Abstract
Purpose
It is unclear which time-points of intradialytic blood pressure (BP) best predict prognosis. Thus, it is important to assess the association between different time-points of intradialytic BP and prognosis in clinical practice.
Methods
We recruited patients who underwent hemodialysis from January 2014 to June 2014. Data about dialysis were collected, including intradialytic BP. Cox regression analysis was performed to examine the association between different time-points of intradialytic BP and clinical events, with a follow-up through December 31, 2019. The primary endpoint was all-cause mortality.
Results
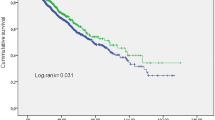
A total of 216 patients were recruited and 62 (30.7%) patients died (6.1 per 100-person year) during the follow-up. Intradialytic SBP varied greatly in fatalities. Univariate and multivariate Cox regression models indicated that the adjusted hazard ratio for death was 1.80 and 5.06 when intradialytic systolic blood pressure (SBP) variation was analyzed in increments of 20 mmHg. Furthermore, we divided intradialytic SBP variation into three categories: < 15 mmHg, 15 ~ 30 mmHg, ≥ 30 mmHg. Kaplan–Meier analysis indicated that both all-cause mortality and cardiovascular mortality increased significantly for patients with intradialytic SBP variation over 30 mmHg (P = 0.006 and 0.021). Univariate and multivariate Cox regression models indicated that the adjusted hazard ratio for death was 3.78 and 12.62 as intradialytic SBP variation ≥ 30 mmHg vs. intradialytic SBP variation < 15 mmHg.
Conclusion
Intradialytic SBP variation, rather than BP of specific intradialytic time-points, has the potential to predict long-term mortality in hemodialysis patients. BP stability is crucial for patients’ prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unstable blood pressure (BP) is a common complication among maintenance hemodialysis (MHD) patients. The prevalence of hypertension in MHD patients was reported as 86% [1]. As for MHD patients, antihypertensive medications and ultrafiltration might lead to lower intradialytic BP. For some patients whose cardiac function fails to compensate, hypotension occurs [2,3,4]. Conversely, others might develop abnormally hypertension due to the activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS), hemodialytic removal of antihypertensive medications, endothelial cell dysfunction, and the release of some pressor neurotransmitters (e.g., endothelin-1) as a result of excessive ultrafiltration [5,6,7,8].
Both intradialytic-hypertension (IDHT) and intradialytic-hypotension (IDH) are associated with higher mortality [9,10,11,12,13]. However, the roles of IDHT and IDH in predicting prognosis are not uniform under different definitions [5, 9]. Furthermore, sometimes patients who felt uncomfortable may not be identified as IDH and IDHT by definition immediately. Therefore, it may be more convenient and accurate to use intradialytic BP alone to evaluate the prognosis.
Studies have already shown that pulse pressure, predialysis systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean artery pressure (MAP) can predict the prognosis of MHD patients [14,15,16,17,18,19]. However, no consensus has been achieved on the time points at which the BP during the hemodialysis (HD) period predicts the prognosis best. The possible reason is that BP varies drastically from patient to patient at different time points. Thus, we designed this study to assess the relationship between intradialytic BP at different time points and mortality to find the ideal index of intradialytic BP as the best predictor of prognosis in MHD patients.
Patients and methods
Patients
Patients enrolled in this study were diagnosed with end-stage kidney disease according to K/DOQI guidelines and started HD before January 2014 in blood purification center, Zhongshan Hospital, Fudan University, Shanghai, China. Inclusion criteria were ① patients older than 18 years old, ② patients without acute heart failure and cardiac dysfunction, ③ patients on MHD for at least 3 months, ④ patients exclusive from the 1st daily shift. They were followed up until December 31, 2019. Patients undergoing dialysis three times a week were treated with standard bicarbonate dialysate (Na+ 138.0 mmol/L, HCO3− 32.0 mmol/L, K+ 2.0 mmol/L, Ca2+ 1.25 mmol/L, Mg2+ 0.5 mmol/L) by low-flux HD using 1.4 m2 dialyzers with synthetic membranes (BLS514SD, Sorin Group Italia, Mirandola, Italy; Polyflux14L, Gambro Dialysatoren GmbH, Hechigen, Germany). The blood flow was 200–280 mL/min, and the dialysate flow was 500 mL/min. Dry weight was monitored in every patient to achieve an edema-free state. The study was approved by our institutional clinical research ethics review board (Ethics Committee of Zhongshan Hospital, Fudan University) and was conducted according to the Declaration of Helsinki principles. Each participant signed an informed consent form before entering the study. The primary endpoint was all-cause mortality. Sudden death was defined as a witnessed death that occurred within one hour after the onset of acute symptoms, with no evidence of accident or violence [20].
