Abstract
An intradialytic systolic blood pressure (SBP) decline, which defines intradialytic hypotension, may be associated with higher all-cause mortality. However, in Japanese patients on hemodialysis (HD), the association between intradialytic SBP decline and patient outcomes is unclear. This retrospective cohort study included 307 Japanese patients undergoing HD over 1 year in three dialysis clinics and evaluated the association between the mean annual intradialytic SBP decline (predialysis SBP-nadir intradialytic SBP) and clinical outcomes, including major adverse cardiovascular events (MACEs; cardiovascular death, nonfatal myocardial infarction or unstable angina, stroke, heart failure, and other severe cardiovascular events requiring hospitalization) by following up for 2 years. The mean annual intradialytic SBP decline was 24.2 (25–75th percentile, 18.3–35.0) mmHg. In the model fully adjusted for intradialytic SBP decline tertile group (T1, <20.4 mmHg; T2, 20.4 to <29.9 mmHg; T3, ≥29.9 mmHg), predialysis SBP, age, sex, HD vintage, Charlson comorbidity index, ultrafiltration rate, use of renin–angiotensin system inhibitors, corrected calcium, phosphorus, human atrial natriuretic peptide, geriatric nutritional risk index, normalized protein catabolism rate, C-reactive protein, hemoglobin, and use of pressor agents, Cox regression analyses showed that the hazard ratio (HR) was significantly higher for T3 than for T1 for MACEs (HR, 2.38; 95% confidence interval 1.12–5.09) and all-cause hospitalization (HR, 1.68; 95% confidence interval 1.03–2.74). Therefore, in Japanese patients on HD, a greater intradialytic SBP decline was associated with worse clinical outcomes. Further studies are warranted to investigate whether interventions to attenuate the intradialytic SBP decline will improve the prognosis of Japanese patients on HD.

Similar content being viewed by others
Introduction
More than 340,000 people are on dialysis in Japan, and more than 40,000 new patients start dialysis yearly. End-stage renal disease (ESRD) requiring renal replacement therapy (RRT) has become a significant public health and social problem [1]. Hemodialysis (HD), which includes conventional HD and hemodiafiltration dialysis (HDF), is the most widespread RRT in Japan [2]. In HD, a significant amount of water is removed in a short period, which may cause a decrease in systolic blood pressure (SBP) during HD. Blood pressure decline during dialysis is closely associated with intradialytic hypotension (IDH), which is defined as a decrease in the intradialytic SBP by a specific amount (20–40 mmHg) or a nadir SBP below a threshold (90–100 mmHg). However, no standard definition has been established [2,3,4,5,6,7]. IDH has been reported to be a significant complication associated with increased cardiovascular and all-cause mortality in patients on HD [8]. Risk factors for IDH include diabetes, cardiovascular complications, malnutrition, high ultrafiltration rate (UFR), and serum osmolality (and its changes) [1, 3,4,5,6,7]. In Japan, where online hemodiafiltration (OL-HDF) and intermittent infusion HDF (I-HDF) are widely used, we reported the possibility of risk factors for greater intradialytic SBP decline, such as the nonuse of calcium channel blockers (CCBs), use of alpha-blockers (ABs), poor nutrition statuses, high serum calcium (Ca)/phosphorus (P) levels, and excessive protein intake [9]. All previous reports on the association between low blood pressure during HD and prognosis were conducted in patients on conventional HD, and there were no data on patients on OL-HDF or I-HDF, which are commonly used in Japan. In addition, in previous studies, IDH and declines in SBP during dialysis have been evaluated or calculated over a short period (3–6 months at most). Given seasonal variations in blood pressure and other clinical and laboratory parameters [9,10,11,12,13,14], this period is insufficient to determine the actual and overall dialysis patterns of patients on HD. Therefore, in this study, we calculated the average annual intradialytic SBP decline in patients on HD, including many OL-HDF and I-HDF patients, taking into account seasonal variations, and examined the relationship between SBP decline during dialysis and clinical outcomes. The primary clinical outcomes were major adverse cardiac events (MACEs) [15]. Secondary endpoints included death or hospitalization due to a decline in activities of daily living (ADL) and all-cause hospitalization [16].
