Abstract
Introduction
Patients with arterial hypertension frequently present with comorbidities that are associated with increased cardiorenal risk, such as metabolic dysfunction-associated fatty liver disease (MAFLD).
Aims
Our study aimed to assess the prevalence and the association of MAFLD with cardiorenal risk markers in newly diagnosed, treatment-naïve hypertensive patients.
Methods
We recruited 281 individuals with new-onset hypertension who were not prescribed any medication. Medical history, clinical examination findings, and laboratory test results were recorded. Liver steatosis was assessed through fatty liver index (FLI) calculation. Patients with FLI ≥ 60 together with one main metabolic abnormality (type 2 diabetes mellitus or overweight/obesity) or at least two metabolic risk abnormalities (increased waist circumference, blood pressure, plasma triglycerides, presence of prediabetes or insulin resistance, decreased plasma high-density lipoprotein) fulfilled the diagnostic criteria for MAFLD.
Results
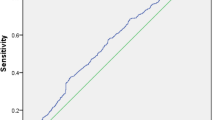
The prevalence of MAFLD in our study population was 28.7%. Individuals with MAFLD were more frequently male and had increased body mass index. Systolic, diastolic, and pulse pressure values were significantly higher in this group of patients. Moreover, lipid, renal, glucose, and inflammatory markers were considerably deranged in patients with MAFLD. After multivariate regression analysis, uric acid, ferritin, and apoE emerged as independent predictors of MAFLD. Area under receiver operating characteristics curve revealed that uric acid had the greatest diagnostic accuracy, with the ideal cutoff being ≥ 5.2 mg/dl (sensitivity: 77.6%, specificity: 76.3%).
Conclusion
MAFLD represents a common comorbidity in hypertensive patients and is associated with markers of cardiorenal risk. Uric acid may be indicative of MAFLD in particular.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Arterial hypertension (AH) represents a common, frequently asymptomatic, noncommunicable disease, that is characterized by worrisome epidemiological trends. Due to the increased life expectancy and the constant exposure to unhealthy lifestyles, the prevalence of AH is rising. This is especially evident in low- and middle-income countries, while the prevalence of AH in high-income countries is moderately decreasing [1]. Moreover, the new definition of hypertension (systolic blood pressure (SBP) ≥ 130 mmHg and/or diastolic BP (DBP) ≥ 80 mmHg meant that the prevalence of hypertension in the USA and China could be as high as 46% [2, 3]. Prompt diagnosis and management of AH is of great importance, since many complications arise from persistently elevated BP, such as ischemic stroke, coronary artery disease, heart failure, and chronic kidney disease (CKD), among others [1].
Patients with AH frequently present with additional comorbidities that are associated with augmented cardiovascular risk, such as metabolic dysfunction-associated fatty liver disease (MAFLD). This newly proposed term, which has replaced the previously used non-alcoholic fatty liver disease, provides positive criteria for hepatic insult due to metabolic derangement [4]. These include the presence of steatosis along with a main metabolic abnormality (overweight/obesity or type 2 diabetes mellitus) or at least two metabolic risk factors [4]. However, the prevalence and the association of MAFLD with cardiorenal risk markers in hypertensive patients is unclear. Therefore, this study aims to determine the prevalence and the association of MAFLD with cardiorenal risk markers in treatment-naive hypertensive patients.
2 Methods
2.1 Study Design
This was a cross-sectional study of 281 persons with new-onset hypertension who attended the Diabetes Outpatient Clinic of Ioannina University Hospital, Ioannina, Greece. Participants were not under any medical treatment. They were interviewed and clinically examined by specialized physicians. Anthropometric measurements and blood samples were collected. The study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist [5]. All individuals were informed about the aims of the study and provided written informed consent. The study was approved by the Ethics Committee of the Ioannina University Hospital and was carried out according to the Declaration of Helsinki (1989).
2.2 Clinical and Biochemical Assessment
Body mass index (BMI) was calculated as the weight in kilograms divided by the height squared in meters. Waist circumference was measured at the midpoint between the inferior border of the costal margin and the anterior superior iliac crest. Three blood pressure readings were obtained in the sitting position of the right arm, with a 5-min interval in rest after every measurement, in two separate visits at least 2 weeks apart. The average of the second and the third systolic and diastolic pressure readings were used. The mean of home blood pressure measurements was taken into account to confirm the diagnosis of hypertension, as well as in cases of potential masked hypertension. The methodology of home blood pressure measurements was in accordance with the latest practice guidelines of the European Society of Hypertension [6]. The diagnosis of arterial hypertension was considered in cases of office blood pressure ≥ 140/90 mmHg or home blood pressure ≥ 135/85 mmHg [7]. Pulse pressure, a marker of arterial stiffness [8], was also evaluated by subtracting DBP from SBP.
