Abstract
The prevalence of nonalcoholic fatty liver disease (NAFLD) has been increasing rapidly worldwide, affecting 25–30% of the population. Fatty liver index (FLI) is a validated marker of NAFLD and can be used as a screening tool for hepatic steatosis. The purpose of the study was to evaluate the relationship between FLI and the risk of major cardiovascular events in never treated hypertensive patients. We included 903 hypertensive patients without a history of cardiovascular disease (mean age 52.7 ± 11.4 years; men 55%; baseline clinic BP 149.8 ± 15.2/95.5 ± 10.1 mmHg). Participants were prospectively evaluated for a mean follow-up period of 5.2 ± 3.2 years with at least one annual visit. Patients were also categorized into two groups using an FLI of 60 units. The incidence of cardiovascular events during follow-up was 8.5% (n = 77). Patients with FLI < 60 (n = 625) had a better BP control compared to their counterparts with FLI ≥ 60 (n = 278) during follow up (43% vs 33%, p = 0.02). Cox-regression analysis indicated that FLI (Hazard Ratio [HR], 1.05; 95% Confidence Interval [CI], 1.03–1.07, p < 0.001), FLI z-scores (HR, 3.66; 95% CI, 2.22–6.04) and high-risk FLI (HR, 7.5; 95% CI, 3.12–18.04) were independent determinants of the outcome after adjustment for baseline and follow-up variables. Stratification by diabetes mellitus indicated that FLI predicted the outcome to a greater extent in those with than those without diabetes (P-interaction < 0.001). In conclusion, FLI has an independent prognostic value for the incidence of cardiovascular events in newly diagnosed, never-treated hypertensive patients. Therefore, FLI might identify higher-risk patients in the primary prevention of hypertension.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been increasingly recognized as a multisystem disease with widespread extrahepatic manifestations [1]. Indeed, the presence and severity of NAFLD are strongly correlated to a heightened risk of cardiovascular disease and metabolic derangement, including diabetes mellitus [1, 2]. Mounting evidence has demonstrated that NAFLD may promote, maintain and perpetuate adverse pathophysiological mechanisms, including low-grade systemic inflammation, renin-angiotensin, sympathetic system activation, and insulin resistance [3]. Although prospective studies have unanimously shown that NAFLD may be independently associated with high-normal blood pressure (BP) or hypertension [4, 5], the exact role of NAFLD in the development of hypertension is not yet fully elucidated and is highly underestimated in the usual clinical practice. Although definitive NAFLD diagnosis mandates the implementation of a liver ultrasound and/or more sophisticated imaging techniques [6], a clinically useful auxiliary marker of NAFLD is the fatty liver index (FLI) which can be easily calculated by using simple laboratory and anthropometric measures [7]. Based on FLI levels, a high probability of underlying NAFLD has been attributed to an FLI measurement of 60 units or higher [7]. As a surrogate marker of NAFLD, the FLI has been used in several epidemiological or clinical studies and is associated with subclinical and clinical organ damage [4, 5, 8].
Although the association between NAFLD and cardiovascular events has been demonstrated in mixed cohorts of patients with and without hypertension [8], no prospective study was conducted on hypertensive individuals to evaluate the effect of FLI or FLI-related high-risk patterns on incident cardiovascular events. Moreover, the previous studies [6, 8] included individuals treated with BP-lowering drugs and individuals without baseline antihypertensive treatment. Therefore, limiting the investigation to newly-diagnosed patients without baseline BP-lowering treatment may avoid bias related to the duration of previous hypertension and the cardiovascular protection legacy of previous BP-lowering drugs. Moreover, a similar BP-lowering strategy can be applied with prespecified follow-up visits and interventions to allow adjustments for the on-treatment clinical characteristics of the participants.