Methods
Study population
HD outpatients from three dialysis clinics (Shibagaki Dialysis Clinic in Jiyugaoka, Shinagawa Togoshi, and Kugahara in Tokyo, Japan) were included in the previous study from April 2019 to March 2020 [9]. Stable patients aged 20 years or older who remained on HD during the entire study period (April 2019 to March 2020) were included in the study. Patients with missing data in any month and patients who did not undergo HD three times a week were excluded, as previously described [9]. This retrospective study used the same population for the analysis. In this study, the 1-year period from April 2019 to March 2020 was the enrollment period, and the 2-year period from April 2020 to March 2022 was the observation period (Fig. 1). Additionally, informed consent for participation in this study was obtained via an opt-out method on the website, and patients who opted out were excluded. The study protocol was independently reviewed and approved by the Keio University Hospital Ethics Committee (approval number: 20221055) and followed the principles of the Declaration of Helsinki.
Study data
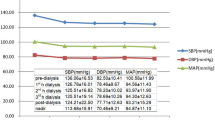
Blood pressure was measured in the supine position, on the arm without vascular access, before puncture and every 60 min after that. Blood pressure was measured with a cuff connected to the dialysis machine, GC-X01 (JMS, Tokyo, Japan), and was recorded automatically. Intradialytic SBP decline was defined as predialysis SBP minus nadir intradialytic SBP, and the annual mean was calculated. In addition, annual means of predialysis SBP, predialysis diastolic blood pressure (DBP), nadir SBP, and ultrafiltration rate (UFR, ml/kg/h) were calculated. Demographic data on age (years), sex, dialysis vintage (years), primary disease leading to ESRD, Charlson comorbidity index (CCI), treatment type, use of antihypertensive medications (CCBs, renin-angiotensin system inhibitors (RASIs), beta-blockers, ABs, and mineralocorticoid receptor antagonists), diuretics (loop diuretics and thiazide diuretics) and other drugs (pressor agents, antiplatelet agents, and statins), and past medical history were recorded in April 2020. In CCI, one point each was assigned for myocardial infarction, congestive heart failure, peripheral arterial disease, dementia, cerebrovascular disease, chronic lung disease, collagen disease, peptic ulcer, mild liver disease, and uncomplicated diabetes mellitus (DM); two points each for hemiplegia, moderate-to-severe renal disease, complicated DM, localized solid cancer, leukemia, and lymphoma; three points for moderate-to-severe liver disease, and 6 points each for metastatic solid cancer and AIDS [17]. However, patients with “6 points” added, i.e., those with metastatic solid cancer or AIDS, were not included in this study. Predialysis serum parameters such as sodium (mEq/l), potassium (mEq/l), total protein (g/dl), albumin (g/dl), urea nitrogen (UN) (mg/dl), creatinine (mg/dl), corrected Ca (mg/dl), P (mg/dl), parathyroid hormone (pg/ml), C-reactive protein (CRP) (mg/dl), and hemoglobin (Hb) (g/dl) were obtained during the first dialysis session in the first week of each month. Data from April 2019 to March 2020 were recorded to calculate annual averages for use in the analyses of this study. Postdialysis serum parameters included human atrial natriuretic peptide (hANP, pg/ml) as a marker of the fluid status. The body mass index (BMI) (kg/m2) was calculated from the height (cm) and weight (kg), the geriatric nutritional risk index (GNRI) from the BMI and serum albumin, the normalized protein catabolic rate (nPCR) (g/kg/day) and single-pool Kt/V from serum UN and BW before and after dialysis, and the session’s length. In addition, the experienced doctors at the dialysis clinics determined the dry weight based on the guidelines [18] by referring to the cardiothoracic ratio, blood pressure during dialysis, home blood pressure, hANP, and edema. For hANP, the median number of measurements was 8 [6,7,8,9,10,11] from April 2019 to March 2020, and the mean value of those measurements was used in the analysis.