We collected the blood samples after an overnight fast. An extended lipid profile including lipoprotein (a), apolipoproteins (ApoA1, ApoB, ApoE), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) was assessed. Low-density lipoprotein cholesterol (LDL-C) concentration was calculated according to the Friedewald formula: LDL-C = TC − HDL-C + (TG/5), when triglycerides value was lower than 400 mg/dl [9]. Regarding glucose-insulin homeostasis markers, we estimated insulin resistance via calculation of the homeostasis model insulin resistance (HOMA-IR = plasma glucose (md/dl) × fasting insulin (mIU/l) ÷ 405) index [10] and the quantitative insulin sensitivity check index (QUICKI = 1/[log fasting insulin (mg/dl) + fasting glucose(μu/ml)]) after the measurement of glucose and insulin [11]. Renal function was estimated by serum urea and creatinine, with the estimation of the glomerular filtration rate (eGFR) according to the CKD-EPI formula [12]. Diagnosis of the metabolic syndrome (MtS) was based on the proposed ATP III criteria [13].
MAFLD was defined according to the recently proposed criteria. Patients should have evidence of hepatic steatosis, determined through imaging techniques, histology, or blood biomarkers/scores. We chose and calculated the fatty liver index (FLI) via the following formula: FLI = ey / (1 + ey) × 100, where y = 0.953 × ln(triglycerides, mg/dl) + 0.139 × BMI, kg/m2 + 0.718 × ln (GGT, U/l) + 0.053 × waist circumference, cm − 15.745. A previous study in Western population shown that at FLI cutoff of ≥ 60, the presence of hepatic steatosis is ruled in [14]. Accordingly, individuals with FLI ≥ 60 fulfilled the diagnostic criteria for MAFLD [4] if they also had at least one of the following: type 2 diabetes mellitus, overweight/obesity, or ≥ 2 metabolic risk abnormalities (increased waist circumference (≥ 102 cm in men, ≥ 88 cm in women), increased blood pressure (≥ 130/85 mmHg or specific drug treatment), increased plasma triglycerides (≥ 150 mg/dl or specific drug treatment), decreased plasma high-density lipoprotein (< 40 mg/dl in men, < 50 mg/dl in women), presence of prediabetes [fasting glucose 100–125 mg/dl or HbA1c 5.7–6.4%), insulin resistance (HOMA-IR ≥ 2.5)].
2.3 Statistical analysis
Continuous variables were tested for normality with the Kolmogorov–Smirnov test and visual inspection of P–P plots. Accordingly, they are presented as mean (standard deviation) if normally distributed, or as median (25th, 75th quartile) if not normally distributed. Categorical variables are displayed as percentages. T-tests were employed to examine the differences between 2 categories of normally distributed continuous data. Differences between categorical variables were tested by forming contingency tables and performing χ2-tests. Logistic regression analysis was used for the multivariate assessment of independent MAFLD predictors in our study population. We also proceeded to a receiver operating characteristics (ROC) curves’ analysis by estimating the area under the ROC curve (AUROC). The ideal cutoff values were chose according to the Youden index [15], with subsequent assessment of its sensitivity and specificity. The statistical calculations were performed in SPSS software (version 25.0; SPSS Inc., Chicago, Illinois, USA). All reported p-values were based on two-sided hypotheses, with a p-value of less than 0.05 being considered statistically significant.
3 Results
3.1 Anthropometric and Clinical Characteristics
In the study population of 281 individuals with treatment-naïve hypertension, 75 were found with MAFLD (prevalence 28.7%) and had significantly higher FLI compared to subjects without MAFLD (77.2 ± 11.0 vs. 27.1 ± 17.3, p < 0.001) (Fig. 1). The baseline characteristics of the study population are presented in Table 1. Patients with MAFLD were more frequently male (66.7% vs. 47.1%, p = 0.004), obese (63.0% vs. 45.3%, p < 0.001), and had much higher rates of metabolic syndrome (78.7% vs. 17.5%, p < 0.001) compared to those without MAFLD. Moreover, they presented with significantly increased office systolic BP (150 ± 23 mmHg vs. 136 ± 25 mmHg, p < 0.001) and diastolic BP (93 ± 12 mmHg vs. 84 ± 14 mmHg, p < 0.001). PP was also considerably higher in individuals with MAFLD (57 ± 18 mmHg vs. 52 ± 15 mmHg, p = 0.01). No differences in mean age or CKD prevalence were detected.
