) in Milli-Q water and then pH and EC were measured using a pH/EC meter.
SSF
Sampling
Sampling was performed at both scales after the complete manual homogenization of all the material in the reactor to get a fully representative sample. Sample size was 2 g of wet material. Total sampled material was less than 10 % of the total mass.
SSF at laboratory scale
A mixture of the substrate with chopsticks (considered as inert material in the fermentation time) was used with a ratio of 1:0.15 (w:w) giving a total weight of 115 g per batch. The mixtures were fermented for 14 days (336 h) in 500 mL Erlenmeyer flasks under constant temperature [4], using a water bath of 55 °C, and air flow of 1.2 mL g−1 DM min−1 (where DM is the dry matter of the soy fibre). Two processes were assessed, ‘standard SSF’ with no inoculation and ‘SSF with inoculation at 0 h’. Samples were collected at 0, 12, 24, 96, 144, 168, 264 and 336 h of the fermentation time in order to obtain the profile of the production of protease.
SSF at bench scale
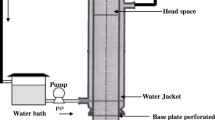
A mixture of the substrate with wood chips was used with a ratio of 1:1 (w:w) giving a total weight of 2300 g per batch. This ratio is increased from lab to bench scale to provide enough porosity and prevent compaction [14]. The mixtures were fermented for 14 days in 10-L air-tight bioreactors, working under near-adiabatic conditions and oxygen controlled aeration (1.1–2.7 mL g−1 DM min−1, oxygen setpoint 12 % in air). These conditions led to an increase in temperature due to the metabolic heat released in the biodegradation process, as occurs with the composting process. The experimental set-up is shown in Fig. 1. Three SSF processes were assessed, ‘standard SSF’, ‘SSF with inoculation at 0 h’ and ‘SSF with inoculation at 24 h’. The formers were performed in order to compare the results with the ones obtained at lab scale. In addition, SSF with inoculation at 24 h allowed for a further assessment of the effect of inoculation on a large scale, especially that SSF in bioreactors is under uncontrolled temperature, which was measured overall the fermentation process. Samples were collected at 0, 96, 168, 264 and 336 h of the fermentation time in order to obtain the profile of the enzyme production.
SSF parameters
Oxygen uptake rate (OUR) in mg O2 g−1 DM h−1, where DM is the dry matter of the soy fibre, was measured over the fermentation time. The area under the OUR curve was also calculated, which represents the total oxygen consumed in the process [16]. The temperature was recorded in the bioreactors during the fermentation and the area below the temperature curve calculated is related to the heat released in the biodegradation in SSF.
Enzyme extraction
Fermented solid material (soy fibre) was mixed thoroughly with 50 mM Tris–HCl (tris(hydroxymethyl)aminomethane) buffer at a pH of 8.10 in a ratio 1:5 (w:v) for a period of 45 min. The extract was separated by centrifugation, using a Beckman J-1200 centrifuge, at 10,000 rpm during 20 min at 4 °C followed by a filtration using a filter paper with a pore size of 0.45 μm. The filtered supernatant was used as the crude enzyme extract for the enzyme assay.
Enzymatic assay
Protease activity was determined using a modified method of Alef and Nannipieri [17]. One millilitre of the enzyme extract was added to 5 mL of 2 % fresh casein solution and incubated at 50 °C during 2 h. One unit of protease activity (U/g DM) is defined as 1 μg of tyrosine released per minute and per gram DM of soy fibre, under the assay conditions.
Data correlation
Sigmaplot 10.0 was used to correlate the protease activities with the OUR and the temperature using standard models. The goodness of fit of the models was evaluated by the correlation coefficient (R 2) and the p value as the main criterion.
Statistical analysis
All SSF experiments and analyses were done in triplicate and therefore, results are represented as their average ± standard deviation. A statistical analysis was performed using the Student’s t test using Minitab Software version 15.1 [18]. A probability level of p < 0.01 was selected to determine statistically significant differences between enzymatic activities.
Results
Soy fibre, the waste that was used as substrate in SSF for the production of protease enzyme, was fully characterised as previously explained and the results are represented in Table 1.
SSF was carried out at laboratory scale as well as at bench scale using 500-mL and 10-L bioreactors with a total batch size of 115 and 2300 g, respectively. The effect of the inoculation of thermophilic bacteria Thermus sp. on the potential of biodegradability and capability of producing protease by the soy fibre was evaluated.
