Abstract
Ankylosing spondylitis (AS) is an autoimmune-related inflammatory arthritis. The association between the DNA methylation and mRNA expression of PDCD1 gene with the susceptibility to AS remains unclear. In this case-control study, the methylation level of PDCD1 promoter was detected in 80 AS patients and 80 healthy controls by MethylTarget method. The transcriptional level of PDCD1 gene was measured in 47 AS patients and 47 healthy controls by real-time quantitative PCR. Finally, 17 methylation sites mapped to one CpG island were detected. Compared to healthy controls, the promoter of PDCD1 was hypermethylated (p < 0.001) and the mRNA expression was downregulated (p < 0.001) in AS patients. Significantly negative correlation was identified between the DNA methylation and mRNA expression of PDCD1 gene (rs = −0.470, p < 0.001). The receiver operating characteristic (ROC) results showed that PDCD1 island had a sensitivity of 61.3% and a specificity of 82.5%, and PDCD1 mRNA had a sensitivity of 87.2% and a specificity of 89.0%. The methylation level of PDCD1 was positively correlated with the ESR, CRP and ASDAS of AS, and was not affected by HLA-B27 status, gender or medicine intake.
Similar content being viewed by others
Introduction
Ankylosing spondylitis (AS) is an autoimmune disease characterized by axial joint inflammation and new bone formation that can eventually lead to spinal deformities, joint stiffness, and even lifelong disability [1]. Extra-articular symptoms also frequently occur in AS, such as psoriasis, uveitis, and intestinal inflammation [2]. AS is estimated to influence 0.1–0.5% of the global population [3]. It usually initially appears during the third decade of life, and causes severe impairment of spinal movement and bodily function [4].
Despite the pathogenesis of AS keeping unclear, susceptibility to AS and the severity of manifestations are remarkably considered heritable [5, 6]. The gene known to be associated with AS is human leukocyte antigen (HLA)-B27, which is positive in almost 95% of patients with AS [7]. However, only about 1–2% of HLA-B27 carriers suffer from AS [8]. Twin and familial studies of AS have illustrated that HLA-B27 accounts for merely 20% of the overall risk of AS, suggesting that other genetic factors are compactly related to AS [9].
The role of epigenetics in the occurrence and development of autoimmune disorders has attracted broad attention [10]. Epigenetics is the study of heritable changes in gene activity or function that are independent of any changes in the DNA sequence itself [11]. Considerable evidence suggests that epigenetic modifications exert a central role in the cellular programming of gene expression [12]. It is sensitive to external stimuli and can mediate the interaction between genes and environment. DNA methylation is the most common epigenetic modification and can be steadily inherited through multiple cell divisions. DNA methylation is a process by which a methyl group was added onto the 5’carbon site of cytosine in cytosine-phosphate-guanosine (CpG) dinucleotides, resulting in the modification of the chromatin structure and ultimately gene silencing [13]. Aberrant DNA methylation has been found to be involved in the pathogenesis of AS and other autoimmune disorders [14,15,16]. For example, the significant hypermethylation of the IFN regulatory factor 8 (IRF8) gene promoter and the downregulation of the mRNA level of the IRF8 gene have been reported in AS patients [17]. In addition, hypomethylation of IL-6 and IL-10 promoters is associated with their elevated expression in peripheral blood mononuclear cells (PBMCs) from patients with rheumatoid arthritis (RA) [18, 19]. These findings indicated that DNA methylation may participate in the occurrence and development of autoimmune diseases by negatively regulating gene transcription.
Programmed Cell Death Protein 1 (PDCD1), also known as CD279, is a member of the CD28/B7 receptor family. PDCD1 is a 55-kDa transmembrane protein with an extracellular IgV-like domain and a 97 amino acid cytoplasmic tail containing an immunotyrosine inhibitory motif and an immunotyrosine switching motif [20]. PDCD1 is primarily expressed on T lymphocytes, B lymphocytes, myeloid cells, natural killer (NK) cells, and thymus cells [21, 22]. Numerous studies have demonstrated that PDCD1 exerts an important role in restraining T cell signal transduction, mediating tolerance mechanisms, and holding immune homeostasis [23]. PDCD1 knockout mice exhibited overactive immune phenotypes [24]. Targeting PDCD1 pathway to treat cancer showed benefits in many patients and has already been applied to clinical cancer therapy [25]. Given that the breakdown of self-tolerance and the self-attack of immune system are the prominent features of autoimmune diseases, PDCD1 may also play a critical role in regulating autoimmune and inflammatory disorders [26, 27]. Published epigenome-wide association studies (EWAS) have identified the association between DNA methylation levels of PDCD1 and various traits including Sjogren syndrome and RA [28, 29]. However, the relationship between PDCD1 promoter methylation, transcript level, and AS susceptibility remains further investigation.