We hypothesize that FLI or high-risk FLI-related patterns may be associated with increased cardiovascular events in newly-diagnosed hypertensive patients without previous exposure to BP-lowering drugs. To test the hypothesis, (1) a prospective cohort was used, (2) FLI was computed at baseline, (3) patients were stratified by FLI risk patterns and received a BP-lowering strategy at baseline, (4) regular annual visits were scheduled during follow-up, and (5) antihypertensive drug treatment escalation together with lifestyle measures was prescribed aiming to achieve optimal BP control, and additional risk factors were adequately treated.
Methods
Study Design and Participants
This prospective study investigated whether FLI, a surrogate marker of NAFLD, predicts fatal or non-fatal cardiovascular events in newly-diagnosed never-treated hypertensive patients. Between 2009 and 2012, we recruited never-treated men and non-pregnant women aged >18 years without a history of cardiovascular disease, referred or self-referred for suspected hypertension to a University hospital outpatient hypertension clinic. Οnly newly-diagnosed patients with sustained hypertension were included in the study, while those with normotension, white-coat, or masked hypertension were excluded. In addition, patients reporting excessive alcohol consumption (i.e., >30 g per day in men and >20 g per day in women) or with a history of cardiovascular disease, secondary hypertension, grade 3 hypertension, treatment with fibrates, or with any other clinically significant concurrent medical condition (thyroidal, psychiatric, neuromuscular, kidney, respiratory, hepatic, or gastrointestinal disease) were also excluded.
Baseline information on medical history was recorded, including cardiovascular risk factors, and clinic and 24-hour ambulatory BP measurements and routine biochemical tests were performed. The annual follow-up visits recorded clinic BP measurements and the follow-up antihypertensive drug treatment. In patients with clinic BP at the intended goal, drug treatment was continued unchanged, whereas it was intensified in those with clinic BP > 140/90 mmHg. Treatment was also changed in case of drug-related adverse effects. Lifestyle interventions were also prescribed on an individual basis, while treatment of additional cardiovascular risk factors was undertaken. Patients with cardiovascular or renal outcomes were identified to terminate their study participation. The hospital ethics committee approved the study protocol, and all participants signed informed consent.
BP measurements
At baseline, clinic BP was measured at 3 separate visits with 1-week intervals between them [9]. At each visit, participants were encouraged to relax for at least 5 min, and subsequently, in a quiet room with a comfortable temperature, an experienced physician, after having explained the procedure to the participants, performed a sitting position three measurements at 1-minute intervals without talking during and between measurements, by a validated upper-arm cuff electronic devices Omron 705IT (Omron Healthcare Europe, Hoofddorp, The Netherlands) with a suitable bladder size. The average BP and heart rate of the last 2 recordings were used.
We recorded clinic BP values in the annual follow-up visits for each patient. The attained follow-up BP was computed and reflected the average systolic or diastolic BP weighted to the elapsed time between visits for all participants. The difference in systolic and diastolic BP between baseline and attained BP during follow-up was also registered for each patient. BP control was considered optimal when clinic BP was less than 140/90 mmHg in 75% or more of the visits.
At baseline, ambulatory BP was recorded for 24 h using validated automated monitors SpaceLabs 90207 over a routine working day (Monday through Friday) and after completing the baseline clinic BP measurements. The cuff with the appropriate bladder size was fixed to the non-dominant arm. The device was set to obtain automatic heart rate and BP readings at 15-minute intervals during the daytime and at 30-minute intervals during the nighttime. Daytime and nighttime were defined using narrow fixed-clock time intervals (from 0600 to 2200 h and from 2200 to 0600 h, respectively). Twenty-four-hour systolic/diastolic BP and heart rate values were the mean of the overall 24-h recordings after artifact editing. More than 70% of successful readings were required to accept a 24-h ambulatory BP recording for analysis. The percentage reduction of daytime vs. night-time systolic BP and/or diastolic BP reflected the magnitude of the respective dipping effect [10].