SSF at laboratory scale
OUR profiles
During the process of fermentation of soy fibre of 336 h (14 days), the OUR was recorded at standard SSF and SSF with inoculation at 0 h (Fig. 2). The trend of the standard OUR profile is explained as follows. The OUR recorded started from 0 mg O2 g−1 DM h−1 with a sharp and continuous increase until reaching a maximum value or a ‘peak’ of a value of around 7 mg O2 g−1 DM h−1, at a time of 24 h. This OUR peak was stable for few hours and then was followed by a sharp decrease until reaching an OUR value of around 4 mg O2 g−1 DM h−1 at 40 h. The OUR gradually decreased over a time of 100 h until reaching an OUR value of 0.3 mg O2 g−1 DM h−1, which is an OUR minimum value at which the OUR stabilises until the end of the process. The total oxygen consumed during the whole fermentation process was in average 480 mg O2/g DM.
The inoculation at 0 h of the substrate used in SSF led to a similar trend in the OUR profile to the one obtained at standard SSF. The differences were observed in a substantially higher peak of 11 mg O2 g−1 DM h−1. This was followed by a gradual and less sharp decrease in the values of OUR, compared to the OUR profile of standard SSF, until reaching an almost stable but still higher minimum value of OUR of 1.1 mg O2 g−1 DM h−1. Consistently, the inoculation led to a substantial increase in the total oxygen consumed to 1013 mg O2/g DM.
Protease activity obtained
The protease enzyme activities measured over the process of fermentation at lab scale are shown in Fig. 2. The enzyme activity showed an increase over the time of fermentation up to maximum values of 5.1 × 102 ± 0.5 × 102 U/g DM for Standard SSF and 8 × 102 ± 0.6 × 102 U/g DM for inoculated SSF, both at 144 h. A substantial decrease occurred at the end of the process.
SSF at bench scale
OUR and temperature profiles
During the process of fermentation in the 10-L bioreactors, OUR and temperature were recorded at standard SSF, SSF with inoculation at 24 h and SSF with inoculation at 0 h (Fig. 3a, b, c).
In general, OUR profiles showed the same trend as previously explained in the case of the lab scale. The inoculation resulted in an increased OUR value. The maximum OUR were 7 mg O2 g−1 DM h−1 at 24 h (Fig. 3a, b) for both, the standard SSF and SSF with inoculation at 24 h. Few hours after the inoculation, OUR profile showed an increase compared to the standard SSF. The inoculation at 0 h led to a substantial increase in the OUR maximum, to 11 mg O2 g−1 DM h−1 at 24 h (Fig. 3c), compared to the standard SSF. The OUR minimum values recorded at the end of the fermentation at 200–336 h increased as a result of the inoculation. In addition, the inoculation led to a significant increase in the total oxygen consumed during the process of SSF. The final OUR and total oxygen consumed were 0.2, 0.4 and 0.8 mg O2 g−1 DM h−1, and 400, 485 and 678 mg O2/g DM for the standard SSF, SSF with inoculation at 24 h and SSF with inoculation at 0 h, respectively.
The temperature measured during the SSF started with 20 °C and then increased over the process of fermentation until reaching a maximum at 50 h (Fig. 3a, b, c). This was followed by a gradual decrease until getting a value around 30 °C, at 200 h, which stabilises until the end of the process. The inoculation with thermophilic bacteria (Fig. 3b, c) led to a significant increase in the maximum temperature to 65 °C, compared to 59 °C for the standard SSF (Fig. 3a). The heat released during the fermentation, which corresponds to the area under the temperature curve, significantly increased as a result of inoculation from 12,496 °C h for the standard SSF to 13,219 °C h for the SSF with inoculation at 24 h. A further increase was observed, up to 14,054 °C h, for the SSF with inoculation at 0 h.
Protease activity obtained
In the 10-L bioreactors, the protease activities measured during the SSF are shown in Fig. 3. A similar trend than those obtained at lab scale was observed. The maximum protease activity obtained was at 96 h with a value of 3.7 × 102 ± 0.2 × 102 U/g DM for the standard SSF and 5 × 102 ± 0.5 × 102 U/g DM for the SSF with inoculation at 24 h. In SSF with inoculation at 0 h, the protease activity had similar values to the SSF with inoculation at 24 h until a time of 96 h, after which, the activity of the former was substantially higher until 336 h. The maximum protease activity obtained of 6.7 × 102 ± 0.3 × 102 U/g DM was observed at 168 h.
Figure 4 summarizes the maximum protease activity obtained at lab and bench scale with and without inoculation and presents the statistical comparison. Higher values were obtained at lab scale compared to bench scale, and in inoculated processes compared to non-inoculated.
Data correlation
The relation between the protease activities obtained and the OUR, at both lab and bench scale, or temperature recorded, in the case of bench scale, along the SSF were studied. Protease activities correlated with OUR with a hyperbolic model with a significant R 2 of 0.81 (Fig. 5a). In the bioreactors, protease activities correlated with the temperature recorded with a linear regression (R 2 = 0.82, Fig. 5b). p-value was below 0.0001 in both cases.