BP measurements
Patients received HD three times per week. They had their BP measured before each session in a sitting position per the standard protocol using an automated stand-alone device or one integrated into the HD machine with an appropriately sized pressure cuff around the non-access upper arm positioned at heart level. The patient must be seated quietly for at least 5 min in a chair and arm supported at heart level. BP should be measured at least 5 min before the needles for dialysis access are placed, which may cause substantial stress in some patients. Caffeine, exercise, and smoking should be avoided for at least 30 min before measurement [21]. Intradialytic BPs were measured at 60-min intervals during all HD treatments in a seated position. Post-HD BP was taken just before detaching the dialysis circuit from the patient following the same protocol. BP was measured at least at five points: ① before HD (predialysis), ② 1 h after HD (1st-hour dialysis), ③ 2 h after HD (2nd-hour dialysis), ④ 3 h after HD (3rd-hour dialysis) and ⑤ after HD with the patient supine (post-dialysis).
When patients suffer from abdominal discomfort, muscle cramps, sighing, anxiety, restlessness, nausea, vomiting, headaches, dizziness, or fainting, BP was taken to get the lowest intra-dialysis BP. If dialysis procedures were uneventful, nadir BP was the lowest BP measured after dialysis initiation.
During dialysis, BP variation was determined by finding the average post-dialysis BP minus average predialysis BP. BP was estimated by averaging all the BP monitored every hour intra-dialysis sessions during the 6-month run-in period before this study.
We divided patients into three groups according to intradialytic SBP variations (absolute pre- to post-SBP change) < 15 mmHg [22], 15–30 mmHg, ≥ 30 mmHg [9, 22] to evaluate the relationship of intradialytic SBP variations and mortality.
Data collection
An independent researcher extracted patient characteristics (demographics, comorbidity, biochemistry, and medication) at the beginning of the run-in phase and the end of the study, and the cause of death from patient charts recorded by their treating physicians.
Biochemical measurements
Blood sampling was performed during a midweek non-dialysis day 8–10 a.m. after 30 min of quiet rest in a semi-recumbent position. Serum albumin, pre-albumin, hemoglobin, and creatinine were measured using standard methods in the routine clinical laboratory. The concentrations of intact parathyroid hormone (iPTH) were measured using electrochemiluminescence immunoassay.
Echocardiography
Transthoracic echocardiographic examinations were conducted using a Philips echocardiographic machine (Philips IE33, Eindhoven, the Netherlands) with a 3.5-MHz multiphase array probe by a single experienced cardiologist during a midweek non-dialysis day, within 2 h after blood sampling, both at the entry and the endpoint of the study. According to the Penn Convention’s recommendations, measurements of the left ventricular internal dimension, interventricular septal thickness, and posterior wall thickness were made at end-diastole. Left ventricular mass was calculated with the Devereux formula. The left ventricular mass index (LVMI) was obtained by dividing left ventricular mass by height in meters rose to the power of 2.7. The left ventricular ejection fraction (EF) was determined by two-dimensional echocardiography.