White-coat hypertension was defined as mean clinic BP ≥ 140/90 mmHg associated with 24-h BP < 130/80 mmHg. Masked hypertension as mean clinic BP < 140/90 mmHg with 24-h BP ≥ 130/80 mmHg, normotension as mean clinic BP < 140/90 mmHg with a 24-h BP < 130/80 mmHg; and sustained hypertension as clinic BP ≥ 140/90 mmHg associated with 24-h BP ≥ 130/80 mmHg [9].
Biochemical examinations and risk factor profile assessment
All subjects underwent standard clinical and laboratory workups recommended for the initial evaluation of subjects with hypertension and to exclude secondary forms of hypertension in case of clinical suspicion [9]. Fasting venous blood was sampled in all patients for routine estimation of glucose, renal function, lipid profile, and gamma glutamyl-transferase (GGT). Glomerular filtration rate (eGFR) was calculated with the Modification Diet Renal Disease (MDRD) equation [11]. Anthropometric measurements were obtained on individuals in light clothing without shoes. We calculated body mass index (BMI) as the body weight/height (kg/m2) and waist-to-hip ratio from the respective circumference measures. FLI was determined according to the following formula: ey/(1 + ey) × 100; where y == 0.953 × log(triglycerides, mg/dL) + 0.139 × BMI, kg/m2 + 0.718 × log(GGT, U/L) + 0.053 × waist circumference, cm–15.745. FLI value 60 or above indicates a high likelihood of underlying NAFLD (i.e., high-risk of NAFLD FLI-related pattern) [7]. Current smokers were defined as those who smoked at least one cigarette or other forms of tobacco daily. Diabetes mellitus was defined as fasting glucose concentration >125 mg/dl on at least two occasions, a diagnostic oral glucose tolerance test, or the use of antidiabetic drugs. Dyslipidemia was defined as low-density lipoprotein cholesterol >160 mg/dl or treatment with lipid-lowering drugs. Metabolic syndrome was defined according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria [12]. The 10-year risk for CV disease was calculated with the atherosclerotic CV disease risk algorithm [13].
Outcome definitions
The primary outcome of interest was the composite of major fatal or non-fatal cardiovascular and renal events. More specifically, the outcome was composed of (1) coronary heart disease defined as fatal or non-fatal myocardial infarction, hospitalized unstable angina, significant coronary artery stenosis diagnosed by angiography or any coronary revascularization procedure; (2) stroke defined as rapid onset of a new neurological deficit persisting at least 24-h unless death supervened confirmed by computed tomography and/or magnetic resonance angiography and/or cerebrovascular angiography findings; (3) heart failure requiring hospitalization; (4) end-stage renal disease, dialysis or renal death; (5) peripheral artery disease requiring revascularization. Transient ischemic attacks and undefined cardiac events such as stable angina or undiagnosed myocardial infarction were not considered among the outcomes. Verified cardiac events alone were subsequently analyzed. A panel of physicians reviewed the hospital records and all other available medical source documents for outcome verification. In dementia or disabling disease cases, information was provided by first-degree relatives. Only the first event was considered in the analysis for patients who experienced more than one non-fatal outcome event.
Statistical analysis
All categorical variables are presented as absolute frequencies and percentages, whereas continuous variables are shown as means values with standard deviations. Significant differences between study subgroups were identified using Student’s independent samples t-test or the chi-squared test where appropriate. We imputed the z-scores of FLI to evaluate the predictive value of the standardized measure in comparison with other categorical variables. Cox proportional hazard analysis was used to examine the effect of FLI as a continuous or categorical variable on the cumulative outcome incidence before and after adjustment for confounders. We ran different crude models, and subsequently, a stepwise inclusion of different baseline and follow-up confounders was used to adjust estimates. Of note that BMI, waist circumference, triglyceride, and GGT levels were not included in the models because they were components of the FLI. The effects from Cox regression models were calculated as hazard ratios (HR) and 95% confidence intervals (CIs). A P value less than 0.05 indicates statistical significance for all binary comparisons in each analysis. In addition, subgroup analyses were made to evaluate whether FLI was differentially associated with the outcome in the presence or not (1) of diabetes mellitus, (2) metabolic syndrome, and (3) baseline drug treatment of diabetes mellitus or dyslipidemia by accounting for the chi-distances of the mean outcome estimates and the Wald statistic. IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, New York, USA) was used for all analyses.
