Abstract
Psoriasis is a common skin disease with the global prevalence of about 2%. Mounting evidence has emerged indicating that there was an association between psoriasis and increased susceptibility to erectile dysfunction (ED). We aimed to assess whether psoriasis was a risk factor for ED through a comprehensive literature review and meta-analysis. The MEDLINE (PubMed), EMBASE, and the Cochrane Library were systematically searched for all studies investigating the erectile function in psoriatic patients. The association between psoriasis and risk of ED was summarized using the odds ratios (OR) with a 95% confidence interval (CI). The protocol for this meta-analysis is available from PROSPERO (CRD42018093025). Overall, 1829449 participants (the mean age ranged from 44 years to 56.3 years) were included from 8 studies (6 cross-sectional, 1 cohort, and 1 case-control study); 39490 of whom were patients with psoriasis, with the mean disease duration from 6 months to 19.9 years. The methodological quality of the 8 included studies was considered to be either moderate or high quality. Synthesis results from the included studies revealed that psoriasis was significantly associated with an increased risk of ED in psoriatic patients (OR = 1.62, 95%CI: 1.37–1.91, P < 0.001; heterogeneity: I2 = 62.6%, P = 0.009). The results were consistent after multivariable adjustment (6 studies; combined OR = 1.5, 95%CI: 1.31–1.72, P < 0.001; heterogeneity: I2 = 53.5%, P = 0.056). Evidence from this meta-analysis indicates that patients with psoriasis have a significantly elevated risk of ED.
Similar content being viewed by others
Introduction
Psoriasis is an immune-mediated dermatosis that is typically characterized by widespread erythematous plaques covered with silvery white scales involving the entire body surface [1]. Psoriasis has been gradually considered as a major global health problem. Epidemiological investigators reported the prevalence of psoriasis in adults varying from 0.91% (United States) up to 8.5% (Norway) [2]. It is suggested that psoriasis is a kind of psychophysiological skin disorder which may affect patients far beyond the skin. Higher risk of developing comorbidities, i.e., cardiovascular disorders (CVD) [3], metabolic syndrome [4], non-alcoholic fatty liver disease [5], Crohn’s disease [6], and cancer [7] was observed among patients with psoriasis than the general population. Moreover, psoriasis is associated with several psychiatric comorbidities, these include, but are not limited to depression, anxiety, poor self-esteem, and even suicidal ideation [8,9,10,11]. All these physical and psychological disorders may have a negative effect on the sufferers’ sexual lives.
A recent epidemiological study described that psoriasis was associated with an increased risk of sexual dysfunction in both female and male subjects (odds ratio = 5.5, 95% confidence interval: 2.6–11.3) [12]. In female patients with psoriasis, Maaty et al.[13]. found that the satisfaction, arousal, and desire significantly decreased with increasing severity of disease, they also observed that patients with genital psoriasis have worse sexual dysfunction than those without genital lesions.
In men patients with psoriasis, the most common complaint among them is erectile difficulties. Based on a large sample case-control study, it has been reported that patients with erectile dysfunction (ED) had a 3.85-fold higher odds in psoriasis subjects than controls [14]. Over the past several years, an increasing number of studies had addressed the relationship between psoriasis and ED [15,16,17]. However, reliable results have been inconsistent with this association. In Goulding et al.’s study [18], though the higher prevalence of ED was observed in men with psoriasis than the controls (58 vs. 49%), the authors concluded that psoriasis per se was not an independent risk factor for ED after conducting a multivariable logistic regression model.
Though a trend toward a higher prevalence of ED in patients with psoriasis was found, the evidence for the potential association is still controversial and the well-established information was still limited. Thus, we conducted a meta-analysis in an attempt to explore whether psoriasis was a risk factor for ED.
Methods
This meta-analysis was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PRISMA checklist was shown in Supplementary Table 1. The protocol for this meta-analysis is available from PROSPERO (CRD42018093025; http://www.crd.york.ac.uk/PROSPERO).