Statistical analysis
Continuous variables were expressed as mean ± Standard Deviation, while categorical variables were appropriately presented as numbers and percentages. Student’s t test was used to compare normal variables, whereas, for categorical variables, chi-square tests were performed, respectively. Repeated ANOVA analysis was used to compare pre-, intra-, post- and nadir dialysis SBP, DBP, and MAP in survival and fatality groups. The effects of SBP, DBP, and MAP on mortality were analyzed using Cox’s proportional hazards models. We constructed a series of models: (1) Model 1 was univariate analysis; (2) Model 2 was adjusted for demographic data, including age, gender, body mass index, dialysis vintage, residual kidney function, and spKt/Vurea; (3) Model 3 was adjusted for demographic data and cardiac conditions (e.g., EF, LVMI, NT-proBNP). SBP was analyzed in increments of 20 mmHg and DBP of 10 mmHg in each model. Two-sided P < 0.05 was considered significant. All analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and 5-year mortality
A total of 216 patients were recruited. 14 (6.5%) were censored during the 5-year observation period because of kidney transplantation and transfer to other HD centers. Ultimately, 202 patients were eligible. During the follow-up, 62 (30.7%) patients died with a mortality rate of 6.1 per 100-persons year, including 22 patients due to fatal cardiovascular events, 18 patients with cerebrovascular events, 9 deaths attributed to severe infection, 8 to sudden death, and 5 to cancer.
Table 1 summarizes the baseline characteristics of the study cohort. Compared to the survivors, the deceased was older, had significantly lower serum albumin, pre-albumin, creatinine, and EF, higher N-terminal pro-B-type natriuretic peptide (NT-proBNP), and more antihypertensive medications.
Intradialytic blood pressure and 5-year mortality
Intradialytic BPs, including predialysis, post-dialysis, 1st-hour dialysis, 2nd-h dialysis, 3rd-h dialysis, nadir BP, BP variation, are listed in Fig. 1. We found no significant differences in predialysis, post-dialysis, 1st-h dialysis, 2nd-h dialysis, 3rd-h dialysis, and nadir BP. At the same time, intradialytic SBP variation was higher in fatalities than in survivors (P < 0.05) (Table 2). Nevertheless, significant differences were not detected in intradialytic DBP and MAP variations and BPs at different time points in the dialysis process.
Predictors for 5-year mortality
Cox’s hazards models were used to evaluate the effects of intradialytic BPs on mortality. We found that after adjusting for demographic data (age, gender, body mass index, dialysis vintage, residual kidney function, spKt/Vurea), intradialytic SBP variation (per 20 mmHg increase) could predict long-term mortality (HR = 2.49, 95% CI 1.07–5.78). Furthermore, after adjusting for demographic data and cardiac conditions (EF, LVMI, NT-proBNP), intradialytic SBP variation (per 20 mmHg increase) was still an independent risk factor for 5-year mortality (HR = 5.06, 95% CI 1.89–13.51) (Table 3). Similar findings were not seen in intradialytic DBP variations and BPs of different dialysis time points, including predialysis BP, post-dialysis BP, and nadir intradialytic BP.
Furthermore, we divided intradialytic SBP variation into three categories: < 15 mmHg, 15–30 mmHg, and ≥ 30 mmHg. Kaplan–Meier survival curves of patients with different levels of SBP variation showed a significant difference in all-cause mortality and cardiovascular mortality among groups (P = 0.006 and 0.021, respectively) (Figs. 2, 3). The adjusted hazard ratio for death was 7.96 (95% CI 1.73–36.56) as intradialytic SBP variation ≥ 30 mmHg vs. intradialytic SBP variation < 15 mmHg in the model, which consists of demographic data (age, gender, body mass index, dialysis vintage, residual kidney function, spKt/Vurea). Moreover, the multivariate Cox regression model, which consists of demographic data and cardiac conditions (EF, LVMI, NT-proBNP), indicated that the adjusted hazard ratio for death was 12.62 (95% CI 2.41–66.10) as intradialytic SBP variation ≥ 30 mmHg vs. intradialytic SBP variation < 15 mmHg. However, no significant difference was found between intradialytic SBP variation 15–30 mmHg and intradialytic SBP variation < 15 mmHg (Table 4).
Patient characteristics of different SBP variation levels
Factors associated with intradialytic SBP variability are listed in Table 5. We found that the patients of intradialytic SBP variation ≥ 30 mmHg were elderly, had higher ultrafiltration rate, larger inter-dialytic weight gain, and less reserved kidney function than patients of intradialytic SBP variation < 15 mmHg (P < 0.05).
Discussion
Our prospective cohort study with a follow-up period of 5 years indicated that intradialytic SBP variation, rather than intradialytic BP at different time points, could predict long-term mortality in patients on MHD, especially in elderly patients and patients with higher ultrafiltration rate, greater inter-dialytic weight gain and less reserved kidney function.