Results
Entire cohort
We initially evaluated 1617 consecutive never-treated white individuals. We excluded 120 patients with white-coat hypertension, 75 patients with masked hypertension, and 122 participants with normotensive or high-normal BP levels. We also excluded patients with a history of cardiovascular disease (n = 80), secondary hypertension (n = 32), grade 3 hypertension (n = 95), those with a history of significant alcohol consumption (n = 30), baseline treatment with a fibrate (n = 28), and any other clinically significant concurrent medical condition (thyroidal, psychiatric, neuromuscular, kidney, respiratory, hepatic, or gastrointestinal disease [n = 33]). Furthermore, 39 individuals, although eligible to participate, refused to give informed consent, and 60 individuals were censored due to follow-up incomplete data. After the implementation of the above during the recruitment period, the population eligible to participate consisted of 903 individuals.
Participants (age 52.7 ± 11.6 years; males 55%; dyslipidemia 44%; diabetes mellitus 10%; smokers 33%; BMI 28.2 ± 4.1 kg/m2) were prospectively evaluated for a mean follow-up period of 5.2 ± 3.2 years. Baseline clinic systolic/diastolic BP was 149.8 ± 15/95.5 ± 10.1 mmHg, and 24-h ambulatory BP was 135.4 ± 12.2/84.2 ± 9.5 mmHg. The average 10-year risk of incident cardiovascular disease for the entire cohort was 5.7%. All patients received antihypertensive treatment (mean number of drugs, 1.9 ± 1.1), and the achieved clinic systolic/diastolic BP during the follow-up period was 135.8 ± 12.5/83.9 ± 9.2 mmHg. Consequently, the average treatment-induced clinic systolic/diastolic BP reduction (i.e., the achieved BP difference between groups during follow-up) was 14 ± 17.8/11.6 ± 11.3 mmHg. The mean number of follow-up visits with registered BP measurements was 5 ± 3.1 per patient. BP control was optimal in 40% of the participants.
Higher vs. lower FLI-related risk subgroups
A high-risk FLI pattern (FLI ≥ 60) was observed in 30.7% (n = 278) of the participants. The demographic and clinical characteristics of the two FLI-related groups are presented in Table 1. Patients with FLI ≥ 60 compared to those with FLI < 60 were younger, more frequently men, had greater body adiposity measures and plasma glucose levels. Although clinic systolic BP was not different between groups, clinic diastolic BP was higher in the high-risk FLI group. The high-risk FLI group had higher 24-h systolic but lower diastolic BP levels than their counterparts with FLI < 60. The average treatment-induced clinic systolic/diastolic BP reduction from baseline was not different between subgroups. In contrast, the attained systolic/diastolic BP difference during follow-up was 1.3/2.4 mmHg greater in the lower than, the higher-risk FLI subgroup. Patients with FLI < 60 had more frequent optimal BP control than their counterparts with FLI ≥ 60 during follow-up (43% vs. 33%, p = 0.02).