Follow-up
Patients were divided into three groups according to the tertile of intradialytic SBP decline. All patients were followed up for 2 years, from April 2020 to March 2022 (observation period). The primary endpoint was the presence of MACEs (cardiovascular death, nonfatal myocardial infarction or unstable angina, stroke, heart failure, and other severe cardiovascular events requiring hospitalization) [15]. Secondary endpoints were death or hospitalization due to a decline in ADL (having difficulty visiting a dialysis clinic) and all-cause hospitalization [16]. Regarding the method of recording clinical data and outcomes, medical clerks, medical engineers, and nurses, who were not directly involved in this study, recorded the data and events each time those events occurred. In this study, we could not follow up the patients further if they stop visiting dialysis clinics, regardless of whether it is due to death or hospitalization caused by a decline in ADL. On the contrary, similar to all-cause mortality, hospitalization due to decreased ADL is an important outcome associated with serious morbidity. Therefore, we combined these two outcomes. For the same reason, a report of Japanese patients with HD set a similar outcome [16].
Statistical analysis
As appropriate, comparisons of baseline variables among the three groups were made via the analysis of variance, Kruskal–Wallis test, or Fisher’s exact test, followed by the post hoc Bonferroni test. The normality of the distribution of continuous variables was examined using the Kolmogorov-Smirnov test. Results are expressed as mean ± standard deviation, median (interquartile range), n (%), or estimates (95% confidence intervals [CI]); P-values are two-sided, and P < 0.05 indicates statistical significance.
Survival curves were plotted via the Kaplan–Meier method and compared using the log-rank test. Log-rank trend tests were also conducted. The Cox proportional hazards model was employed to determine hazard ratios (HRs) with 95% CIs for survival. In the multivariate fully adjusted model, intradialytic SBP decline tertile groups, predialysis SBP, age, sex, HD vintage, CCI, UFR, RASI use, serum corrected Ca/P, hANP, nPCR, GNRI, CRP, Hb, and use of pressor agents were included as independent variables. Furthermore, we modeled the nonlinear associations between the intradialytic SBP decline and outcomes by Cox regression models using restricted cubic splines with four knots (5th, 35th, 65th, and 95th percentiles). Moreover, since the death and/or hospitalization due to a decline in ADL are competing risk events against MACEs and all-cause hospitalization, the cumulative incidence rates considering competing risks were compared using Gray’s test. The Fine–Gray subdistribution hazards model was used in the multivariate model as a sensitivity analysis for MACEs and all-cause hospitalization, together with the standard Cox regression model for cause-specific hazards. Finally, a subgroup analysis was performed for the presence of DM and predialysis SBP using the Cox proportional hazards model. Predialysis SBP was divided into three groups based on tertiles for the analysis. All statistical analyses were performed using EZR (Saitama Medical University Hospital, Saitama, Japan), a graphical user interface of R (The R Foundation for Statistical Computing, Vienna, 12 Austria) [19], and Stata version 17.0 (Stata Corporation, College Station, TX, USA).
Results
Baseline characteristics
Among the 379 patients who underwent HD at Shibagaki dialysis clinics in Jiyugaoka, Shinagawa Togoshi, and Kugahara in April 2019, 17 patients who underwent HD at a frequency other than three times per week did not meet the inclusion criteria, and 55 patients with missing data in any month were excluded, including patients who died (n = 32) during the 1-year study period. As a result, 307 patients were included in the analysis (Fig. 1). Among the 307 patients included in the previous study, no one opted out, and all the patients were enrolled in the analyses of this study. The intradialytic SBP decline of the participants was 24.2 (18.3–35.0) mmHg, and the patients were divided into three groups according to their tertiles: T1: <20.4 mmHg; T2: 20.4 to <29.9 mmHg; T3: ≥29.9 mmHg. Baseline characteristics for each group are listed in Tables 1 and 2. Age (P = 0.001), DKD (P < 0.001), nephrosclerosis (P = 0.001), autosomal dominant polycystic kidney disease (P = 0.005), CCI (P < 0.001), I-HDF (P = 0.02), AB use (P = 0.01), pressor agent use (P = 0.004), predialysis SBP (P < 0.001), predialysis DBP (P < 0.001), creatinine (P = 0.03), and BMI (P = 0.008) were significantly different among the groups. A total of 47,219 HD sessions (153.5 ± 6.4 sessions/patient) were performed during the 1-year enrollment period. IDH was detected only in 2 (0–7) sessions/patient, representing 1.3% (0–4.5%) of all sessions in the 1-year cohort.