Data sources and searches
MEDLINE (PubMed), EMBASE, and the Cochrane Library were systematically searched from the inception dates of the databases to 7 March 2018. The search was restricted to human participants and the English language. The subject headings and the text keywords were used for the search. We used the search strategy for MEDLINE including the MeSH and text words was (((((((“Erectile Dysfunction”[Mesh]) OR sexual dysfunction, physiological) OR sexual dysfunctions, psychological) OR sexual dysfunction) OR sexual function) OR impotence)) AND ((((((“Psoriasis”[Mesh]) OR psoriasis) OR (pustulosis of palms and soles)) OR pustulosis palmaris et plantaris) OR palmoplantaris pustulosis) OR (pustular psoriasis of palms and soles)). Literature search, study selection, quality assessment, and data extraction were performed by two authors (S.K Z. and J.M W.) independently. Any disagreements were resolved by consensus or consultation with a third author (ZGZ).
Measurement of psoriasis and ED
Definitions of psoriasis and ED were in line with the international classification of diseases codes. Patients with psoriasis were diagnosed based on clinical symptoms, dermatological characteristics, and the clinical diagnosing criteria of psoriasis. ED (also known as impotence) was defined as the recurrent or persistent inability to attain or maintain an erection sufficient for satisfactory sexual performance [19]. We included the studies in which ED was measured by using any of the validated methods, including questionnaires, physical examination, and clinical assessment of erectile function, i.e., the 5-item International Index of Erectile Dysfunction (IIEF-5) questionnaire, the International Index of Erectile Function questionnaire (IIEF), and the pertinent history and physical examination.
Study selection
Any available epidemiologic evidence reported the association between psoriasis and risk of ED were included in the presented study. Participants were limited to the broad-spectrum population diagnosed with psoriasis and ED. According to the patient, intervention, comparison, outcome and study design (PICOS), the question that guided this meta-analysis was: Does psoriasis increase the risk of ED? The PICOS evidence was composed of the following combinations: an adult patient with ED or impotence (P); a history of psoriasis (I); compared with the general or non-psoriasis population (C); the diagnosis of ED or impotence (O); all study designs were accepted (S). In addition, those studies on the pertinent subjects which provided relative risk, hazard ratio, and odds ratios (OR) with 95% confidence intervals (CI) or adequate data to enable calculation of these efficiency values were also included. The exclusion criteria included the following: (1) female subjects; (2) the control population data were not available; (3) review articles, comments, meeting abstracts, editorials, letters, and case reports, etc.; (4) duplicated data; (5) animal experiments; (6) studies with <10 participants; (7) previous publications of the same clinical trial.
Data extraction
A standardized data collection form was conducted to extract the following pertinent information: the first authors’ names, year of publication, country of origin, study design, mean disease duration, the demographic and age of case and the control sample, methods of psoriasis and ED ascertainment, the OR with CI, and variable adjustment for confounding factors.
Quality assessment
The methodological quality evaluation of the cross-sectional study was based on cross-sectional study quality methodology checklist (low quality = 0–3, moderate quality = 4–7, high quality = 8–11). The quality of case-control or cohort study was in line with the Newcastle–Ottawa Scale (NOS, low quality = 0–3, moderate quality = 4–6, high quality = 7–9). The grading of recommendations assessment, development, and evaluation (GRADE) approach was conducted to exert the absolute estimates of the risk of ED in men with psoriasis, assess and rank the overall quality of the evidence.
Statistical analyses
The strength of association between psoriasis and the risk of ED in the included studies was estimated using OR and its 95% CI. Results with a two-tail P value < 0.05 were regarded as statistically significant. The heterogeneity of included studies was evaluated by using the I2 statistic and the Cochrane Q statistic (I2 > 50% was considered of substantial heterogeneity; a P value of Q test < 0.10 was considered statistically significant). On account of the high likelihood of between-study variance for differences in study design and study population, a random effects model rather than a fixed effects model was employed. Subgroup analyses were utilized to further investigate the origin of heterogeneity. Sensitivity analyses were applied to detect the potential source of heterogeneity by omitting studies one by one and evaluating the resulting effect. Publication bias analysis was assessed by the Begg’s and Egger’s test. The current statistical analysis was performed using the Stata (version 13.0, Stata Corp LP, College Station, Texas, USA).