BP has a periodic change related to the change in blood volume during the HD period. In general, the patient’s BP is highest before dialysis. During the dialysis session, the BP drops slowly as the blood volume decreases gradually with ultrafiltration. After dialysis, the blood volume increases gradually with water and sodium retention, and the BP rises slowly until the next dialysis. For patients with hypertension, BP in the course of HD might have an inevitable decline. However, an excessive decrease in BP during the HD process may decrease blood flow of tissues and organs and affect patients’ prognosis.
The relationship between BP and survival in MHD patients is still controversial. On the one hand, the prevalence of hypertension in MHD patients is very high; on the other hand, hypotension is the most common complication in HD patients [23]. Most studies suggest that IDH may be a potential confounding factor for poor prognosis in dialysis patients [9,10,11]. IDH causes discomfort and increases the risk of death. IDH affects not only the conventional HD procedures but also severely reduces patients’ quality of life and lifespan. Common risk factors for IDH may counteract hypovolemia during dialysis by triggering cardiovascular hemodynamic mechanisms, such as plasma reperfusion, cardiac perfusion, and sympathetic nervous system activity [23, 24]. The incidence of IDH in patients with different diagnostic criteria was significantly different, and the incidence of IDH in diverse populations with similar definition was also considerably different [10, 11, 23, 24]. As for the paradoxical IDHT, initial research focused on potential biologic mechanisms to explain the acute rise in BP, particularly the role of endothelin-1 (ET-1) [6]. There is strong evidence that extracellular volume excess is a consistent phenotype in patients with IDHT. Patients with recurrent IDHT have been characterized as patients with lower baseline weight and small inter-dialytic weight gain, resulting in masked chronic extracellular volume excess [25]. IDHT broadly refers to BP increases from predialysis to post-dialysis or intradialysis, but there remains heterogeneity in how IDHT is classified in the literature. Unlike the general population [26], the direct link between elevated BP and cardiovascular mortality in dialysis patients has not been revealed [1, 27, 28]. However, most studies have shown that IDHT may be an essential risk factor in MHD patients [12, 13, 29]. Thus, the definitions of IDH and IDHT have limitations in predicting prognosis.
Some studies have shown that low pre- and post-dialysis BP is associated with increased mortality. In contrast, other studies have shown that post-dialysis BP has a worse prognosis than patients with elevated predialysis BP [13, 28]. Hara et al. [15] figured out that predialysis BP was independently associated with all-cause mortality and cardiovascular events among Japanese dialysis patients. Latterly, Tsuruya et al. [18] discovered that post-dialysis BP was a better predictor of mortality than predialysis BP in Japanese HD patients. We intended to determine whether the intradialytic BP can reflect the prognosis directly and which time points of the intradialytic BP can reflect the prognosis best. In our study, we found that intradialytic SBP varied greatly in fatalities. Similar findings were not seen in intradialytic DBP and MAP variations and BPs of different dialysis time points, including predialysis BP, post-dialysis BP, and nadir intradialytic BP.
BP variability might be caused by impaired endothelial function, increased inflammation, increased stress in blood vessel walls, impaired baroreceptor function, and increased sympathetic nervous system activity [30]. Changes in dialysis solution and osmotic pressure can cause random BP fluctuations in MHD patients. Vascular stiffness, reduced cardiac output, and common dysautonomia and new hormone imbalances in MHD patients may amplify these fluctuations so as to make hemodynamic changes easier. Before and after HD, BP variations reflect the volume fluctuation during HD and the cardiovascular compensatory mechanism. When BP fluctuates too much, the relative target organs will be damaged. There is some evidence that, for MHD patients, SBP variability during treatment is independent of the mean SBP, and that even with the antihypertensive drug, SBP is also a strong predictor of stroke and transient cerebral ischemia [30]. Among HD patients, long-term BP variability is measured with pre-HD BPs and is associated with cardiovascular morbidity and mortality [31, 32]. Recent data suggest that short-term BP variability, considered intradialytic BP fluctuations, is a risk factor for cardiovascular events among HD patients [33]. Studies have also found that increased SBP variability in MHD patients was an independent risk factor for hospitalization, all-cause mortality, and cardiovascular mortality [34, 35]. After adjusting for the confounding factors as demographic data and cardiac functions, we found that intradialytic SBP variation was still a predictor for 5-year mortality. Flythe et al. [9] showed larger drops of SBP ≥ 30 mmHg were only found to be associated with mortality in combination with nadir SBP < 90 mmHg in both the Hemodialysis (HEMO) Study cohort and their large dialysis organization (LDO) cohorts. In an observational study using data from a US-based LDO, ΔiSBP was defined as pre-HD SBP minus nadir SBP. ΔiSBP was divided into six categories: < 15, 16–20, 21–30, 31–40, 41–50 and > 51 mmHg [22]. Therefore, we divided our patients into three groups according to intradialytic SBP variations: < 15 mmHg, 15–30 mmHg, and ≥ 30 mmHg. Kaplan–Meier survival curves showed a worse survival rate in patients with intradialytic SBP variation ≥ 30 mmHg than with intradialytic SBP variation < 15 mmHg, both in all-cause mortality and cardiovascular mortality. Therefore, intradialytic BP stability is crucial to patients’ outcomes.