Association between tertile groups of intradialytic SBP decline and the development of MACEs
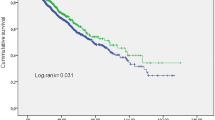
The intradialytic SBP decline was divided into three groups according to the tertile, T1, T2, and T3, from the smallest decline to the largest. During the 2-year observation period, 18 (18%) patients in T1, 23 (23%) in T2, and 36 (35%) in T3, and a total of 77 (25%) developed MACEs (cardiovascular death: 3 patients, nonfatal myocardial infarction or unstable angina: 15 patients, stroke: 12 patients, heart failure: 26 patients, other severe cardiovascular events requiring hospitalization: 21 patients). Among those MACEs, 24 (31%) occurred in spring, 18 (23%) in summer, 14 (18%) in fall, and 21 (27%) in winter. The log-rank test showed a significant difference in the incidence of MACEs in the three groups (P = 0.008), with the Bonferroni post hoc test showing a significantly higher incidence of MACEs in T3 compared to T1 (P = 0.009). The log-lank trend test showed that the greater the intradialytic SBP decline, the greater the incidence of MACEs (P = 0.003) (Fig. 2A). Furthermore, the cumulative incidence of MACEs differed significantly between the three groups (Gray’s test, P = 0.01), with a significantly higher incidence in T3 than in T1 using the Bonferroni correction (P = 0.01) (Fig. 2B). The present study is characterized by fewer conventional HD and more OL-HDF and I-HDF. The intradialytic SBP decline values were 25.8 ± 10.1, 27.5 ± 13.4, and 29.5 ± 17.3 mmHg in patients on HD, OL-HDF, and I-HDF, respectively, and no significant difference was found for each treatment modality (P = 0.45). MACEs occurred in 4 (31%) patients on conventional HD, 49 (27%) on predilution OL-HDF, and 24 (22%) on I-HDF, and the log-rank test showed no significant difference by treatment groups (P = 0.64).
In the multivariate analysis, the Cox proportional hazards model and the Fine–Gray subdistribution hazards model were performed. In the Cox proportional hazards model, the HR for MACEs was significantly higher for T3 than for T1 (HR 2.38 [95% CI 1.12–5.09]) (Fig. 3A). In the Fine–Gray analysis, the HR for MACEs increased for T3 compared with that for T1 (HR 2.07 [95% CI 0.98–4.40]), although this was not statistically significant (Fig. 3B).
Association between the intradialytic systolic blood pressure decline tertile groups (T1, T2, and T3) and the primary outcome (MACEs) and secondary outcomes using the Cox proportional hazards model (A, C, and D) and Fine–Gray subdistribution hazards model (B, E). *P < 0.05 versus T1. Black circles represent HRs; solid lines and error bars represent 95% confidence intervals. CI confidence interval, MACEs major adverse cardiovascular events, SBP systolic blood pressure
Besides, restricted cubic spline curves were developed for the HR for the incidence of MACEs according to intradialytic SBP decline. In the unadjusted model, HR tended to increase when intradialytic SBP decline was less than 20 mmHg; however, this finding was not statistically significant (Fig. 4A). In the fully adjusted model, the HR curve was almost horizontal below 1 when the intradialytic SBP decline was less than 20 mmHg whereas the HR was significantly increased when the intradialytic SBP was greater than 24.2 mmHg (Fig. 4B).