Results
Literature search
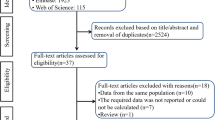
Through a broad selection of the databases, a total of 297 relevant articles were identified in the initial search (97 articles from MEDLINE, 115 articles from EMBASE, and 85 articles from the Cochrane Library). Of these articles, underwent the title and abstract review, 245 were excluded after removing duplicates, and irrelevant articles, leaving 52 pertinent articles for further full-text review. Among them, 8 studies were excluded due to the control group was not available; 15 for failing to meet the inclusion criteria; 10 for inappropriate grouping; and 11 for insufficient outcome data. Lists of full-text excluded studies with reasons for exclusion are presented in Supplementary Table 2. Finally, 8 observational studies [18, 20,21,22,23,24,25,26] (6 cross-sectional studies [18, 21,22,23,24, 26], 1 cohort study [20] and 1 case-control study [25]) met the pre-defined eligibility criteria were included in this study. The selection process was illustrated in Fig. 1.
Study characteristic
The 8 studies which published between 2010 and 2017 years have been enrolled a total of 1,829,449 participants, 39,490 of whom were psoriasis patients while the remaining 1,789,959 individuals were healthy controls. The mean age of the individuals among studies ranged from 44 years to 56.3 years. Six studies [18, 21,22,23, 25, 26] were conducted in Europe and 2 studies in Asia [20, 24]. The mean disease duration of psoriasis ranged from 6 months to 19.9 years. And 6 studies [18, 20, 21, 24,25,26] reported the adjusted OR with 95%CI. The detail characteristics of the 8 studies were summarized in Table 1.
Study quality and overall quality of the evidence
The results of the methodological quality assessment of the 6 cross-sectional studies were listed in Supplementary Table 3, 3 studies [23, 24, 26] were judged of high quality and 3 studies [18, 21, 22] were considered with moderate quality. Using the NOS, 1 cohort study [20] was assessed to be high quality and 1 case-control study [25] was evaluated to be medium quality (Supplementary Table 4). The GRADE-relevant outcomes revealed that the rate of events of ED on average in patients with psoriasis was 4037/39490 (10.2%), whereas the control was 151710/1789959 (8.5%); the absolute effect of psoriasis on ED was 46 more per 1000 (from 28 more to 66 more); and the overall quality of the evidence was judged as moderate (Table 2).
Synthesis of results
As shown in Fig. 2, synthesis of results from 8 studies showed that the OR of psoriasis in patients with ED versus comparators was 1.62 (95%CI: 1.37–1.91, P < 0.001; heterogeneity: I2 = 62.6%, P = 0.009). The outcomes were consistent when restriction to multivariable adjustment (6 studies; combined OR = 1.5, 95%CI: 1.31–1.72, P < 0.001; heterogeneity: I2 = 53.5%, P = 0.056) (Fig. 3).
Subgroup analyses
To further elicit the relationship between psoriasis and the risk of ED and detect the source heterogeneity, subgroup analyses were performed based on mean disease duration of psoriasis, sample size, geographical area, publication year, and study design (Table 3). On account of the little variance in mean age among studies (ranged from 44 years to 56.3 years), thus we did not perform the subgroup analysis on age.
Stratified analysis by the mean disease duration of psoriasis revealed that duration ≥ 10 years [18, 21, 22, 24] exhibited an increased odds of ED compared with non-psoriasis (OR = 1.76, 95% CI: 1.35–2.29, P < 0.001) and no substantial heterogeneity was detected; whereas when limited to < 10 years [20, 23], the results did not show this association (OR = 1.99, 95% CI: 0.74–5.34, P = 0.171).
Subgroup analysis by the sample size revealed that studies with the total participants < 300 [18, 22, 23, 25] (OR = 2.36, 95% CI: 1.45–3.85) had a higher risk of ED compared with >300 participants [20, 21, 24, 26] (OR = 1.49, 95% CI: 1.28–1.73). There was no significant heterogeneity in studies of <300 participants (I2 = 43.9%, P = 0.148), while substantial heterogeneity was observed in studies > 300 participants (I2 = 67.4%, P = 0.027).
Subgroup analysis based on the geographical area showed that the association between psoriasis and ED was more stronger in studies conducted in Europe region [18, 21,22,23, 25, 26] (RR = 1.94, 95% CI: 1.46–2.59, heterogeneity: I2 = 53.7%, P = 0.056) than those studies conducted in Asia region [20, 24] (OR = 1.31, 95% CI: 1.14–1.5, heterogeneity: I2 = 4.5%, P = 0.306). On the other hand, a stronger association was detected in the publication year during 2016–2017 [23,24,25,26] (OR = 1.84, 95%CI: 1.36–2.49 than those during 2010–2014 [18, 20,21,22] (OR = 1.61, 95%CI: 1.14–2.28). Stratified analysis by the study design revealed that a higher risk of ED among patients with psoriasis was observed (OR = 1.78, 95% CI, 1.42–2.24) in cross-sectional studies, while this association was not presented for cohort/case-control studies (OR = 1.57, 95%CI: 0.87–2.83).