Factors associated with intradialytic SBP variability were found to be greater dialytic fluid removal and rate, as well as older age and dialysis vintage [36]. When intravascular volume status is disturbed, BP exerts an upward and downward force, resulting in hemodynamic instability in the context of atherosclerosis, coronary artery disease, myocardial stunning, and autonomic dysfunction [37, 38]. Leypoldt et al. [39] evaluated the association between volume status and SBP. The decrease of SBP during HD is related to reducing body weight and blood volume during HD. Thus, fewer reductions in body weight caused high pre- and post-dialysis SBP. BP instability may be a potential pathway for the association between high ultrafiltration rates and all-cause and cardiovascular mortality [40,41,42]. In our study, we also found that patients of intradialytic SBP variation ≥ 30 mmHg were elderly, had higher ultrafiltration rate, larger inter-dialytic weight gain, and less reserved kidney function than patients of intradialytic SBP variation < 15 mmHg. For patients with greater inter-dialytic weight gain and higher ultrafiltration rates, individualized HD modality, like frequent or long-term dialysis, should be taken.
In this study, we comprehensively assess the optimal intradialytic BP to indicate long-term prognosis in MHD patients. We first testified the contribution of intradialytic BP, including predialysis, 1st-h dialysis, 2nd-h dialysis, 3rd-h dialysis, post-dialysis, nadir BP, and BP variation to patients’ outcomes. Also, we explored the characteristics of patients with significant BP variability in clinical practice. Furthermore, we have a long-term follow-up period.
However, our study has several limitations. One of these is the small sample size and selection bias (patients were all from Asia, they were chosen from one shift and during a specific period across the year), the results might not be validated in other populations. Nevertheless, the study patients were free of acute heart failure and cardiac dysfunction, and we did not include information on diastolic function. Further research might target whether our study results could be testified in MHD patients with various comorbidities. An additional limitation is that we did not evaluate the effect of different vascular access types on patients' hemodynamic stability. Fourth, the relevance of dialysis unit BP might be overlooked in recent years, since the interdialytic BP measurement had the prognostic superiority. Home BP monitoring (HBPM) and ambulatory BP monitoring (ABPM) are undoubtedly at the core of interdialytic BP management in the HD population [43]. However, their superiority was hampered by the low adherence rate among HD patients [44]. Peridialysis BP had its unraveled convenience and can be readily collected in the clinical setting. If patients are unwilling to perform HBPM or ABPM regularly, dialysis unit BP would be the only choice for the clinicians. From this perspective, having a good understanding of the peridialysis BP mortality associations had crucial clinical significance. Indeed, not having incorporated out-of-dialysis BP is part of the study limitations. Ambulatory BP monitoring might be taken into consideration in our future studies.