3.2 The Association of MAFLD with Blood Biomarkers
The association of MAFLD prevalence with markers of lipidemia, glucose and insulin homeostasis, liver function, and renal function are displayed in Table 2. Individuals with MAFLD had greater abnormalities in common and extended lipid panel parameters, such as ApoA1, ApoB, ApoE, and Lp(a). Uric acid was also significantly higher in subjects with MAFLD [6.0 (5.3, 6.8) mg/dl vs. 4.2 (3.4, 5.2) mg/dl, p < 0.001]. MAFLD individuals also had significantly impaired glucose levels and greater degrees of insulin resistance evidenced by HOMA-IR index and QUICKI. Moving to liver enzymes, higher levels of transaminases, alkaline phosphatase and gamma glutamyl transferase were observed in MAFLD individuals. Greater abnormalities in kidney function markers were noted in the subgroup of individuals with MAFLD. Finally, we detected higher levels of white blood cells, ferritin, and fibrinogen in the group of MAFLD (Fig. 2).
Differences in a white blood cells (WBC), b ferritin, and c fibrinogen between hypertensive subjects with and without metabolic dysfunction-associated fatty liver disease (MAFLD). Filled circles represent the mean and error bars the 95% confidence intervals. *Statistically significant difference (p < 0.05). **Statistically significant difference (p < 0.001)
3.3 Predictors of MAFLD in Treatment-Naïve Hypertensive Patients
We next examined the association of MAFLD with significantly different variables. Parameters included in the definition of MAFLD were excluded from this analysis. According to our findings, apoE (odds ratio 1.09, 95% confidence interval 1.02–1.17, p = 0.02), uric acid (odds ratio 2.77, 95% confidence interval 1.63–4.70, p < 0.001), and ferritin (odds ratio 1.01, 95% confidence interval 1.00–1.02, p = 0.03) emerged as independent predictors of MAFLD in our study population (Table 3).
Finally, we assessed the diagnostic performance of these parameters regarding the presence of MAFLD through ROC curve analysis (Fig. 3). Uric acid had great diagnostic accuracy (AUROC 0.829, p < 0.001), while apoE (AUROC 0.692, p < 0.001) and ferritin (AUROC 0.738, p < 0.001) had decent diagnostic accuracy. Specifically for uric acid, values above or equal 5.2 mg/dl were indicative of MAFLD with a sensitivity and specificity of 77.6% and 76.3%, respectively.
4 Discussion
In this cross-sectional study, MAFLD was detected in a significant proportion of treatment-naïve, hypertensive patients. According to our results, MAFLD was associated with several demographic and clinical characteristics, such as male sex, SBP, DBP, and pulse pressure. Concerning cardiovascular risk markers, there was a greater magnitude in lipid abnormalities, hyperuricemia, hyperglycemia, insulin resistance, disrupted liver biochemistry, and renal impairment in hypertensives with MAFLD. Moreover, increases in ferritin and fibrinogen were also noted. Finally, we found that uric acid was the most accurate predictor of MAFLD in this population, with the ideal cutoff being ≥ 5.2 mg/dl.
MAFLD, a recently proposed term aiming to better characterize the liver pathology associated with metabolic deregulation, is being thoroughly investigated regarding its hepatic and extrahepatic complications, namely cardiovascular and kidney diseases [16, 17]. It appears that its presence may signify an augmented risk for incident cardiovascular events [18], mandating the need for prompt identification. Recent studies have shown that MAFLD may be a more appropriate prognostic indicator of incident cardiovascular and chronic kidney disease compared to non-alcoholic fatty liver disease (NAFLD) [19,20,21]. Moreover, medications with pleiotropic effects such as sodium-glucose cotransporter-2 inhibitors [22,23,24], glucagon-like peptide-1 receptor agonists [25,26,27,28], and melatonin [29,30,31] may even improve this poor prognosis by inducing MAFLD regression, as shown in preclinical and clinical studies.