Sensitivity analysis
To confirm the robustness of the synthetic effect estimate and assess the influence of individual study on the overall risk of ED, a sensitivity analysis was performed. After omitting any of the studies, there was no substantial change on the new overall pooled OR, which ranged from 1.53 (95%CI: 1.32–1.77) to 1.84 (95%CI: 1.36–2.47) (Table 4 and Supplementary Figure 1). On the other hand, similar heterogeneity was obtained after each study exclusion, the I2 ranged from 44.5 to 67.9%. These outcomes indicated that no single study dominated the overall pooled OR and heterogeneity.
Publication bias
Visualization of the funnel plot indicated that both Begg’s rank correlation test and Egger’s linear regression yielded non-significant publication bias among the included studies (Begg’s, P > |z| = 0.386; Egger, P > |t| = 1.04, 95%CI: −0.97–2.41) (Supplementary Figure 2).
Discussion
Over the past few decades, a growing number of epidemiological studies have assessed the association between psoriasis and risk of ED but provided inconsistent results. Based on all of the available epidemiological evidence related to this topic, the current meta-analysis indicated a 1.62-fold increased risk of ED among subjects with psoriasis compared with those without psoriasis. Similarly, such an association between psoriasis and high risk of ED was consistent when conducted the multivariable adjustment analysis for confounding factors (OR = 1.5, 95%CI: 1.31–1.72). Ascertained by GRADEpro, the rate of events of ED on average in patients with psoriasis and without psoriasis was 10.2 and 8.5%, respectively; the absolute effect was 46 more per 1000. These results were in line with a recent meta-analysis reported by Wu et al. [27] and some other relevant studies which did not meet our inclusion criteria [8, 14,15,16,17, 28]. We also performed subgroup analyses to assess the degree to which potential confounders might have influenced these findings. Sensitivity analyses indicated that the quantification of the risk for the ED remained significantly higher in nearly all of the studies, suggesting that our findings in the present meta-analysis were robust. However, substantial heterogeneity has also appeared in the current study. The different mean disease duration of psoriasis, sample size, geographical area, study design, and varied characteristics of subjects could all be partly responsible for the heterogeneity. Of note, both Wu et al.’s study [27] and our study found a potential relationship between psoriasis and ED, but our study have a more restricted inclusion criteria (9 included studies in Wu et al.’ study and 8 included studies in the present study) and have been conducted more subgroup analyses (i.e., mean disease duration, sample size, geographical area, publication year, and study design) to further detect the heterogeneity, all of which might make the elaborate explanations on this topic.
ED, a multifactorial condition, can be affected by biological, psychological and interpersonal determinants [29]. The development of ED can be attributed to vascular, neuronal, hormonal, and metabolic factors, ultimately caused endothelial and smooth-muscle dysfunction [30]. A variety of independent risk factors have been confirmed for ED development, i.e., aging, cardiovascular disease, diabetes mellitus, dyslipidemia, and depression [30]. Nevertheless, some other potential risk factors have gradually been recognized to increase susceptibility to ED, such as some certain skin diseases [31, 32].
There has long been a recognition that skin disorder may have a negative effect on sexual aspects of the patient’s life [33]. Psoriasis is a common skin disorder with visible disfiguration, which is associated with a physical burden as well as a psychological burden, and ultimately has a deleterious effect on the sufferer’s sexual health. Psoriasis Area Severity Index (PASI) has been used to evaluate the severity of psoriasis, which was obtained by weighting the erythema, desquamation, induration, infiltration or thickness in the body surface area [34]. IIEF, a multidimensional scale for assessment of erectile dysfunction, is a widely used validation questionnaire for assessing the erectile function domains [35]. It was reported that IIEF score had a negative correlation with PASI scores in clinical practice [22]. In line with this finding, Egeberg et al.[26]. found that ED was significantly increased in men with psoriasis, the adjusted OR was slightly increased in severe psoriasis group than mild psoriasis group (1.13 versus 1.17). However, in a more recent study developed by Bardazzi et al.[23]., they found that a higher prevalence of ED was observed in patients with mild psoriasis compared to those patients with severe psoriasis (56.67 versus 46.68%).