Relation between the OUR or temperature and protease activities obtained during the process of fermentation in both the 500-mL and 10-L bioreactors (a) or only in the 10-L bioreactors (b), respectively. All individual data for each replicate has been plotted and different symbols are used for inoculated and non-inoculated systems
Discussion
SSF performance and protease production
In the current study, soy fibre was chosen to act as substrate in SSF for the production of protease enzymes due to its ability to produce such enzymes as previously reported in our laboratories [4].
The data of OUR obtained, in the 500-mL and 10-L bioreactors (Figs. 2, 3), are used to express the biodegradation in organic solid materials as a result of the microbial growth during the process of fermentation [16]. As observed, the OUR and also the temperature profiles were similar to those previously reported in SSF experiments of soy fibre and other organic wastes such as coffee husk and hair wastes [4, 5]. During the fermentation, these parameters rose due to the biodegradation of the organic material until reaching a maximum and then decreased to almost constant minimum values. This is an indication that the waste material was fully stabilised after 336 h (14 days) of fermentation, as it has been previously reported [16]. Therefore, after the extraction of the enzyme, the soy fibre was stabilised and finally can be used as organic amendment for soils. The inoculation of thermophilic bacteria led to higher total oxygen consumed and heat released of the process of fermentation. This is an indication that the inoculum increased the substrate degradation during the fermentation.
In the current study, the protease activity obtained reached values up to 500 U/g DM at lab scale and 350 U/g DM at bench scale for the standard SSF (Figs. 2, 3). These values were relatively high compared to previous works on SSF for the enzyme production using other substrates, e.g., chicken feather and deoiled J. curcas [11, 12]. This could be due to the fact that soy fibre has considerably a high protein and/or amino acid content and nitrogen available [19].
The profile of protease production in the bioreactors showed that the enzyme synthesis coincided with the rise of temperature (thermophilic phase, at temperatures over 50 °C) that is inherent to an adiabatic biodegradation process of biodegradable organic matter. In addition, a positive correlation was found between the enzyme activity and temperature measured during the process of fermentation (Fig. 5b). These might be an indication that mainly thermophilic flora are responsible for the enzyme production, as it has been previously reported in a study on the protease production using soy fibre in SSF on a bench scale [4]. In comparison to this previous study, where SSF was also carried out at the same laboratory scale at 37 °C, much higher enzyme activities were obtained using the same scale but at 55 °C. This further supports the fact that mainly thermophilic microbial growth could be responsible for the protease production. The inoculation of the thermophilic bacteria Thermus sp. led to a substantial increase in the protease activity up to a maximum of 800 and 670 U/g DM at the lab and bench scale (Figs. 2, 3), respectively. Consequently, the autochthonous microorganisms of soybean fibre may be more efficient in the enzyme production, in the presence of the bacteria of the inoculum. Also, another factor may be that Thermus bacteria produced additional protease especially that the bacterial optimum growth occurs at thermophilic temperatures.
Comparison between lab and bench scale
Comparing both work scales, i.e., lab (115 g, controlled temperature at 55 °C) and bench (2300 g, uncontrolled temperature), similar values for maximum biological activity measured as OUR were observed. However, overall activity as given by the total oxygen consumed decreased 17 and 33 % for standard and inoculated at 0 h SSF. Consistently, specific protease activity was significantly reduced in a 30 and 16 % in the standard and inoculated at 0 h SSF, respectively. This can be attributed to the changes in temperature produced at bench scale that affect microorganisms growth and performance, together with a higher heterogeneity found at bench scale for composition and temperature gradients. Temperature could be controlled in the bioreactor to reduce the temperature gradients in time however this strategy would lead to a higher processing cost. Nonetheless, the inoculation led to highly consistent and scalable SSF processes with high reproducibility. From the perspective of bioreactor design and operation a possible approach to improve process in the long term operation is sequential batch or fed-batch operation strategies as proposed by Cheirsilp and Kitcha [20], although this is out of the scope of this paper.
Finally the good correlations found in this work for protease activity with OUR and temperature might allow for the prediction of the protease activities overall a fermentation process.
Future research should explore the long-term operation conditions and the possibility of undertaking high scale SSF reactors to obtain full information on process parameters and enzyme yield. The characterisation and potential applications of proteases obtained by SSF, especially when inoculation with thermophilic bacteria is performed, can be investigated that will allow for detailed economical an environmental assessments. Finally, from microbiological perspectives, the microbial community can be characterised in order to fundamentally understand the enzyme production during SSF processes and the interactions between the autochthonous microorganisms of soy bean fibre and the inoculated strains. Other approaches can consist on isolating protease-producer strains from soy fibre.