In this case-control study, we explored the association of PDCD1 gene with AS susceptibility in eastern Chinese Han population based on the methylation and transcriptome signatures. Moreover, the correlation between the methylation and mRNA expression of PDCD1 gene as well as the clinical index of AS patients was also assessed.
Materials and methods
Human subjects
During July 2021 to August 2021, confirmed AS cases and healthy subjects were consecutively recruited from the Department of Rheumatology and Immunology, the First Affiliated Hospital of Anhui Medical University (Hefei, Anhui, China), and the medical examination center of the same hospital, respectively. AS cases were clinical diagnosed according to the New York criteria amended by the American College of Rheumatology in 1984. AS cases and healthy controls (HCs) were matched according to age and gender. Five milliliters peripheral venous blood was extracted from all the participants. Demographic and clinical information, such as age, sex, height, weight, HLA-B27 status, disease course, serum erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), were obtained from structured questionnaires and patients’ medical records. Additionally, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score (ASDAS) were used to evaluate disease activity. Bath Ankylosing Spondylitis Functional Index (BASFI) was applied to assess functional disabilities. The current study was in line with the Declaration of Helsinki and supported by the ethics committee of Anhui Medical University. Signed informed consents were acquired from the whole participants prior to registration.
DNA methylation detection
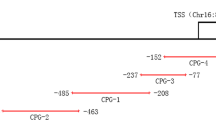
Genomic DNA was isolated from 2 ml peripheral blood of AS cases and HCs using the Qiagen® FlexiGene® DNA kit under the manufacturer’s instruction, and then stored at −80 °C before detecting DNA methylation. After quality control, DNA samples were diluted to 20 ng/ul for quantitative methylation analysis. The CpG islands located in the promoter region of PDCD1 gene were selected from 2 k upstream of the transcription start site (TSS) to 1 k downstream of the first exon based on the criteria as follows: (1) excessive than 200 bp length; (2) 0.6 or higher ratio of observed or expected dinucleotides CpG; (3) 50% or higher cytosine-guanine content. Finally, 17 CpG methylation sites from one island of the PDCD1 promoter were selected and sequenced (Supplementary Table S1).
Genomic DNA (400 ng) was treated with sodium bisulfite using EZ DNA MethylationTM-GOLD Kit (ZYMO RESEARCH, CA, USA) based on the manufacturer’s instructions, which will transform unmethylated cytosine to uracil. Samples with DNA bisulfite conversion rate below 98% were filtered out and all samples were eligible in this study. The optimized primer combinations were used for multiple PCR amplification of bisulfite modified DNA sequences. PCR amplicons (170–270 bp) were separated by agarose electrophoresis and purified using the QIAquick Gel Extraction kit (QIAGEN, Hilden, Germany), and PDCD1 methylation test was carried out by using Illumina Hiseq/Miseq 2000 according to the manufacturer’s protocol. The sequences of sense and antisense primers for PDCD1 were as follows: forward: 5’-TGAAATTGTTGATATTAGTGATGAGATG-3’, reverse: 5’- CCACCCACACAACCTCAC-3’. The methylation level of the PDCD1 promoter was analyzed using MethylTarget™ (Genesky Biotechnologies Inc., Shanghai, China).