Different comorbidities and treatments of the disease rather than psoriasis can modulate the ED risk [36]. In the present study, 6 studies provided the OR of multivariable adjustment for confounding factors, i.e., age, hypertension, systemic treatment, obesity (body mass index), diabetes mellitus, dyslipidemia, depression, anxiety, smoking, alcohol, genital lesions, and drug usage, etc. Moreover, these 6 studies also have the strict exclusion criteria in both the study group and control group. Therefore, the synthesis effect estimate in the current study seems reliable and robust. Though the overall OR adjusted for known potential causes for ED decreased slightly compared with the complete analysis (1.5 versus 1.62), our study indicated that patients with psoriasis were associated with a 50% increase in ED risk than non-psoriasis after adequate confounder adjustment.
In the present study, subgroup analysis by mean disease duration revealed that duration ≥ 10 years had been confirmed the relationship between psoriasis and ED, while no positive association was found in duration < 10 years. However, Cabete et al.[21]. observed an increased prevalence of ED in patients with psoriasis especially in those had a severe symptom of psoriasis, but they found that psoriasis duration did not statistically contribute to ED in these subjects. Stratified analysis by sample size revealed that more significant association was found in participants < 300 groups compared with >300 participants groups. It is possible that studies with small sample sizes are prone to generate the detection bias, selection bias, and performance bias. Another explanation could be the quality of the included studies with a small sample were lower than those with a large sample. Therefore, additional well-designed studies are still warranted to validate this finding. In the current study, we also observed that studies conducted in Europe region have a stronger association between psoriasis and ED than Asia region. This might probably due to the high prevalence of psoriasis was detected in the Europe countries. It was reported that the prevalence of psoriasis in adults was estimated as 3.73, 8.5, 3.1, and 5.2% in Denmark [37], Norway [38], Italy [39], and France [40], respectively; while in China [41] and Chinese Taipei [42] was 0.2–1.5% and 0.23%, respectively. It was also reported that the prevalence of psoriasis among the Caucasian population and Mongoloid population was 1–2% and 0.3%, respectively [20].
Though our meta-analysis has linked psoriasis to ED, no clear-cut pathogenic and etiological mechanism has been identified to elucidate this potential association. It was suggested that the etiology of ED in psoriasis patients may be multifactorial, including physical, organic and/or psychogenic factors [20, 43]. The connecting link between psoriasis and CVD may be a conceivable contributor [18]. Accumulating evidence has emerged demonstrating that psoriasis is an independent risk factor for the development of CVD [36]. Compared to the general population, patients with psoriasis were reported that have a higher risk of atherosclerosis and hypertension, both of which were recognized as the risk factors affecting ED [18, 21]. Psoriasis has been speculated to be associated with systemic chronic vascular inflammation and endothelial dysfunction [44]. It is known that endothelial dysfunction is the shared pathogenic mechanism between ED and CVD [45]. Endothelial dysfunction can reduce the bioavailability of nitric oxide and ultimately lead to ED [46]. On the other hand, patients with psoriasis had increased susceptibility to metabolic syndrome compared with the general population [47]. Intriguingly, an association between metabolic syndrome and ED has been confirmed in some studies [48, 49]. The relationship network has been linked metabolic syndrome to endothelial dysfunction, nitric oxide dysfunction, as well as vascular insufficiency, and that all of these pathogenic conditions might be involved in the development of psoriasis and ED [50]. Yet in several studies reported the mechanism underlying linkage between ED and psoriasis might contribute to systemic inflammation. Patients with psoriasis likely have a high level of pro-inflammatory cytokines, including leukotrienes, reactive oxygen species, TNF-α, IFN-γ, and Th-1, which may subsequently contribute to endothelial dysfunction—central to the pathogenesis of ED [22, 51,52,53].