Restricted cubic splines showing the hazard ratio for major adverse cardiovascular events according to intradialytic systolic blood pressure decline in the unadjusted (A) and fully adjusted model (B). In splines, solid lines represent the hazard ratio, and dashed lines represent the 95% confidence interval
Association between tertile groups of intradialytic SBP decline and secondary outcomes
We first examined the association between the intradialytic SBP decline and death or hospitalization due to a decline in ADL. During the 2-year observation period, 14 (14%) patients in T1, 19 (18%) patients in T2, and 23 (23%) patients in T3, and a total of 56 (18%) patients experienced death or hospitalization due to an ADL decline. A log-rank test showed no significant difference in the incidence of death or hospitalization due to a decline in ADL among the three groups (P = 0.26) (Supplementary Fig. 1A). In the multivariate analysis, the Cox proportional hazards model was performed. The Cox proportional hazards model showed that for death or hospitalization due to a decline in ADL, the HR was significantly increased at T2 compared with that at T1 (HR 2.16 [95% CI 1.05–4.46]) (Fig. 3C). Next, cubic spline curves of HR for death or hospitalization due to a decline in ADL according to the intradialytic SBP decline were created. Although the HR curve did not exceed 1 from 24.2 to 50 mmHg in some areas in the unadjusted model (Supplementary Fig. 2A), in the fully adjusted model, the greater the intradialytic SBP decline, the higher the HR; however, this association was not statistically significant (Supplementary Fig. 2B).
Next, we examined the association between the intradialytic SBP decline and all-cause hospitalization, which included 61 (60%) patients in T1, 60 (59%) in T2, 63 (61%) in T3, and a total of 184 (60%) experienced all-cause hospitalization during the 2-year observation period. Log-rank tests showed no significant difference in the incidence of all-cause hospitalization between the three groups (P = 0.58) (Supplementary Fig. 1B). In the multivariate analysis, the Cox proportional hazards model and the Fine–Gray subdistribution hazards model were performed. The Cox proportional hazards model showed that for all-cause hospitalization, the HR was significantly higher at T3 than at T1 (HR 1.68 [95% CI 1.03–2.74]) (Fig. 3D). The Fine–Gray subdistribution hazards model revealed that for all-cause hospitalization, the HR was significantly higher at T3 than at T1 (HR 2.23 [95% CI 1.05–4.75]) (Fig. 3E). Besides, cubic spline curves were created for the HR for all-cause hospitalization according to the intradialytic SBP decline. In both the unadjusted and fully adjusted models, the HR increased with greater intradialytic SBP decline, and the increase in HR became statistically significant when the intradialytic SBP decline exceeded 24.2 mmHg (Supplementary Fig. 2C and 2D).
Subgroup analyses by DM and predialysis SBP
First, we performed subgroup analyses in the DM and non-DM groups with the Cox proportional hazards model. We found a significant HR increase at T3 compared with that at T1 only in the DM group for any outcomes of MACEs (HR 4.22 [95% CI 1.14–15.55]), death or hospitalization due to a decline in ADL (HR 5.02 [95% CI 1.19–21.24]), and all-cause hospitalization (HR 3.80 [95% CI 1.54–9.36]). On the contrary, in the tests for interaction between DM and intradialytic SBP decline for each outcome in T3 compared with that in T1, no interaction was found for MACEs (P = 0.59) and all-cause hospitalization (P = 0.37), whereas a significant interaction was found for death or hospitalization due to a decline in ADL (P = 0.02) (Supplementary Fig. 3).
Then, patients were divided into three groups by tertile of predialysis SBP. For MACEs and death or hospitalization due to a decline in ADL, HR was significantly higher at T3 than at T1 only in the group with higher predialysis SBP (predialysis SBP ≥ 159 mmHg) (MACEs, HR 3.59 [95% CI 1.10–11.7]; death or hospitalization due to a decline in ADL, HR 5.02 [95% CI 1.45–17.31]). While assessing the interaction between predialysis systolic blood pressure and intradialytic SBP decline for each outcome, no interaction was found for either outcome in T3 and T1 (Supplementary Fig. 3). However, these results should be cautiously interpreted because of the limited sample size.