Real-time quantitative PCR (RT-qPCR)
Peripheral blood mononuclear cells (PBMCs) were freshly separated from 3 ml peripheral blood of AS patients and HCs by Ficoll-Hypaque density gradient centrifugation method within 4 h. Total RNA was extracted from cells using the miRNeasy Mini Kit (Qiagen, Germany). RNA concentration and purity were detected using a NanoDrop™2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Eligible RNA samples were then reverse-transcribed into complementary DNA (cDNA) using a PrimeScript™ RT reagent kit (Takara Bio Inc., Japan). The relative expression level of PDCD1 mRNA was measured in the Real-Time quantitative PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq II (Takara Bio, Japan). 2−∆∆Ct method normalized to endogenous control was used for calculating the relative expression level of PDCD1. The sequences of sense and antisense primers for PDCD1 and β-actin are as follows: PDCD1, forward: 5’-CCAGGATGGTTCTTAGACTCCC-3’, reverse: 5’-TTTAGCACGAAGCTCTCCGAT-3’; β-actin, forward: 5’-TGACGTGGACATCCGCAAAG-3’, reverse: 5’-CTGGAAGGTGGACAGCGAGG-3’ [30,31,32].
Statistical analysis
SPSS 23.0 software (SPSS Inc, Chicago, IL, USA) was chosen for statistical analysis of the data, and GraphPad Prism 5.01 software (GraphPad Inc, CA, USA) was applied for plotting. Continuous data conforming to normal distribution were represented by mean ± standard deviation (SD), and independent t-test was used for comparison between the two groups; skewness distribution data were described by median and interquartile (IQR), and comparison between the two groups was conducted by Mann–Whitney U test. Categorical data were presented in absolute numbers and percentages, and compared with Pearson chi-square test. Spearman’s rank correlation coefficient test was conducted to investigate the association between PDCD1 gene and clinical index. The predictive performance of PDCD1 as a biomarker for AS was evaluated by receiver operating characteristic (ROC) curve and area under curve (AUC). A two-side p value <0.05 was defined as statistically significant.
Results
Characteristics of study subjects
In the first stage, the DNA methylation levels of PDCD1 gene were detected in 80 AS patients and 80 HCs. In both groups, there were 60 (75.00%) males and 20 (25.00%) females. The mean age of AS and HCs were 35.14 ± 9.93 and 34.83 ± 9.52, respectively, and no significant differences were found in either age (p = 0.839) or gender (p = 1.000). In the second stage, 47 AS patients and 47 HCs were randomly selected from the first stage to detect the mRNA expression of PDCD1 gene. There were 37 (78.72%) males and 10 (21.28%) females in both groups. The mean age of AS and HCs were 34.66 ± 8.62 and 36.00 ± 8.99, respectively. The age (p = 0.462) and gender (p = 1.000) of AS patients were also comparable to those of HCs. The other specific demographic and clinical features of all participants are summarized in Table 1.
Methylation level of PDCD1
The methylation level of each CpG site was calculated by the ratio of methylated cytosine to total detected cytosine. A total of 17 CpG sites at one CpG island in the promoter region of PDCD1 gene were detected to be methylated. The result showed that all 17 CpG sites were significantly hypermethylated in AS patients compared with HCs (Supplementary Table S1). To further understand the overall methylation status of PDCD1 gene in AS patients, the methylation level of CpG island was determined by calculating the average methylation level of all CpG sites. Compared with HCs, the CpG island in the promoter region of PDCD1 gene was also highly methylated in AS patients (AS vs. HCs: 73.53% (67.41–79.19%) vs. 65.67% (56.50–70.70%), p < 0.001, Fig. 1). To assess the potential of PDCD1 gene methylation as a biomarker for AS, ROC curve analysis was conducted on the CpG island. The AUC of ROC curve was 0.764 (95% CI = 0.690–0.837, p < 0.001) with a sensitivity of 61.3% and a specificity of 82.5% (Fig. 2A).
Subgroup analysis of PDCD1 methylation
It is well known that the most significant heritability of AS comes from the B27 antigen and the male population. In addition, drug use may affect DNA methylation levels. Thus, subgroup analysis was performed by HLA-B27 antigen, gender and medicine intake to further explore the role of PDCD1 methylation in AS. The results showed that the methylation levels in the promoter region of PDCD1 gene in HLA-B27-positive patients and HLA-B27-negative patients were all significantly higher than that in healthy subjects (HLA-B27(+) vs. HC: 73.15% (65.26%–78.55%) vs. 65.67% (56.50%–70.70%), p < 0.001; HLA-B27(−) vs. HC: 75.83% (68.92%–79.45%) vs. 65.67% (56.50%–70.70%), p < 0.001, respectively) (Fig. 3A, B). Moreover, in the subgroup analysis of gender, the hypermethylation of PDCD1 promoter was also detected in both male and female AS patients (AS-male vs. HC-male: 73.34% (66.52%–79.29%) vs. 62.88% (55.59%–70.70%), p < 0.001; AS-female vs. HC-female: 75.28% (68.69%–78.93%) vs. 67.79% (60.94%–70.81%), p = 0.003, respectively) (Fig. 3C, D). However, no significant differences were found between treatment groups in the current study (Supplementary Table S2).