Extensive analyses were carried out that psoriasis may be not per se a risk factor for ED, but the concomitant psychological distress [20, 54]. Psoriasis, especially in the presence of genital lesions, can cause an alteration in body image thus cause feelings of depression, anxiety, low self-esteem, stigmatization, and decreased confidence; all these psychological impairments could lead to ED [25, 43, 55]. On the other hand, there are reports [56, 57] revealing that some treatments prescribed for psoriasis might also attribute to the development of ED, such as methotrexate, retinoids, etc. It has been speculated that methotrexate could disrupt the balance of estrogen–testosterone, leading to ED [58]. However, some systemic anti-psoriatic therapies can have beneficial effects on the erectile function of men with psoriasis, i.e., anti-TNF-α, interleukin-23 inhibitors, etc[59,60,61]. Since the pathogenesis of ED in psoriatic patients attributed to various factors, thus a better alternative management for those patients should employ a combined approach, such as medical, psychological, and behavioral interventions together.
This systematic review and meta-analysis have been summarized all available evidence to confirm the association between psoriasis and the risks of developing ED. Nevertheless, we acknowledged there was an inherent limitation in the current study. As with any meta-analysis, substantial heterogeneity across included studies was detected. Subsequently, subgroup analysis shown that no substantial heterogeneity was observed in the subgroup of ≥ 10 years mean disease duration, sample size < 300 participants, study those studies conducted in Asia, and cross-sectional study design. Furthermore, no significant heterogeneity was detected when eliminated the study of Chen et al. during sensitivity analyses. As a result, high-quality well-designed cohort studies with large sample size are still warranted to validate the evidence of psoriasis predisposing to the development of ED.
Conclusions
In summary, our meta-analysis indicates a potential hazardous effect of psoriasis for developing ED. After multivariable adjustment for the confounding factors, psoriasis and ED was still closely associated, which reminds both dermatologists and urologists should realize the relationship between psoriasis and ED and provide specific treatments for those patients when necessary.
References
Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983–94.
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–85.
Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol. 2018;79:345–52.
Fernandez-Armenteros JM, Gomez-Arbones X, Buti-Soler M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M, et al. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol. 2018. https://doi.org/10.1111/jdv.15159.
Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30:282–7.
Egeberg A, Mallbris L, Warren RB, Bachelez H, Gislason GH, Hansen PR, et al. Association between psoriasis and inflammatory bowel disease: a danish nationwide cohort study. Br J Dermatol. 2016;175:487–92.
Jensen P, Egeberg A, Gislason G, Thyssen JP, Skov L. Risk of uncommon cancers in patients with psoriasis: a danish nationwide cohort study. J Eur Acad Dermatol Venereol. 2018;32:601–5.
Turel EA, Temeltas G, Deveci A, Dinc G, Guler HB, Ozturkcan S. Sexual dysfunction in patients with psoriasis. J Dermatol. 2006;33:772–8.
Parisi R, Webb RT, Kleyn CE, Carr MJ, Kapur N, Griffiths C, et al. Psychiatric morbidity and suicidal behaviour in psoriasis: a primary care cohort study. Br J Dermatol 2018. https://doi.org/10.1111/bjd.17004.
Nazik H, Nazik S, Gul FC. Body image, self-esteem, and quality of life in patients with psoriasis. Indian Dermatol Online J. 2017;8:343–6.
Pompili M, Innamorati M, Trovarelli S, Narci si A, Bellini S, Orsini D, et al. Suicide risk and psychiatric comorbidity in patients with psoriasis. J Int Med Res. 2016;44(1 suppl):61–66.
Molina-Leyva A, Almodovar-Real A, Carrascosa JC, Molina-Leyva I, Naranjo-Sintes R, Jimenez-Moleon JJ. Distribution pattern of psoriasis, anxiety and depression as possible causes of sexual dysfunction in patients with moderate to severe psoriasis. Bras Dermatol. 2015;90:338–45.
Maaty AS, Gomaa AH, Mohammed GF, Youssef IM, Eyada MM. Assessment of female sexual function in patients with psoriasis. J Sex Med. 2013;10:1545–8.
Chung SD, Keller JJ, Chu TW, Lin HC. Psoriasis and the risk of erectile dysfunction: a population-based case-control study. J Sex Med. 2012;9:130–5.
Ferreira BR, Pio-Abreu JL, Reis JP, Figueiredo A. Analysis of the prevalence of mental disorders in psoriasis: the relevance of psychiatric assessment in dermatology. Psychiatr Danub. 2017;29:401–6.
Meeuwis KA, de Hullu JA, van de Nieuwenhof HP, Evers AW, Massuger LF, van de Kerkhof PC, et al. Quality of life and sexual health in patients with genital psoriasis. Br J Dermatol. 2011;164:1247–55.