Discussion
This study analyzed a total of 47,219 HD sessions in 307 patients who had been on HD for 1 year, with an annual mean intradialytic SBP decline of 24.2 (18.3–35.0) mmHg from April 2019 to March 2020. The log-rank and Gray’s tests showed a significant increase in the risk of MACEs in the T3 group with the largest intradialytic SBP decline, compared to the T1 group with the smallest one. Additionally, in a fully adjusted multivariate model, the T3 group was significantly associated with an increase in the rate of occurrence of MACEs and all-cause hospitalization compared with the T1 group. The Cox regression models using restricted cubic splines also supported these results. In this study, MACEs occurred in 25% of the patients during the 2-year observation period, which is not exceptionally high considering the previously reported prevalence rates [20, 21].
Previous reports have shown an increase in mortality with intradialytic SBP declines ≥30–50 mmHg [22,23,24,25,26]. A study conducted in Japan also found an increase in the mortality rate with intradialytic SBP declines of ≥40 mmHg [22]. On the other hand, another report suggested that the mortality rate increases with an intradialytic SBP decline of <15 mmHg [25], indicating that patients with less intradialytic SBP decline may also have a worse prognosis. However, in the present study, the log-rank trend test and the Cox proportional hazards model indicated that the incidence of MACEs was significantly higher at T3 than at T1. Besides, the Cox proportional hazards model showed that the HR for all-cause hospitalization was significantly higher at T3 than at T1. Therefore, the results of this study do not rule out the possibility that the reduction of intradialytic SBP decline may improve the prognosis of patients on HD, although further intervention studies are required as this is an observational study. To minimize intradialytic SBP decline, first of all, intradialytic weight gain should be reduced by restricting salt intake, which leads to slower UFR. Additionally, a previous retrospective study suggested that CCB use, the appropriate control of chronic kidney disease-mineral bone disorders (especially serum phosphorus levels), avoidance of AB use, and not excessive protein intake are associated with ameliorations in the intradialytic SBP decline [9]. Therefore, these interventions may improve the prognosis of patients on HD by preventing intradialytic SBP decline.
The mechanism by which greater intradialytic SBP decline is associated with worse prognosis, including MACEs, is not precise. In a previous report [27], a greater intradialytic SBP decline was associated with the development of regional wall motion abnormalities, and consequently, with a lower left ventricular ejection fraction. Concerning IDH, a study reported that organ ischemia due to blood pressure decline affects various outcomes, including cardiovascular complications [28]. Although organ blood flow is determined by systemic blood pressure, vascular organ resistance, and capillary function [29], patients on HD may be more susceptible to organ ischemia due to vascular stenosis and reduced capillary function. Such tissue ischemia may cause further hypotension by releasing adenosine, an endogenous vasodilator, leading to a vicious cycle and worsening prognosis [30].
Although previous studies have shown that mortality is also associated with IDH and blood pressure variability during dialysis [22,23,24,25,26], the present study found only a significant increase in HR for death or hospitalization due to a decline in ADL at T2 compared with T1 in the fully adjusted model. This might suggest that among the explanatory variables in the fully adjusted model, age and CCI were more closely and significantly associated with the increased risk of death or hospitalization due to a decline in ADL, compared with intradialytic SBP decline (age, HR 1.04 [95% CI 1.01–1.07]; comorbidity index, HR 1.33 [95% CI 1.09–1.61]). In addition, unlike previous reports, this study considered death and hospitalization due to a decline in ADL as the same outcome, which may have blunted the association between intradialytic SBP decline and all-cause mortality.