A HLA-B27 (+) AS patients vs. healthy controls; B HLA-B27 (−) AS patients vs. healthy controls; C male AS patients vs. male healthy controls; D female AS patients vs. female healthy controls. Mann–Whitney U test was performed, data were expressed as median and interquartile range, p < 0.05 was considered significant.
mRNA expression level of PDCD1
We further measured the mRNA expression level of PDCD1 gene in the PBMCs of 47 AS patients and 47 HCs using RT-qPCR technology. The relative expression level of PDCD1 gene was significantly decreased in AS patients than that in HCs (AS vs. HCs: 0.586 (0.330–0.888) vs. 1.991 (1.243–3.442), p < 0.001) (Fig. 4A). ROC results showed that the AUC was 0.909 (95% CI = 0.852–0.967, p < 0.001) for the PDCD1 mRNA level with a sensitivity of 87.2% and a specificity of 80.9% (Fig. 2B). There was a significantly negative correlation between the DNA methylation level of PDCD1 promoter and the expression level of PDCD1 gene (rs = −0.470, p < 0.001, Fig. 4B).
A The expression level of PDCD1 mRNA in AS patients and healthy controls, Mann–Whitney U test was performed, data were expressed as median and interquartile range; B The correlation analysis between methylation level and mRNA level of PDCD1, Spearman’s rank correlation coefficient test was conducted. p < 0.05 was considered statistically significant.
Methylation level and clinical characteristics
We analyzed the associations between the DNA methylation and mRNA levels of PDCD1 gene and clinical manifestations, and the results were provided in Table 2. The DNA methylation level of PDCD1 promoter was positively correlated with the ESR (rs = 0.306, p = 0.006), CRP (rs = 0.306, p = 0.006) and ASDAS (rs = 0.287, p = 0.012) of AS patients. Moreover, there was no significant correlation between the PDCD1 mRNA and clinical characteristics of AS.
Discussion
Epigenetics, which refers to the functional modification of DNA without changing the sequence, can be inherited from one cell cycle to the next and is largely responsible for the expression of cell-specific genes [33]. DNA methylation is one of the best studied and understood epigenetic modifications, which plays a key role in regulating gene transcription and nuclear tissue, and ultimately affecting cellular function [29]. Emerging evidence has linked the aberrant DNA methylation with the pathogenesis and development of AS [34]. A genome-wide DNA methylation profile analysis of 5 AS patients and 5 healthy individuals identified 1915 differentially methylated CpG sites mapped to 1214 genes in the peripheral blood of AS, including PDCD1. However, the adjusted P value was not significant and data on the transcription level of PDCD1 gene were lacking [35].
In the current study, we investigated the relationship between the DNA methylation and mRNA expression of PDCD1 gene and the susceptibility to AS. In the first stage, a total of 80 AS patients and 80 HCs were included to detect the methylation level of PDCD1 promoter, and the result showed that all 17 CpG sites and the CpG island in the promoter region of PDCD1 gene were highly methylated in AS. In the second stage, 47 AS patients and 47 HCs were randomly selected from the first stage to measure the mRNA expression of PDCD1. We found that the mRNA level of PDCD1 was significant lower in AS patients compared with HCs, and there was a significantly negative correlation between the DNA methylation and mRNA expression level of PDCD1 gene, further supporting of the functional relevance of PDCD1 in AS. Moreover, subsequent analyses indicated that the methylation level of PDCD1 gene was positively correlated with the ESR, CRP and ASDAS of AS, and not influenced by HLA-B27, gender and medicine use.