Prognosis of higher vs. lower risk FLI patterns
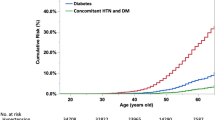
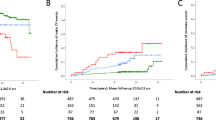
The incidence of the composite outcome in the total population was 8.5% (n = 77), while the fatality rate among composite outcome cases was 22% (n = 17). Coronary heart disease events were 60% (n = 46; 23 myocardial infarctions, 16 percutaneous coronary interventions, and 7 cases of coronary artery bypass), while strokes were 40% (n = 31). No cases of end-stage renal disease occurred. The rate of outcome incidence was 8-fold greater in patients with high-risk FLI compared to their lower-risk counterparts (60/278 vs. 17/625, respectively). The predictive value of FLI before and after adjustment for confounders is presented in Table 2 and Supplementary Table 1. For a standardized increase of FLI (SD = 25.5 units), the outcome was also increased by 3.7-fold (95% CI, 2.2–6.0) after full adjustment for baseline and on-treatment variables. In addition, the high-risk compared to the lower-risk FLI pattern predicted the outcome incidence (Table 2, Fig. 1) significantly before and after adjustment for the same confounders. Although FLI predicted the outcome to a greater extent in patients with than without diabetes mellitus, a differential outcome prediction was not observed between patients with or without (1) metabolic syndrome and (2) baseline statins or antidiabetic drugs (Table 3).
Discussion
In newly diagnosed hypertensive patients without a history of cardiovascular disease, FLI was associated with an increased risk of cardiovascular events after adjustment for baseline and follow-up clinical characteristics. The FLI high-risk pattern (FLI ≥ 60 units) for underlying NAFLD was associated with a 7.5-fold higher outcome rate than the lower-risk FLI pattern. However, even within the low-risk FLI pattern of NAFLD (FLI < 60 units), there was a 5% greater risk of incident cardiovascular events for each unit increase of FLI. Our findings may refine the cardiovascular risk stratification of newly-diagnosed hypertensive patients.
Although the definite diagnosis of NAFLD requires the implementation of alternative imaging techniques, including liver ultrasounds [6, 8], the imputation of a surrogate marker of NAFLD, namely FLI [7], may help clinicians visiting patients with cardiovascular risk factors like hypertension to increase the clinical suspicion of underlying NAFLD. Different studies have suggested that NAFLD is associated with a higher risk of incident cardiovascular events [8]. However, these studies were performed independently of hypertension or included hypertensive patients with and without drug treatment. Our study is the first to predict the effect of the high-risk FLI pattern on cardiovascular events in newly-diagnosed hypertension without baseline antihypertensive drug treatment. Newly-diagnosed patients are usually characterized by low to moderate cardiovascular risk; however, to avoid bias related to the different levels of baseline cardiovascular risk, we focused our investigation on patients without a history of cardiovascular events. Furthermore, since antihypertensive drugs may slow the progression of atherosclerosis, patients without drug exposure were eligible to participate [14, 15]. Finally, an important and unique aspect of our study was that patients were scheduled to adhere to follow-up visits every year in which a valid BP measurement was available, while the average follow-up BP was computed and weighted by the time interval between the follow-up visits. Thus, we adjusted our risk estimates according to baseline and on-treatment clinical characteristics, including the optimal BP control rate during follow-up.
The imputed 10-year risk of fatal and nonfatal cardiovascular events [13] in our cohort was 16.3%, but this risk was 8-fold lower in patients at low risk than those at high risk of NAFLD. The relative risk of incident cardiovascular events between the two groups was slightly reduced to 7.5 after adjustment for baseline and on-treatment clinical characteristics. However, FLI used as a continuous variable was linearly associated with the outcome, while for every 25 units of FLI increment (one standard deviation), a 3.7-fold increase in the outcome was observed. We also examined whether an FLI increase in patients with diabetes mellitus or metabolic syndrome was associated with a greater risk of cardiovascular events than in patients without diabetes mellitus or metabolic syndrome.