Saad F, Haider A, Gooren L. Hypogonadal men with psoriasis benefit from long-term testosterone replacement therapy—a series of 15 case reports. Andrologia. 2016;48:341–6.
Goulding JM, Price CL, Defty CL, Hulangamuwa CS, Bader E, Ahmed I. Erectile dysfunction in patients with psoriasis: increased prevalence, an unmet need, and a chance to intervene. Br J Dermatol. 2011;164:103–9.
Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira EJ, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–607.
Chen YJ, Chen CC, Lin MW, Chen TJ, Li CY, Hwang CY, et al. Increased risk of sexual dysfunction in male patients with psoriasis: a nationwide population-based follow-up study. J Sex Med. 2013;10:1212–8.
Cabete J, Torres T, Vilarinho T, Ferreira A, Selores M. Erectile dysfunction in psoriasis patients. Eur J Dermatol. 2014;24:482–6.
Tasliyurt T, Bilir Y, Sahin S, Seckin HY, Kaya SU, Sivgin H, et al. Erectile dysfunction in patients with psoriasis: potential impact of the metabolic syndrome. Eur Rev Med Pharmacol Sci. 2014;18:581–6.
Bardazzi F, Odorici G, Ferrara F, Magnano M, Balestri R, Patrizi A. Sex and the PASI: patients affected by a mild form of psoriasis are more predisposed to have a more severe form of erectile dysfunction. J Eur Acad Dermatol Venereol. 2016;30:1342–8.
Ji S, Zang Z, Ma H, Gu M, Han Y, Wang L, et al. Erectile dysfunction in patients with plaque psoriasis: the relation of depression and cardiovascular factors. Int J Impot Res. 2016;28:96–100.
Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, Ruiz-Carrascosa JC, Naranjo-Sintes R, Jimenez-Moleon JJ. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047–55.
Egeberg A, Hansen PR, Gislason GH, Skov L, Thyssen JP. Erectile dysfunction in male adults with atopic dermatitis and psoriasis. J Sex Med. 2017;14:380–6.
Wu T, Duan X, Chen S, Chen X, Yu R, Yu X. Association between psoriasis and erectile dysfunction: a meta-analysis. J Sex Med. 2018;15:839–47.
Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, Naranjo-Sintes R, Serrano-Ortega S, Jimenez-Moleon JJ. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882–9.
Althof SE, Needle RB. Psychological factors associated with male sexual dysfunction: screening and treatment for the urologist. Urol Clin North Am. 2011;38:141–6.
Hackett G, Kirby M, Wylie K, Heald A, Ossei-Gerning N, Edwards D, et al. British society for sexual medicine guidelines on the management of erectile dysfunction in men-2017. J Sex Med. 2018;15:430–57.
Juan CK, Chen HJ, Shen JL, Kao CH. Lichen simplex chronicus associated with erectile dysfunction: a population-based retrospective cohort study. PLoS ONE. 2015;10:e128869.
Chung SD, Keller JJ, Lin HC. Association of erectile dysfunction with atopic dermatitis: a population-based case-control study. J Sex Med. 2012;9:679–85.
Heseltine GF. The site of onset of eczema and personality trait differences: an exploratory study. J Psychosom Res. 1963;7:241–6.
Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194–9.
Rosen RC, Cappelleri JC, Gendrano NR. The international index of erectile function (iief): a state-of-the-science review. Int J Impot Res. 2002;14:226–44.
Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014–24.
Brandrup F, Green A. The prevalence of psoriasis in Denmark. Acta Derm Venereol. 1981;61:344–6.
Bo K, Thoresen M, Dalgard F. Smokers report more psoriasis, but not atopic dermatitis or hand eczema: results from a Norwegian population survey among adults. Dermatology. 2008;216:40–45.
Naldi L, Colombo P, Placchesi EB, Piccitto R, Chatenoud L, La Vecchia C. Study design and preliminary results from the pilot phase of the PraKtis study: self-reported diagnoses of selected skin diseases in a representative sample of the Italian population. Dermatology. 2004;208:38–42.
Wolkenstein P, Revuz J, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S. Psoriasis in France and associated risk factors: results of a case-control study based on a large community survey. Dermatology. 2009;218:103–9.
Farber EM, Nall L. Psoriasis in the tropics. Epidemiol, Genet, Clin, Ther Asp Dermatol Clin. 1994;12:805–16.