The novelty of this study compared to previous reports will be discussed as follows. First, all previous studies included patients on conventional HD [22,23,24,25,26], and there have been no data on patients on OL-HDF or I-HDF (a total of 96% in this study). Patients who underwent OL- and I-HDF are theoretically less susceptible to intradialytic SBP declines than those who underwent conventional HD [2, 31]. In our study, the T3 group exhibited a significantly worse prognosis; however, the intradialytic SBP decline in the group was only ≥29.9 mmHg. Additionally, in the restricted cubic spline curves, the HR for MACEs and all-cause hospitalization significantly increased when the intradialytic SBP decline was greater than 24.2 mmHg. These cut-off values were smaller than previously reported risks of intradialytic SBP decline of ≥40–50 mmHg [22,23,24,25,26]: this may be due to the large proportion of OL- and I-HDF patients included in our study. Second, previous reports calculated intradialytic SBP declines from the period of 3–6 months at most [22,23,24,25,26]. However, this calculation method did not consider their seasonal variations. Actually, predialysis SBP and intradialytic SBP declines in patients on HD, like blood pressure in the general population, show a consistent seasonal pattern, peaking in winter and falling nadir in summer [9,10,11,12,13,14]. Therefore, it is essential to use annual averages of intradialytic SBP declines to consider seasonal variations, as was done in the present study. Finally, we obtained intradialytic SBP and UFR data for all HD sessions and monthly clinical and laboratory parameters for all participants for 1 year and used the averages of these values for analyses, enhancing this study’s internal validity. However, the present study has several limitations. First, this is a retrospective observational study and not an intervention study. Therefore, it was impossible to determine a causal relationship between the intradialytic SBP decline and any outcomes. Second, the covariates such as home blood pressure measurements, dietary behaviors, and exercise habits that might have been associated with intradialytic blood pressure could not be obtained. Third, excluding patients who did not continue HD for 1 year may have introduced some selection bias during patient screening, making it difficult to extrapolate the results of this study to patients with a life expectancy of less than 1 year. Fourth, it is impossible to eliminate the effect of medications to treat cardiovascular diseases or procedures for IDH such as lower extremity elevation, infusion, and ultrafiltration rate, on outcomes. Finally, the sample size was also not large. However, we performed multivariate analyses adjusted for sufficient covariates, including age, CCI, pressor agent use, and predialysis SBP, which were significantly different among the tertile groups and may be associated with the outcomes.
In conclusion, this was the first observational study to examine the association between the intradialytic SBP decline and MACEs and all-cause hospitalization using annually averaged values to accommodate seasonal variations. This study showed that in Japanese patients on HD, mainly on OL-HDF and I-HDF, the mean annual decline in the intradialytic SBP was significantly associated with increased MACEs and all-cause hospitalization even in the fully adjusted model. Although avoiding the intradialytic SBP decline may improve the prognosis, including MACEs, in patients on HD, a prospective study is needed to demonstrate a causal relationship.
References
Hanafusa N, Abe M, Joki N, Hoshino J, Kikuchi K, Goto S, et al. 2020 Annual dialysis data report, JSDT Renal Data Registry. Nihon Toseki Igakkai Zasshi. 2021;54:611–57.
Kikuchi K, Hamano T, Wada A, Nakai S, Masakane I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019;95:929–38.
Cho A, Lee YK, Oh J, Yoon JW, Shin DH, Jeon HJ, et al. The relationship between intradialytic hypotension and vascular calcification in hemodialysis patients. PLoS One. 2017;12:e0185846.
Mc Causland FR, Brunelli SM, Waikar SS. Dialysis dose and intradialytic hypotension: results from the HEMO study. Am J Nephrol. 2013;38:388–96.
Mc Causland FR, Waikar SS. Association of predialysis calculated plasma osmolarity with intradialytic blood pressure decline. Am J Kidney Dis. 2015;66:499–506.
Okoye OC, Slater HE, Rajora N. Prevalence and risk factors of intra-dialytic hypotension: a 5 year retrospective report from a single Nigerian Centre. Pan Afr Med J. 2017;28:62.
Yu J, Liu Z, Shen B, Teng J, Zou J, Ding X. Intradialytic hypotension as an independent risk factor for long-term mortality in maintaining hemodialysis patients: a 5-year follow-up cohort study. Blood Purif. 2018;45:320–6.
Kanbay M, Ertuglu LA, Afsar B, Ozdogan E, Siriopol D, Covic A, et al. An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J. 2020;13:981–93.