PDCD1 is an inhibitory receptor that plays an important role in suppressing immune response and promoting self-tolerance by regulating T cell activity, activating antigen-specific T cell apoptosis and inhibiting regulatory T cell apoptosis [36]. Increasing evidence supports the role of PDCD1 pathway in the prevention of autoimmune diseases [37]. For example, PDCD1 knockout mice on the C57BL/6 background developed lupus like glomerulonephritis, while premorbid BWF1 mice treated with anti-PDCD1 were protected from lupus nephritis [38, 39]. The serum levels of soluble PDCD1 and the mRNA expression of PDCD1 were downregulated in the peripheral blood of RA, and knockout of the PDCD1 gene in mice increased the incidence and severity of collagen II-induced arthritis [40, 41]. The majority of the studies on the role of PDCD1 in AS focus on gene polymorphisms, and several single nucleotide polymorphisms have been reported to be correlated with the risk of AS [42, 43]. In this study, we performed gene-specific methylation and RT-qPCR assays, and the hypermethylation of PDCD1 promoter as well as its downregulated mRNA expression were detected. The main finding is that the aberrant DNA methylation and expression level of PDCD1 gene may participate in the pathogenesis of AS, and PDCD1 may be a promising biomarker for AS. The lack of same observation in previous published EWAS may be due to differences in race and sample size [15, 35].
Several limitations of this study are worth considering. Firstly, this was a single-center case-control study based on one hospital, and the positive rate of HLA-B27 was relatively low in the included AS patients, so selection bias may be inevitable. Secondly, we only detected the mRNA expression level of PDCD1 gene, but not its protein level. Thirdly, functional studies on the role of PDCD1 in AS are lacking in this study. Fourthly, β-actin was used as an endogenous control without validation. Thus, multi-center studies with larger sample sizes are required to deeply investigate the role of PDCD1 in AS to provide more comprehensive evidence.
Conclusion
DNA methylation and mRNA expression of PDCD1 gene were significantly associated with the susceptibility to AS. The hypermethylation of the PDCD1 gene promoter and the downregulation of its transcription may be involved in the pathogenesis of AS. PDCD1 may be a promising biomarker for the diagnosis and evaluation of AS.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–74.
de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18:196.
Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res. 2019;71:1285–99.
Ritchlin C, Adamopoulos IE. Axial spondyloarthritis: new advances in diagnosis and management. BMJ. 2021;372:m4447.
Gao S, Zhang J, Xu T, Xun C, Cao R, Guo H, et al. Associations of toll-like receptor 4 and 2 gene polymorphisms with susceptibility to ankylosing spondylitis: a meta-analysis. Int J immunogenet. 2021;48:219–28.
Chen Y, Yang H, Xu S, Shen J, Xu W, Shao M, et al. Association analysis of B7-H3 and B7-H4 gene single nucleotide polymorphisms in susceptibility to ankylosing spondylitis in eastern Chinese Han population. Int J immunogenet. 2021;48:500–9.
Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 2010;6:399–405.
Zhu W, He X, Cheng K, Zhang L, Chen D, Wang X, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res. 2019;7:22.
Hanson A, Brown MA. Genetics and the causes of ankylosing spondylitis. Rheum Dis Clin North Am. 2017;43:401–14.
Li J, Li L, Wang Y, Huang G, Li X, Xie Z, et al. Insights into the role of DNA methylation in immune cell development and autoimmune disease. Front Cell Dev Biol. 2021;9:757318.
Yang H, Chen Y, Xu W, Shao M, Deng J, Xu S, et al. Epigenetics of ankylosing spondylitis: recent developments. Int J Rheum Dis. 2021;24:487–93.
Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity. 2010;105:4–13.
Meng H, Cao Y, Qin J, Song X, Zhang Q, Shi Y, et al. DNA methylation, its mediators and genome integrity. Int J Biol Sci. 2015;11:604–17.
Kyrgios I, Fragou A, Kotanidou EP, Mouzaki K, Efraimidou S, Tzimagiorgis G, et al. DNA methylation analysis within the IL2RA gene promoter in youth with autoimmune thyroid disease. Eur J Clin Investig. 2020;50:e13199.