In our population of hypertensive patients, the prevalence of MAFLD was approximately 29%, which is lower than other reports in different populations [32, 33]. To our knowledge, however, this is the first report of MAFLD prevalence and correlation with risk markers in treatment-naïve hypertensive subjects. The association between MAFLD and arterial hypertension has a strong pathophysiologic basis. Among the common pathways involved are oxidative stress, endothelial dysfunction, and gut dysbiosis [34,35,36,37,38,39]. Moreover, the role of inflammation is central, and might be implicated in the risk of poor cardiorenal prognosis surrounding those entities. Inflammation could initiate a deleterious cascade involving promotion of endothelial dysfunction and platelet activation [38, 40, 41], ultimately leading to atherothrombotic complications [42, 43]. In our study, inflammatory markers such as ferritin and fibrinogen were significantly elevated in hypertensive patients with MAFLD compared to those without MAFLD. Ferritin has been associated with incident cardiovascular and renal disease [44,45,46], while the same could be argued for fibrinogen [47,48,49,50,51].
Significant abnormalities in the extended lipid profile of the hypertensive individuals with MAFLD were also noted, including apoA1, apoB, apoE, and Lp(a). ApoA1 has been found to be a stronger predictor of cardiovascular disease and mortality in elderly men when compared to HDL-C and LDL-C. ApoB measurement may also be superior to conventional dyslipidemia, as its lowering was associated with a greater reduction of major adverse cardiovascular event rate, even after accounting for the reductions in non-HDL-C and LDL-C [52]. Increased apoB was associated with the risk of major adverse cardiovascular events in the Swedish AMORIS cohort study [53]. Moving to Lp(a), it represents a risk factor for cardiovascular disease even at very low levels of LDL-C [54]. Those markers have been implicated also in CKD [55, 56]. On the other hand, apoE, which was found elevated in the subgroup of hypertensive patients with MAFLD, has not been associated with cardiorenal complications [57].
Uric acid appears to be crucial in metabolic dysregulation and has been recently linked with MtS incidence in the study of Cicero et al. of 923 elderly individuals in the Brisighella Heart Study [58]. In fact, serum uric acid may also be considered in the definition of MtS, as it can provide incremental and independent prognostic information [59]. Several reports have additionally highlighted uric acid’s importance regarding the incidence of cardiovascular and renal complications, with the landmark URRAH project providing the evidence for lower thresholds in patient risk stratification [60,61,62,63]. Concerning MAFLD, serum uric acid has been associated with NAFLD consistently in previous reports, and this association appears to persist in MAFLD [64,65,66,67]. Xing et al. reported that uric acid normalized to renal function was an independent predictor of MAFLD in individuals with type 2 diabetes mellitus [68]. Based on the multivariate and ROC curve analysis of our study, uric acid emerged as the most potent predictor of MAFLD in hypertensive participants. Therefore, its measurement may be considered essential in newly diagnosed hypertensive patients, with values exceeding 5.2 mg/dl being indicative of MAFLD presence.
Our study has some limitations. First and foremost, the limited sample size does not allow for safe conclusions to be drawn, while the cross-sectional design is inappropriate for establishing cause-and-effect relationships. Moreover, the incidence of MAFLD in newly diagnosed hypertensive patients could not be determined through this study. Thus, our study serves the purpose of hypothesis generation, and future longitudinal studies should further assess the potential role of MAFLD in the progression of cardiovascular and renal disease. Additionally, as the participants were treatment-naïve, we were not able to evaluate any associations of modern cardiorenal pharmacotherapy with the prevalence of MAFLD. Regarding the definition of MAFLD, the presence of steatosis was defined according to a non-invasive, non-imaging risk score, the FLI, according to the latest MAFLD definition. Therefore, there is a chance for potential misclassification of the study population into the MAFLD and non-MAFLD subgroups. However, FLI is simple to obtain in every day clinical practice and can rule in the presence of ultrasonographic steatosis at a cutoff of ≥ 60 according to the study of Bedogni et al. [14]. We should also state that the FLI-derived MAFLD diagnosis has a moderate agreement with the liver elastography-derived MAFLD diagnosis, as we recently reported in an analysis of the National Health and Nutrition Examination Survey 2017–2020 [69]. As far as the studied markers are concerned, we have not examined other established, non-traditional markers of cardiovascular and renal disease prognosis in this study, such as pulse wave velocity and flow-mediated dilation. Finally, residual confounding on the multivariate associations could be present, thus the results should be interpreted with caution and further studies are required to serve as a validation.
Regarding clinical implications, since MAFLD may be present in a significant proportion of newly diagnosed hypertensive individuals, the measurement of FLI and the application of MAFLD diagnostic criteria in this group of patients could be suggested. Uric acid should be included in the initial investigations and values ≥ 5.2 mg/dl may indicate the coexistence of MAFLD. Future, adequately-sized, prospective studies should validate our results regarding the prevalence of MAFLD in hypertensive individuals and the importance of uric acid as a predictor. Moreover, the knowledge should be extended to novel agents, such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists, to determine whether they are efficacious in preventing MAFLD development, halting its progression, or even induce its regression.