We also stratified our results to patients with diabetes mellitus or metabolic syndrome, conditions both characterized by a higher level of cardiovascular risk within the primary prevention setting [9]. Our finding that in patients with diabetes mellitus, for one unit of FLI increase, the rate of events was more than 3-fold greater compared to patients without diabetes mellitus might be driven by insulin resistance of the visceral adipose tissue. Especially in diabetes mellitus within adipocytes, insulin-inhibited lipase is rather dysfunctional, resulting in increased levels of free fatty acids that may increase very-low-density lipoprotein cholesterol levels contributing to the progression of atherosclerosis [16, 17]. By contrast, in our study, the presence or not of metabolic syndrome was not associated with a differential propensity of FLI to predict cardiovascular outcomes, at least partly because the features of metabolic syndrome and NAFLD are highly interrelated [18]. In addition, patients with or without baseline exposure to medications for lifestyle-related diseases (i.e., hypertension, diabetes mellitus, and dyslipidemia) yielded no different prediction of FLI on the outcome.
We have previously demonstrated in a cohort of newly diagnosed hypertensive patients without diabetes mellitus that those patients at high risk of NAFLD compared with their lower risk counterparts had a 2.3 times higher likelihood of hypertension-mediated organ damage. FLI was linearly related to the presence of any subclinical damage at the heart level (i.e., increased left ventricular mass), the kidney (i.e., increased urinary albumin excretion), or microcirculation (i.e., capillary rarefaction), and large arteries (by means of aortic stiffness) [19]. The activation of the renin-angiotensin system, along with the hepatic release of pro-inflammatory mediators, may jointly mediate a detrimental effect on the myocardium, including left ventricular hypertrophy [3]. The deficiency of angiotensin (1–7)/Mas axis components observed in different insulin resistance states, including NAFLD, may downregulate the antifibrotic or antiproliferative effects and promote cardiac hypertrophy [20, 21]. The mechanisms mentioned above may also promote vascular deterioration in different domains, including the kidney and the heart.
The synergistic or additive effect of NAFLD and hypertension should be evaluated in future prospective studies with different arms: (1) NAFLD (or high-risk FLI pattern alone), (2) hypertension alone, (3) NAFLD (or high-risk FLI pattern) and hypertension, and (4) no NAFLD (or low-risk FLI pattern) and normal BP levels. Moreover, another setting for future investigation might be the recurrent cardiovascular events in optimally treated secondary prevention hypertensive patients with or without NAFLD (or an FLI-related high vs. lower-risk pattern). Finally, future studies may consider standardized interventions for NAFLD like (1) lifestyle/dietary modification, (2) exercise programs, or (3) potential pharmacological treatment by different antidiabetic drug classes, to be combined with established risk factor treatment strategies against hypertension, dyslipidemia, or diabetes mellitus [6].
We acknowledge the following limitations of our investigation. First, our results cannot be generalized to other races or ethnic groups because we used only white individuals. Although we performed ambulatory BP measurements to exclude white-coat and masked hypertension at baseline, follow-up ambulatory BP was not available to refine BP control estimates. The study population was of low/moderate baseline CV risk; additional risk factors were adequately treated, and thus, the findings cannot be extended or reproduced to higher-risk populations. Despite the low rate of patients who were lost during follow-up, including those with incomplete data (almost 3.7%), these patients were not calculated in our analysis. Since no registered data about menopausal status were available, the confounding effect of menopause on outcome incidence remains unknown. Although we did not follow a prespecified protocol for treating hypertension, we aimed to reduce BP levels below the target recommended by current guidelines according to the physicians’ discretion. A cause-effect relationship between the high-risk FLI pattern and cardiovascular events cannot be claimed in our study because of (1) potential unmeasured confounding, (2) the unknown duration of increased FLI levels in different individuals, and (3) the effects of antihypertensive drug treatment on the outcome during follow-up. Although we acknowledge that FLI has a suboptimal accuracy in diagnosing NAFLD compared to standard imaging techniques, when tested as a continuous measure was also able to predict the outcome.
Conclusion
High-risk compared to the lower-risk FLI pattern and FLI were independently associated with future cardiovascular events in newly-diagnosed never-treated hypertensive patients. This finding may refine cardiovascular risk stratification in the primary prevention of hypertensive patients.