Furthermore, we did not use objective methods (e.g., bio-impedance, lung ultrasound) to evaluate fluid status. Assessment of volume balance in HD patients with clinical criteria (e.g., peripheral edema or signs of lung congestion) has limitations. Objective evaluation of the volume status may be an excellent way to minimize intradialytic BP variability and improve outcomes. Previous efforts using biomarkers (e.g., renin, aldosterone, natriuretic peptides, and others) or ultrasonographic measurement of inferior vena cava diameter were largely inaccurate or impractical [45]. The use of bioimpedance analysis was explored in a pilot study [46]. A recent study showed that a lung-ultrasound-guided strategy to evaluate volume excess and guide dry-weight probing was associated with significantly lower intradialytic, interdialytic, daytime, and nighttime ambulatory BP levels [47]. Further researches need to apply objective methods to estimate volume excess and dry weight, which is more accurate.
Conclusions
Intradialytic SBP variation, rather than BPs of specific intradialytic time-points, can be an optimal metric to evaluate long-term prognosis in MHD patients. Patients with elder age, higher ultrafiltration rate, greater inter-dialytic weight gain, and less reserved kidney function tend to suffer more significant intradialytic BP variation. So modest variation in SBP after HD might be associated with the highest survival. Individualized treatments such as proper setting ultrafiltration rate and controlling inter-dialytic weight gain should be taken to achieve BP stability for better outcomes.
References
Agarwal and Rajiv (2005) Hypertension and survival in chronic hemodialysis patients-past lessons and future opportunities. Kidney Int 67(1):1–13. https://doi.org/10.1111/j.1523-1755.2005.00050.x
Daugirdas JT (2001) Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis 38(4 Suppl 4):S11-17. https://doi.org/10.1053/ajkd.2001.28090
Sherman RA (2002) Intradialytic hypotension: an overview of recent, unresolved and overlooked issues. Semin Dialysis 15(3):141–143. https://doi.org/10.1046/j.1525-139x.2002.00002.x
Andrulli S, Colzani S, Mascia F, Lucchi L, Stipo L, Bigi MC, Crepaldi M, Redaelli B, Albertazzi A, Locatelli F (2002) The role of blood volume reduction in the genesis of intradialytic hypotension. Am J Kidney Dis 40(6):1244–1254. https://doi.org/10.1053/ajkd.2002.36894
Inrig JK (2010) Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 55(3):580–589. https://doi.org/10.1053/j.ajkd.2009.08.013
Teng J, Tian J, Lv WL, Zhang XY, Zou JZ, Fang Y, Yu J, Shen B, Liu ZH, Ding XQ (2015) Inappropriately elevated endothelin-1 plays a role in the pathogenesis of intradialytic hypertension. Hemodial Int 19(2):279–286. https://doi.org/10.1111/hdi.12238
Agarwal R, Light RP (2010) Intradialytic hypertension is a marker of volume excess. Nephrol Dialysis Transplant 25(10):3355–3361. https://doi.org/10.1093/ndt/gfq210
Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu CY, Chung HM, Liu CP, Fang HC (2006) Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 69(10):1833–1838. https://doi.org/10.1038/sj.ki.5000266
Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM (2015) Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26(3):724–734. https://doi.org/10.1681/ASN.2014020222
Yu J, Liu Z, Shen B, Teng J, Zou J, Ding X (2018) Intradialytic hypotension as an independent risk factor for long-term mortality in maintaining hemodialysis patients: a 5-year follow-up cohort study. Blood Purif 45(4):320–326. https://doi.org/10.1159/000486231
Stefansson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stalhammar NO (2014) Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9(12):2124–2132. https://doi.org/10.2215/CJN.02680314
Inrig JK, Patel UD, Toto RD, Szczech LA (2009) Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 54(5):881–890. https://doi.org/10.1053/j.ajkd.2009.05.012
Yang CY, Yang WC, Lin YP (2012) Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four-year prospective observational cohort study. BMC Nephrol 13:12. https://doi.org/10.1186/1471-2369-13-12
Lertdumrongluk P, Streja E, Rhee CM, Sim JJ, Gillen D, Kovesdy CP, Kalantar-Zadeh K (2015) Changes in pulse pressure during hemodialysis treatment and survival in maintenance dialysis patients. Clin J Am Soc Nephrol 10(7):1179–1191. https://doi.org/10.2215/CJN.09000914