5 Conclusions
Metabolic dysfunction-associated fatty liver disease is a frequently encountered comorbidity in newly diagnosed, treatment-naïve hypertensive patients. It is associated with greater abnormalities in markers of cardiorenal risk including lipid profile, glucose and insulin homeostasis, liver biochemistry, renal function, and inflammatory markers. Uric acid may be the most potent predictor of metabolic dysfunction-associated fatty liver disease in this group of patients, with a cutoff of ≥ 5.2 mg/dl. Future studies are needed to validate our findings and improve our knowledge on this deleterious association.
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37. https://doi.org/10.1038/s41581-019-0244-2.
Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults: an analysis of national data. JAMA Cardiol. 2018;3:572–81. https://doi.org/10.1001/jamacardio.2018.1240.
Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R, China Hypertension Survey I. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation. 2018;137:2344–56. https://doi.org/10.1161/CIRCULATIONAHA.117.032380.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Jarvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. https://doi.org/10.1016/j.jhep.2020.03.039.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. https://doi.org/10.1136/bmj.39335.541782.AD.
Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, Persu A, Mancia G, Kreutz R, European Society of Hypertension C, the European Society of Hypertension Working Group on Blood Pressure M, Cardiovascular V. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–302. https://doi.org/10.1097/HJH.0000000000002843.
Williams B, Mancia G, Spiering W, AgabitiRosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Niiranen TJ, Kalesan B, Mitchell GF, Vasan RS. Relative contributions of pulse pressure and arterial stiffness to cardiovascular disease. Hypertension. 2019;73:712–7. https://doi.org/10.1161/HYPERTENSIONAHA.118.12289.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. https://doi.org/10.1007/BF00280883.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. https://doi.org/10.1210/jcem.85.7.6661.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97. https://doi.org/10.1001/jama.285.19.2486
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. https://doi.org/10.1186/1471-230X-6-33.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. https://doi.org/10.1002/bimj.200410135.
Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic dysfunction-associated fatty liver disease in the National Health and Nutrition Examination Survey 2017–2020: epidemiology, clinical correlates, and the role of diagnostic scores. Metabolites. 2022;12:1070.
Theofilis P, Vordoni A, Kalaitzidis RG. Interplay between metabolic dysfunction-associated fatty liver disease and chronic kidney disease: epidemiology, pathophysiologic mechanisms, and treatment considerations. World J Gastroenterol. 2022;28:5691–706.
Mantovani A, Csermely A, Tilg H, Byrne CD, Targher G. Comparative effects of non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease on risk of incident cardiovascular events: a meta-analysis of about 13 million individuals. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-328224.
Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, Kim SU, Lee JI. MAFLD predicts the risk of cardiovascular disease better than NAFLD in asymptomatic subjects with health check-ups. Dig Dis Sci. 2022;67:4919–28. https://doi.org/10.1007/s10620-022-07508-6.
Tanaka M, Mori K, Takahashi S, Higashiura Y, Ohnishi H, Hanawa N, Furuhashi M. Metabolic dysfunction-associated fatty liver disease predicts new onset of chronic kidney disease better than does fatty liver or nonalcoholic fatty liver disease. Nephrol Dial Transplant. 2022. https://doi.org/10.1093/ndt/gfac188.
Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, Wei CP, Chin YH, Tay P, Lim G, Tan DJH, Lim WH, Chan KE, Teng M, Tan E, Tamaki N, Huang DQ, Siddiqui MS, Young DY, Noureddin M, Muthiah MD. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. A Meta-analysis and systematic review of 12,620,736 Individuals. Endocr Pract. 2022;28:667–72. https://doi.org/10.1016/j.eprac.2022.03.016.
Morishita A, Tadokoro T, Fujihara S, Iwama H, Oura K, Fujita K, Tani J, Takuma K, Nakahara M, Shi T, Haba R, Okano K, Nishiyama A, Ono M, Himoto T, Masaki T. Ipragliflozin attenuates non-alcoholic steatohepatitis development in an animal model. PLoS ONE. 2022;17:e0261310. https://doi.org/10.1371/journal.pone.0261310.