References
Zhang XJ, She ZG, Li H. Time to step-up the fight against NAFLD. Hepatology 2018;67:2068–71.
Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of nonalcoholic fatty liver disease in China from 2008 to 2018: a systematic review and metaanalysis. Hepatology 2019;70:1119–33.
Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol. 2018;30:979–85.
Bonnet F, Gastaldelli A, Pihan-Le Bars F, Natali A, Roussel R, Petrie J, et al. D.E.S.I.R., RISC Study Groups. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J Hypertens. 2017;35:493–500.
Huh JH, Ahn SV, Koh SB, Choi E, Kim JY, Sung KC, et al. A Prospective Study of fatty liver index and incident hypertension: the KoGES-ARIRANG Study. PLoS One. 2015;10:e0143560.
Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168–e185.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;2:6–33.
Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res. 2020;126:679–704.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Authors/Task Force Members:. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68.
Clase CM, Kiberd BA, Garg AX. Relationship between glomerular filtration rate and the prevalence of metabolic abnormalities: results from the Third National Health and Nutrition Examination Survey (NHANES III). Nephron Clin Pr. 2007;105:178–84.
Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation 2002;106:3143–421.
Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SM, et al. Use of risk assessment tools to guide decision making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73:3153–67.
Thomopoulos C, Antza C. Legacy effect on mortality in antihypertensive drug comparison trials: a meta-analysis. J Hypertens. 2019;37:1532–4.
Hirakawa Y, Arima H, Rodgers A, Woodward M, Chalmers J. Cumulative in-trial and posttrial effects of blood pressure and lipid lowering: systematic review and meta-analysis. J Hypertens. 2017;35:905–13.
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–7.
Lan YL, Lou JC, Lyu W, Zhang B. Update on the synergistic effect of HSL and insulin in the treatment of metabolic disorders. Ther Adv Endocrinol Metab. 2019;10:2042018819877300.
Godoy-Matos AF, Silva Junior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60.
Siafi E, Andrikou I, Konstantinidis D, Kakouri N, Iliakis P, Koskinas J, et al. Fatty liver index and hypertension-mediated organ damage in never-treated hypertensive patients without diabetes mellitus. J Hypertens. 2021;39:2470–7.
Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-mas receptor axis. Hypertens Res. 2009;32:533–6.
Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16:120–6.
Author information
Authors and Affiliations
Contributions
ES, CTh, CTs, DK, and IA, contributed to the conception or the design of the present work. ES, NK, FT, MK, CF, IZ, EM, SD, IL, and SP contributed to the data acquisition. ES, CTh, CTs, and IA, contributed to data analysis and interpretation of the results. ES and CTh, drafted the first version of the manuscript. CTh and CTs. critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of interest
CTh reports honoraria for lectures or consultancy fees from Menarini, Medtronic, Berlin-Chemie, Sanofi, and AstraZeneca. CTs, reports honoraria for advisory boards and lectures from Medtronic, Servier, Bayer, Menarini, Novartis, AstraZeneca, Boehringer, Pfizer, Pythagoras, Sanofi, and Amgen. All other authors report no conflict. However, all authors have not received any funding directly related to the subject or content of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siafi, E., Andrikou, I., Thomopoulos, C. et al. Fatty liver index and cardiovascular outcomes in never-treated hypertensive patients: a prospective cohort. Hypertens Res 46, 119–127 (2023). https://doi.org/10.1038/s41440-022-01015-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01015-x
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
High red meat consumption among PNPLA3 polymorphism carriers is associated with NAFLD in a multi-center cross-sectional study
European Journal of Clinical Nutrition (2024)
-
Mechanisms underlying the bidirectional association between nonalcoholic fatty liver disease and hypertension
Hypertension Research (2023)
-
Fatty liver index predicts the development of hypertension in a Japanese general population with and without dysglycemia
Hypertension Research (2023)
-
Fatty liver index has potential as a predictor of hypertension in the Japanese general population
Hypertension Research (2023)