Hara M, Tanaka S, Taniguchi M, Fujisaki K, Torisu K, Masutani K, Hirakata H, Nakano T, Tsuruya K, Kitazono T (2018) Prognostic value of predialysis blood pressure and risk threshold on clinical outcomes in hemodialysis patients: the Q-Cohort Study. Medicine 97(51):e13485. https://doi.org/10.1097/MD.0000000000013485
Rootjes PA, de Roij van Zuijdewijn CLM, Grooteman MPC, Bots ML, Canaud B, Blankestijn PJ, van Ittersum FJ, Maduell F, Morena M, Peters SAE, Davenport A, Vernooij RWM, Nube MJ, Investigators HDFPP (2020) Long-term peridialytic blood pressure patterns in patients treated by He. Kidney Int Rep 5(4):503–510. https://doi.org/10.1016/j.ekir.2020.01.007
Han YC, Tu Y, Zhou LT, Pan MM, Wang B, Liu H, Tang RN, Liu BC (2019) Peridialysis BP levels and risk of all-cause mortality: a dose-response meta-analysis. J Hum Hypertens 33(1):41–49. https://doi.org/10.1038/s41371-018-0103-9
Tsuruya K, Kanda E, Nomura T, Iseki K, Hirakata H (2020) Postdialysis blood pressure is a better predictor of mortality than predialysis blood pressure in Japanese hemodialysis patients: the Japan Dialysis Outcomes and Practice Patterns Study. Hypertens Res. https://doi.org/10.1038/s41440-020-0425-1
Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB (1999) The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 159(17):2004–2009. https://doi.org/10.1001/archinte.159.17.2004
Tatsuya S, Yoshiharu T, Masamitsu F, Enyu I (2004) Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66(3):1212–1220. https://doi.org/10.1111/j.1523-1755.2004.00812.x
K/DOQI Workgroup (2005) K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45(null): S1–153
Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, Amin A, Kovesdy CP, Sim JJ, Kalantar-Zadeh K (2018) Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dialysis Transplant 33(1):149–159. https://doi.org/10.1093/ndt/gfx037
Assimon MM, Flythe JE (2015) Intradialytic blood pressure abnormalities: the highs, the lows and all that lies between. Am J Nephrol 42(5):337–350. https://doi.org/10.1159/000441982
van der Sande FM, Dekker MJ, Leunissen KML, Kooman JP (2018) Novel insights into the pathogenesis and prevention of intradialytic hypotension. Blood Purif 45(1–3):230–235. https://doi.org/10.1159/000485160
Nongnuch A, Campbell N, Stern E, El-Kateb S, Fuentes L, Davenport A (2015) Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int 87(2):452–457. https://doi.org/10.1038/ki.2014.276
Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D (2001) Impact of high-normal blood pressure on the risk of cardiovascular disease. New Engl J Med 345(18):1291–1297. https://doi.org/10.1056/NEJMoa003417
Foley RN, Herzog CA, Collins AJ (2002) Blood pressure and long-term mortality in United States hemodialysis patients USRDS Waves 3 and 4 study. Kidney Int 62(5):1784–1790. https://doi.org/10.1046/j.1523-1755.2002.00636.x
Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P (1998) “U” curve association of blood pressure and mortality in hemodialysis patients. Medical directors of dialysis clinic, Inc. Kidney Int 54(2):561–569. https://doi.org/10.1046/j.1523-1755.1998.00005.x
Losito A, Del Vecchio L, Del Rosso G, Locatelli F (2016) Postdialysis hypertension: associated factors, patient profiles, and cardiovascular mortality. Am J Hypertens 29(6):684–689. https://doi.org/10.1093/ajh/hpv162
Rothwell PM (2010) Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. The Lancet 375(9718):938–948. https://doi.org/10.1016/s0140-6736(10)60309-1
Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, Chertow GM (2014) Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J Hum Hypertens 28(1):18–24. https://doi.org/10.1038/jhh.2013.49
Shafi T, Sozio SM, Bandeen-Roche KJ, Ephraim PL, Luly JR, St Peter WL, McDermott A, Scialla JJ, Crews DC, Tangri N, Miskulin DC, Michels WM, Jaar BG, Herzog CA, Zager PG, Meyer KB, Wu AW, Boulware LE (2014) Predialysis systolic BP variability and outcomes in hemodialysis patients. J Am Soc Nephrol 25(4):799–809. https://doi.org/10.1681/ASN.2013060667
Flythe JE, Inrig JK, Shafi T, Chang TI, Cape K, Dinesh K, Kunaparaju S, Brunelli SM (2013) Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis 61(6):966–974. https://doi.org/10.1053/j.ajkd.2012.12.023