Akuta N, Kawamura Y, Fujiyama S, Saito S, Muraishi N, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kumada H. Favorable impact of long-term SGLT2 inhibitor for NAFLD complicated by diabetes mellitus: a 5-year follow-up study. Hepatol Commun. 2022;6:2286–97. https://doi.org/10.1002/hep4.2005.
Takahashi H, Kessoku T, Kawanaka M, Nonaka M, Hyogo H, Fujii H, Nakajima T, Imajo K, Tanaka K, Kubotsu Y, Isoda H, Oeda S, Kurai O, Yoneda M, Ono M, Kitajima Y, Tajiri R, Takamori A, Kawaguchi A, Aishima S, Kage M, Nakajima A, Eguchi Y, Anzai K. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol Commun. 2022;6:120–32. https://doi.org/10.1002/hep4.1696.
Li X, Wu X, Jia Y, Fu J, Zhang L, Jiang T, Liu J, Wang G. Liraglutide decreases liver fat content and serum fibroblast growth factor 21 levels in newly diagnosed overweight patients with type 2 diabetes and nonalcoholic fatty liver disease. J Diabetes Res. 2021;2021:3715026. https://doi.org/10.1155/2021/3715026.
Morieri ML, Targher G, Lapolla A, D’Ambrosio M, Tadiotto F, Rigato M, Frison V, Paccagnella A, Simioni N, Avogaro A, Fadini GP. Changes in markers of hepatic steatosis and fibrosis in patients with type 2 diabetes during treatment with glucagon-like peptide-1 receptor agonists. A multicenter retrospective longitudinal study. Nutr Metab Cardiovasc Dis. 2021;31:3474–83. https://doi.org/10.1016/j.numecd.2021.08.049.
Jianping W, Xuelian Z, Anjiang W, Haiying X. Efficacy and safety of glucagon-like peptide-1 receptor agonists in the treatment of metabolic associated fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:586–93. https://doi.org/10.1097/MCG.0000000000001556.
Dutta D, Kumar M, Shivaprasad KS, Kumar A, Sharma M. Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: a meta-analysis. Diabetes Metab Syndr. 2022;16:102539. https://doi.org/10.1016/j.dsx.2022.102539.
Saha M, Manna K, Das SK. Melatonin suppresses NLRP3 inflammasome activation via TLR4/NF-kappaB and P2X7R signaling in high-fat diet-induced murine NASH model. J Inflamm Res. 2022;15:3235–58. https://doi.org/10.2147/JIR.S343236.
Pakravan H, Ahmadian M, Fani A, Aghaee D, Brumanad S, Pakzad B. The effects of melatonin in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Adv Biomed Res. 2017;6:40. https://doi.org/10.4103/2277-9175.204593.
Akhavan Rezayat A, Ghasemi Nour M, Bondarsahebi Y, Hozhabrossadati SA, Amirkhanlou F, Akhavan Rezayat S, Kiani M, Imani B. The effects of melatonin therapy on the treatment of patients with Non-alcoholic steatohepatitis: a systematic review and Meta-analysis on clinical trial studies. Eur J Pharmacol. 2021;905:174154. https://doi.org/10.1016/j.ejphar.2021.174154.
Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, Bruno MJ, de Knegt RJ, Cao W, Peppelenbosch MP, Ghanbari M, Li Z, Pan Q. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022;20:e573–82. https://doi.org/10.1016/j.cgh.2021.02.030.
Ruiz-Manriquez J, Olivas-Martinez A, Chávez-García LC, Fernández-Ramírez A, Moctezuma-Velazquez C, Kauffman-Ortega E, Castro-Narro G, Astudillo-García F, Escalona-Nandez I, Aguilar-Salinas CA, Navarro-Alvarez N, Torre A. Prevalence of metabolic-associated fatty liver disease in Mexico and development of a screening tool: the MAFLD-S score. Gastro Hep Adv. 2022;1:352–8. https://doi.org/10.1016/j.gastha.2021.12.011.
Theofilis P, Vordoni A, Nakas N, Kalaitzidis RG. Endothelial dysfunction in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Life (Basel). 2022;12:718. https://doi.org/10.3390/life12050718.
Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. https://doi.org/10.1155/2018/9547613.
Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:e1900257. https://doi.org/10.1002/mnfr.201900257.
Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. https://doi.org/10.1038/hr.2010.264.
Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9:781. https://doi.org/10.3390/biomedicines9070781.
Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, Rosshart SP, Forslund SK, Muller DN. The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. 2021;128:934–50. https://doi.org/10.1161/CIRCRESAHA.121.318065.