Chang YT, Chen TJ, Liu PC, Chen YC, Chen YJ, Huang YL, et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Derm Venereol. 2009;89:262–6.
Molina-Leyva A, Jimenez-Moleon JJ, Naranjo-Sintes R, Ruiz-Carrascosa JC. Sexual dysfunction in psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:649–55.
Martyn-Simmons CL, Ranawaka RR, Chowienczyk P, Crook MA, Marber MS, Smith CH, et al. A prospective case-controlled cohort study of endothelial function in patients with moderate to severe psoriasis. Br J Dermatol. 2011;164:26–32.
Kostis JB, Jackson G, Rosen R, Barrett-Connor E, Billups K, Burnett AL, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol. 2005;96(12B):85M–93M.
Gao L, Zhao Z, Guo F, Liu Y, Guo J, Zhao Y, et al. Association of endothelial nitric oxide synthase polymorphisms with an increased risk of erectile dysfunction. Asian J Androl. 2017;19:330–7.
Rodriguez-Zuniga M, Garcia-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657–66.
Besiroglu H, Otunctemur A, Ozbek E. The relationship between metabolic syndrome, its components, and erectile dysfunction: a systematic review and a meta-analysis of observational studies. J Sex Med. 2015;12:1309–18.
Maseroli E, Corona G, Rastrelli G, Lotti F, Cipriani S, Forti G, et al. Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: a comparative study. J Sex Med. 2015;12:956–65.
Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–32.
De Simone C, Di Giorgio A, Sisto T, Carbone A, Ghitti F, Tondi P, et al. Endothelial dysfunction in psoriasis patients: cross-sectional case-control study. Eur J Dermatol. 2011;21:510–4.
Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem. 2010;25:388–92.
Zindanci I, Albayrak O, Kavala M, Kocaturk E, Can B, Sudogan S, et al. Prevalence of metabolic syndrome in patients with psoriasis. ScientificWorldJournal. 2012;2012:312463.
Mercan S, Altunay IK, Demir B, Akpinar A, Kayaoglu S. Sexual dysfunctions in patients with neurodermatitis and psoriasis. J Sex Marital Ther. 2008;34:160–8.
Kurizky PS, Mota LM. Sexual dysfunction in patients with psoriasis and psoriatic arthritis-a systematic review. Rev Bras Reumatol. 2012;52:943–8.
Wylie G, Evans CD, Gupta G. Reduced libido and erectile dysfunction: rarely reported side-effects of methotrexate. Clin Exp Dermatol. 2009;34:e234.
Reynolds OD. Erectile dysfunction in etretinate treatment. Arch Dermatol. 1991;127:425–6.
Aguirre MA, Velez A, Romero M, Collantes E. Gynecomastia and sexual impotence associated with methotrexate treatment. J Rheumatol. 2002;29:1793–4.
Cohen-Barak E, Sah M, Kerner M, Rozenman D, Ziv M. Impact of antipsoriatic therapy on endothelial function. Br J Dermatol. 2015;173:1440–6.
Guenther L, Han C, Szapary P, Poulin Y, Bourcier M, Ortonne JP, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851–7.
Ruiz-Villaverde R, Sanchez-Cano D, Rodrigo JR, Gutierrez CV. Pilot study of sexual dysfunction in patients with psoriasis: influence of biologic therapy. Indian J Dermatol. 2011;56:694–9.
Acknowledgements
This work was supported by the grants from Science and Technology Planning Project of Guangdong Province (No.2017B030314108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, S., Wang, J., Xie, Q. et al. High prevalence of erectile dysfunction in men with psoriasis: evidence from a systematic review and meta-analysis. Int J Impot Res 31, 74–84 (2019). https://doi.org/10.1038/s41443-018-0093-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-018-0093-8
- Springer Nature Limited
This article is cited by
-
Female sexual dysfunction in psoriasis: a systematic review and meta-analysis using the Female Sexual Function Index
International Journal of Impotence Research (2024)
-
Erectile dysfunction and metabolic syndrome components in obese men with psoriasis: response to a 12-week randomized controlled lifestyle modification program (exercise with diet restriction)
Irish Journal of Medical Science (1971 -) (2024)
-
Genital Psoriasis: Impact on Quality of Life and Treatment Options
American Journal of Clinical Dermatology (2019)