Lu J, Zhu M, Liu S, Zhu M, Pang H, Lin X, Ni Z, Qian J, Cai H, Zhang W (2017) The relationship between survival rate and intradialytic blood pressure changes in maintenance hemodialysis patients. Ren Fail 39(1):417–422. https://doi.org/10.1080/0886022X.2017.1305407
Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, Toto R, Himmelfarb J, Winchester JF, Stivelman J, Lindsay RM, Szczech LA (2007) Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 71(5):454–461. https://doi.org/10.1038/sj.ki.5002077
Flythe JE, Kunaparaju S, Dinesh K, Cape K, Feldman HI, Brunelli SM (2012) Factors associated with intradialytic systolic blood pressure variability. Am J Kidney Dis 59(3):409–418. https://doi.org/10.1053/j.ajkd.2011.11.026
Burton JO, Jefferies HJ, Selby NM, McIntyre CW (2009) Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 4(5):914–920. https://doi.org/10.2215/CJN.03900808
Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW (2010) Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodial Int 14(1):18–28. https://doi.org/10.1111/j.1542-4758.2009.00403.x
Leypoldt JK, Cheung AK, Delmez JA, Gassman JJ, Levin NW, Lewis JA, Lewis JL, Rocco MV (2002) Relationship between volume status and blood pressure during chronic hemodialysis. Kidney Int 61(1):266–275. https://doi.org/10.1046/j.1523-1755.2002.00099.x
Flythe JE, Kimmel SE, Brunelli SM (2011) Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79(2):250–257. https://doi.org/10.1038/ki.2010.383
Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G (2007) Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dialysis Transplant 22(12):3547–3552. https://doi.org/10.1093/ndt/gfm466
Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK (2006) Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 69(7):1222–1228. https://doi.org/10.1038/sj.ki.5000186
Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG (2014) Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dialysis Transplant 29(3):672–681. https://doi.org/10.1093/ndt/gft515
Miskulin DC, Gassman J, Schrader R, Gul A, Jhamb M, Ploth DW, Negrea L, Kwong RY, Levey AS, Singh AK, Harford A, Paine S, Kendrick C, Rahman M, Zager P (2018) BP in dialysis: results of a pilot study. J Am Soc Nephrol 29(1):307–316. https://doi.org/10.1681/ASN.2017020135
Rajiv A, Andersen MJ, Howard PJ (2008) On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3(1):153–158. https://doi.org/10.2215/CJN.03650807
Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, Covic AC (2014) Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis 64(1):111–118. https://doi.org/10.1053/j.ajkd.2014.01.420
Loutradis C, Sarafidis PA, Ekart R, Papadopoulos C, Sachpekidis V, Alexandrou ME, Papadopoulou D, Efstratiadis G, Papagianni A, London G, Zoccali C (2019) The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: a randomized controlled trial. Kidney Int 95(6):1505–1513. https://doi.org/10.1016/j.kint.2019.02.018
Acknowledgements
The authors are grateful to all participating patients and dialysis staff of Blood Purification Center, Division of Nephrology, Zhongshan Hospital, Fudan University. This study is supported by the National Natural Science Foundation of China (Grant no. 81903969), the Youth Foundation of Zhongshan Hospital (Grant no. 2019ZSQN22), Shanghai "science and technology innovation plan" technical standard project (No. 19DZ2205600), as well as by Shanghai Municipal Hospital Frontier Technology Project supported by Shanghai ShenKang Hospital Development Center (No. SHDC12018127).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any competing interests to declare, financial or otherwise.
Ethical approval
The study was approved by our institution’s clinical research ethics review board (Ethics Committee of Zhongshan Hospital, Fudan University) and was conducted according to the Declaration of Helsinki principles. Written informed consent was obtained from all participants.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Chen, X., Wang, Y. et al. Intradialytic systolic blood pressure variation can predict long-term mortality in patients on maintenance hemodialysis. Int Urol Nephrol 53, 785–795 (2021). https://doi.org/10.1007/s11255-020-02701-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02701-w