Oikonomou E, Leopoulou M, Theofilis P, Antonopoulos AS, Siasos G, Latsios G, Mystakidi VC, Antoniades C, Tousoulis D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications. Atherosclerosis. 2020;309:16–26. https://doi.org/10.1016/j.atherosclerosis.2020.07.027.
Theofilis P, Sagris M, Antonopoulos AS, Oikonomou E, Tsioufis C, Tousoulis D. Inflammatory mediators of platelet activation: focus on atherosclerosis and COVID-19. Int J Mol Sci. 2021;22:11170. https://doi.org/10.3390/ijms222011170.
Sagris M, Theofilis P, Antonopoulos AS, Oikonomou E, Paschaliori C, Galiatsatos N, Tsioufis K, Tousoulis D. Inflammation in coronary microvascular dysfunction. Int J Mol Sci. 2021;22:13471. https://doi.org/10.3390/ijms222413471.
Sagris M, Theofilis P, Antonopoulos AS, Tsioufis C, Oikonomou E, Antoniades C, Crea F, Kaski JC, Tousoulis D. Inflammatory mechanisms in COVID-19 and atherosclerosis: current pharmaceutical perspectives. Int J Mol Sci. 2021;22:6607. https://doi.org/10.3390/ijms22126607.
Suarez-Ortegon MF, McLachlan S, Fernandez-Real JM, Tuomainen TP, Aregbesola A, Wild SH. Serum ferritin and incident cardiometabolic diseases in Scottish adults. Cardiovasc Diabetol. 2022;21:26. https://doi.org/10.1186/s12933-022-01450-7.
Kang HT, Linton JA, Kwon SK, Park BJ, Lee JH. Ferritin level is positively associated with chronic kidney disease in Korean men, based on the 2010–2012 Korean National Health and Nutrition Examination Survey. Int J Environ Res Public Health. 2016. https://doi.org/10.3390/ijerph13111058.
Wu YH, Wang SY, Li MX, He H, Yin WJ, Guo YH, Zhang HQ, Sun ZM, Zhang D, Wang X, Sun SY, Tang SX, Du R, Zhang CH. Serum ferritin independently predicts the incidence of chronic kidney disease in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:99–105. https://doi.org/10.2147/DMSO.S228335.
Fibrinogen Studies C, Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D’Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. https://doi.org/10.1001/jama.294.14.1799.
Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. https://doi.org/10.1056/NEJMoa1107477.
Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. https://doi.org/10.1161/01.cir.0000042700.48769.59.
Goicoechea M, de Vinuesa SG, Gomez-Campdera F, Aragoncillo I, Verdalles U, Mosse A, Luno J. Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl. 2008. https://doi.org/10.1038/ki.2008.519.
Huang MJ, Wei RB, Wang Y, Su TY, Di P, Li QP, Yang X, Li P, Chen XM. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. 2017;7:e014294. https://doi.org/10.1136/bmjopen-2016-014294.
Hagstrom E, Steg PG, Szarek M, Bhatt DL, Bittner VA, Danchin N, Diaz R, Goodman SG, Harrington RA, Jukema JW, Liberopoulos E, Marx N, McGinniss J, Manvelian G, Pordy R, Scemama M, White HD, Zeiher AM, Schwartz GG, Investigators OO. Apolipoprotein B, residual cardiovascular risk after acute coronary syndrome, and effects of alirocumab. Circulation. 2022;146:657–72. https://doi.org/10.1161/CIRCULATIONAHA.121.057807.
Walldius G, de Faire U, Alfredsson L, Leander K, Westerholm P, Malmstrom H, Ivert T, Hammar N. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: a cohort study. PLoS Med. 2021;18:e1003853. https://doi.org/10.1371/journal.pmed.1003853.
Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, McNeal CJ, Moriarty PM, Natarajan P, Nordestgaard BG, Parhofer KG, Virani SS, von Eckardstein A, Watts GF, Stock JK, Ray KK, Tokgozoglu LS, Catapano AL. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac361.
Goek ON, Kottgen A, Hoogeveen RC, Ballantyne CM, Coresh J, Astor BC. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol Dial Transplant. 2012;27:2839–47. https://doi.org/10.1093/ndt/gfr795.
Hopewell JC, Haynes R, Baigent C. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res. 2018;59:577–85. https://doi.org/10.1194/jlr.R083626.
Sofat R, Cooper JA, Kumari M, Casas JP, Mitchell JP, Acharya J, Thom S, Hughes AD, Humphries SE, Hingorani AD. Circulating apolipoprotein E concentration and cardiovascular disease risk: meta-analysis of results from three studies. PLoS Med. 2016;13:e1002146. https://doi.org/10.1371/journal.pmed.1002146.