Uchiyama K, Shibagaki K, Yanai A, Kusahana E, Nakayama T, Morimoto K, et al. Seasonal variation and predictors of intradialytic blood pressure decline: a retrospective cohort study. Hypertens Res. 2021;44:1417–27.
Argilés A, Mourad G, Mion C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N. Engl J Med. 1998;339:1364–70.
Tozawa M, Iseki K, Iseki C, Morita O, Yoshi S, Fukiyama K. Seasonal blood pressure and body weight variation in patients on chronic hemodialysis. Am J Nephrol. 1999;19:660–7.
Spósito M, Nieto FJ, Ventura JE. Seasonal variations of blood pressure and overhydration in patients on chronic hemodialysis. Am J Kidney Dis. 2000;35:812–8.
Cheung AK, Yan G, Greene T, Daugirdas JT, Dwyer JT, Levin NW, et al. Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol. 2002;13:2345–52.
Usvyat LA, Carter M, Thijssen S, Kooman JP, Van Der Sande FM, Zabetakis P, et al. Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin J Am Soc Nephrol. 2012;7:108–15.
Yamaguchi J, Hagiwara N, Ogawa H, Koyanagi R, Kasanuki H, Takagi A, et al. Effect of amlodipine+ candesartan on cardiovascular events in hypertensive patients with coronary artery disease (from The Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] Study). Am J Cardiol. 2010;106:819–24.
Ogawa C, Tsuchiya K, Maeda K. High serum magnesium levels are associated with favorable prognoses in diabetic hemodialysis patients, retrospective observational study. PLoS One. 2020;15:e0238763.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Mizuguchi J, Tomo T, Masakane I, Watanabe Y, Kawanishi H, Akiba T, et al. JSDT [Guidelines for maintenance hemodialysis: hemodialysis prescriptions]. J Jpn Soc Dial Ther. 2013;46:587–632.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the dialysis outcomes and practice patterns study (DOPPS). J Am Soc Nephrol. 2003;14:3270–7.
Okamura A, Okura H, Iwai S, Sakagami A, Kamon D, Hashimoto Y, et al. Incidence and prognostic impact of the calcified nodule in coronary artery disease patients with end-stage renal disease on dialysis. Heart Vessels. 2022;37:1662–8.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–20.
Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–34.
Lu J, Zhu M, Liu S, Zhu M, Pang H, Lin X, et al. The relationship between survival rate and intradialytic blood pressure changes in maintenance hemodialysis patients. Ren Fail. 2017;39:417–22.
Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transpl. 2018;33:149–59.
Yu J, Chen X, Wang Y, Liu Z, Shen B, Teng J, et al. Intradialytic systolic blood pressure variation can predict long-term mortality in patients on maintenance hemodialysis. Int Urol Nephrol. 2021;53:785–95.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20.
Sars B, van der Sande FM, Kooman JP. Intradialytic hypotension: mechanisms and outcome. Blood Purif. 2020;49:158–67.
Magder SA. The highs and lows of blood pressure: toward meaningful clinical targets in patients with shock. Crit Care Med. 2014;42:1241–51.
Franssen CFM. Adenosine and dialysis hypotension. Kidney Int. 2006;69:789–91.
Mineshima M, Takahashi S, Tomo T, Kawanishi H, Kawaguchi H, Minakuchi J, et al. A clinical significance of intermittent infusion hemodiafiltration using backfiltration of ultrapure dialysis fluid compared to hemodialysis: a multicenter randomized controlled crossover trial. Blood Purif. 2019;48:368–81.
Acknowledgements
The authors thank all the participants of this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
This study and all its protocols were reviewed and approved by the ethics committee of our hospital, and informed consent was obtained from all patients prior to their participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, R., Uchiyama, K., Washida, N. et al. Mean annual intradialytic blood pressure decline and cardiovascular events in Japanese patients on maintenance hemodialysis. Hypertens Res 46, 1536–1546 (2023). https://doi.org/10.1038/s41440-023-01228-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01228-8
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Blood pressure management in hemodialysis patients
Hypertension Research (2023)