Conclusions
The waste soy fibre demonstrated the ability to be a suitable source for protease production through SSF in the 500-mL and 10-L bioreactors. The results revealed that higher enzyme yields can be obtained with an inoculation of thermophilic bacteria Thermus sp. Consistent and reproducible results for OUR and enzyme activities were obtained when scaling-up the SSF from a batch size of 115–2300 g. This suggests that the proposed SSF processes could be easily scalable under non-sterile, near adiabatic conditions. In addition, high correlations were found between the protease activities obtained and the parameters (oxygen uptake and temperature) measured during the fermentation. These correlations might allow for the prediction of the protease activities overall a fermentation process of soy fibre.
As a final conclusion, it can be stated that it is possible to perform SSF processes at bench scale with inoculation under non-sterile conditions obtaining similar results to those found at the lab scale in terms of protease production. Temperature is a key parameter in scaling up the SSF process.
References
Pandey A, Soccol C, Larroche C (2008) Current developments in solid-state fermentation. Springer, Asiatech Publishers Inc, New Delhi
Singhania RR, Patel AK, Soccol C, Pandey A (2009) Recent advances in solid-state fermentation. Biochem Eng J 44:13–18
Salihu A, Alam Z, Abdulkarim I, Salleh H (2012) Lipase production: an insight in the utilization of renewable agricultural residues. Res Cons Recyc 58:36–44
Abraham J, Gea T, Sánchez A (2013) Potential of the solid-state fermentation of soy fibre residues by native microbial populations for bench-scale alkaline protease production. Biochem Eng J 74:15–19
Abraham J, Gea T, Sánchez A (2014) Substitution of chemical dehairing by proteases from solid- state fermentation of hair wastes. J Cleaner Prod 74:191–198
Sukumaran RK, Patel AK, Larroche C, Pandey A (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Tech 46:541–549
Turk B (2006) Targeting proteases: successes, failures and future prospects. Nature Rev Drug Disc 5:785–798
El-Bakry M, Abraham J, Cerda A, Barrena R, Ponsá S, Gea T, Sánchez A (2015) From wastes to high value added products: novel aspects of SSF in the production of enzymes. Crit Rev Env Sci Technol available on-line: doi:10.1080/10643389.2015.1010423
Vijayaraghavana P, Vincent G (2012) Cow dung as a novel, inexpensive substrate for the production of a halo-tolerant alkaline protease by Halomonas sp. PV1 for eco-friendly Applications. Biochem Eng J 69:57–60
Mukherjee AK, Adhikari K, Rai SK (2008) Production of alkaline protease by a thermophilic Bacillus subtilis under solid-state fermentation (SSF) condition using Imperata cylindrica grass and potato peel as low-cost medium: characterization and application of enzyme in detergent formulation. Biochem Eng J 39:353–361
Rai SK, Konwarh R, Mukherjee AK (2009) Purification, characterization and biotechnological application of an alkaline-keratinase produced by Bacillus subtilis RM-01 in solid-state fermentation using chicken-feather as substrate. Biochem Eng J 45:218–225
Thanapimmetha A, Luadsongkrama A, Titapiwatanakunc B, Srinophakun P (2012) Value added waste of Jatropha curcas residue: optimization of protease production in solid state fermentation by Taguchi DOE methodology. Ind Crops Prod 37:1–5
Gaur R, Tiwari S, Sharma (2014) Isolation and Characterization of Thermotolerant Alkaline Serine Protease of Bacillus sp. P-02. Am J Food Tech 9:246–956
Ruggieri F, Gea T, Artola A, Sánchez A (2009) Air filled porosity measurements by air pycnometry in the composting process: a review and a correlation analysis. Biores Technol 100:2655–2666
The US Department of Agriculture and The US Composting Council. Test Methods for the Examination of Composting and Compost (2001) Houston: Edaphos International
Ponsá S, Gea T, Sánchez A (2010) Different indices to express biodegradability in organic solid wastes. J Env Qual 39:706–712
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press Limited, San Diego
Minitab, Minitab 15.1 reference manual (2007) Minitab Inc
García MC, Torreu M, Marina ML, Laborda F (1997) Composition and characterization of soybean and related products. Crit Rev Food Sci Nut 37:361–391
Cheirsilp B, Kitcha S (2015) Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: fed-batch and repeated-batch fermentations. Ind Crops Prod 66:73–80
Acknowledgments
This study was financially supported by the Spanish Ministerio de Economía y Competitividad (Project CTM2012-33663). Mamdouh El-Bakry was recipient of a postdoctoral fellowship from Universitat Autònoma de Barcelona.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Bakry, M., Gea, T. & Sánchez, A. Inoculation effect of thermophilic microorganisms on protease production through solid-state fermentation under non-sterile conditions at lab and bench scale (SSF). Bioprocess Biosyst Eng 39, 585–592 (2016). https://doi.org/10.1007/s00449-016-1540-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1540-x