Cicero AFG, Fogacci F, Giovannini M, Grandi E, Rosticci M, D’Addato S, Borghi C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci Rep. 2018;8:11529. https://doi.org/10.1038/s41598-018-29955-w.
Pugliese NR, Mengozzi A, Virdis A, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell’Oro R, Bruno B, Lippa L, D’Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G, Palatini P, Galletti F, Ferri C, Desideri G, Viazzi F, Pontremoli R, Muiesan ML, Grassi G, Masi S, Borghi C, Working Group on Uric A, Cardiovascular Risk of the Italian Society of H. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol. 2021;110:1073–82. https://doi.org/10.1007/s00392-021-01815-0.
Theofilis P, Tsimihodimos V, Vordoni A, Kalaitzidis RG. Serum uric acid levels and cardiometabolic profile in middle-aged, treatment-naive hypertensive patients. High Blood Press Cardiovasc Prev. 2022;29:367–74. https://doi.org/10.1007/s40292-022-00522-9.
Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell’Oro R, Bruno B, Lippa L, D’Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G, Palatini P, Galletti F, Ferri C, Desideri G, Viazzi F, Pontremoli R, Muiesan ML, Grassi G, Borghi C, from the Working Group on Uric A, Cardiovascular Risk of the Italian Society of H. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75:302–8. https://doi.org/10.1161/HYPERTENSIONAHA.119.13643.
Son YB, Yang JH, Kim MG, Jo SK, Cho WY, Oh SW. The effect of baseline serum uric acid on chronic kidney disease in normotensive, normoglycemic, and non-obese individuals: a health checkup cohort study. PLoS ONE. 2021;16:e0244106. https://doi.org/10.1371/journal.pone.0244106.
Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric Acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71:362–70. https://doi.org/10.1053/j.ajkd.2017.08.017.
Yu H, Zhao L, Liu L, Li Y, Sun J, Liu Y. Relationship between serum uric acid level and nonalcoholic fatty liver disease in type 2 diabetes patients. Medicine (Baltimore). 2021;100:e26946. https://doi.org/10.1097/MD.0000000000026946.
Bao T, Ying Z, Gong L, Du J, Ji G, Li Z, Gao W, Jiang X, Yang H, Huang Y, Tang H. Association between serum uric acid and nonalcoholic fatty liver disease in nonobese postmenopausal women: a cross-sectional study. Sci Rep. 2020;10:10072. https://doi.org/10.1038/s41598-020-66931-9.
Wei F, Li J, Chen C, Zhang K, Cao L, Wang X, Ma J, Feng S, Li WD. Higher serum uric acid level predicts non-alcoholic fatty liver disease: a 4-year prospective cohort study. Front Endocrinol (Lausanne). 2020;11:179. https://doi.org/10.3389/fendo.2020.00179.
Oral A, Sahin T, Turker F, Kocak E. Relationship between serum uric acid levels and nonalcoholic fatty liver disease in non-obese patients. Medicina (Kaunas). 2019. https://doi.org/10.3390/medicina55090600.
Xing Y, Chen J, Liu J, Song G, Ma H. Relationship between serum uric acid-to-creatinine ratio and the risk of metabolic-associated fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:257–67. https://doi.org/10.2147/DMSO.S350468.
Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic dysfunction-associated fatty liver disease in the National Health and Nutrition Examination Survey 2017–2020: epidemiology, clinical correlates, and the role of diagnostic scores. Metabolites. 2022. https://doi.org/10.3390/metabo12111070.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Research involving human participants
The study was approved by the Ethics Committee of the Ioannina University Hospital and was carried out according to the Declaration of Helsinki (1989).
Informed consent
All individuals were informed about the aims of the study and provided written informed consent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
PT interpreted the results and drafted the manuscript. VT conceived and designed the study and revised the manuscript. AV interpreted the results and revised the manuscript. RGK conceived and designed the study, acquired the data, interpreted the results and drafted the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theofilis, P., Vordoni, A., Tsimihodimos, V. et al. Metabolic Dysfunction-Associated Fatty Liver Disease in Newly Diagnosed, Treatment-Naive Hypertensive Patients and Its Association with Cardiorenal Risk Markers. High Blood Press Cardiovasc Prev 30, 63–72 (2023). https://doi.org/10.1007/s40292-023-00558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00558-5