Coit P, Kaushik P, Caplan L, Kerr GS, Walsh JA, Dubreuil M, et al. Genome-wide DNA methylation analysis in ankylosing spondylitis identifies HLA-B*27 dependent and independent DNA methylation changes in whole blood. J Autoimmun. 2019;102:126–32.
Chen Y, Wu Y, Yang H, Wang J, Kong J, Yu L, et al. DNA methylation and mRNA expression of B7-H3 gene in ankylosing spondylitis: a case-control study. Immunol Invest. 2022;51:2025–34.
Chen M, Wu M, Hu X, Yang J, Han R, Ma Y, et al. Ankylosing spondylitis is associated with aberrant DNA methylation of IFN regulatory factor 8 gene promoter region. Clin Rheumatol. 2019;38:2161–69.
Fu LH, Ma CL, Cong B, Li SJ, Chen HY, Zhang JG. Hypomethylation of proximal CpG motif of interleukin-10 promoter regulates its expression in human rheumatoid arthritis. Acta Pharmacol Sin. 2011;32:1373–80.
Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–93.
Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–71.
Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ. Emerging concepts in PD-1 checkpoint biology. Semin Immunol. 2021;52:101480.
Liu MY, Klement JD, Langan CJ, van Riggelen J, Liu K. Expression regulation and function of PD-1 and PD-L1 in T lymphoma cells. Cell Immunol. 2021;366:104397.
Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37.
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22.
Jachetti E, Sangaletti S, Chiodoni C, Ferrara R, Colombo MP. Modulation of PD-1/PD-L1 axis in myeloid-derived suppressor cells by anti-cancer treatments. Cell Immunol. 2021;362:104301.
Zhao P, Wang P, Dong S, Zhou Z, Cao Y, Yagita H, et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat Biomed Eng. 2019;3:292–305.
Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72–9.
Björk A, Richardsdotter Andersson E, Imgenberg-Kreuz J, Thorlacius GE, Mofors J, Syvänen AC, et al. Protein and DNA methylation-based scores as surrogate markers for interferon system activation in patients with primary Sjögren’s syndrome. RMD Open. 2020;6:e000995.
Xiao M, Zheng X, Li X, Wu X, Huang Y, Wei Q, et al. Integrative blood-derived epigenetic and transcriptomic analysis reveals the potential regulatory role of DNA methylation in ankylosing spondylitis. Arthritis Res Ther. 2022;24:15.
Wu J, Deng LJ, Xia YR, Leng RX, Fan YG, Pan HF, et al. Involvement of N6-methyladenosine modifications of long noncoding RNAs in systemic lupus erythematosus. Mol Immunol. 2022;143:77–84.
Wu J, Zhang TP, Zhao YL, Li BZ, Leng RX, Pan HF, et al. Decreased H19, GAS5, and linc0597 expression and association analysis of related gene polymorphisms in rheumatoid arthritis. Biomolecules. 2019;10:55.
Tang YP, Zhang QB, Dai F, Liao X, Dong ZR, Yi T, et al. Circular RNAs in peripheral blood mononuclear cells from ankylosing spondylitis. Chin Med J. 2021;134:2573–82.
Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev. 2012;11:604–9.
Ma Y, Fan D, Xu S, Deng J, Gao X, Guan S, et al. Ankylosing spondylitis patients display aberrant ERAP1 gene DNA methylation and expression. Immunol Invest. 2022;51:1548–60.
Hao J, Liu Y, Xu J, Wang W, Wen Y, He A, et al. Genome-wide DNA methylation profile analysis identifies differentially methylated loci associated with ankylosis spondylitis. Arthritis Res Ther. 2017;19:177.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34.
Zamani MR, Aslani S, Salmaninejad A, Javan MR, Rezaei N. PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol. 2016;310:27–41.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Wong M, La Cava A, Singh RP, Hahn BH. Blockade of programmed death-1 in young (New Zealand black x New Zealand white)F1 mice promotes the activity of suppressive CD8+ T cells that protect from lupus-like disease. J Immunol. 2010;185:6563–71.
Li S, Liao W, Chen M, Shan S, Song Y, Zhang S, et al. Expression of programmed death-1 (PD-1) on CD4+ and CD8+ T cells in rheumatoid arthritis. Inflammation. 2014;37:116–21.
Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010;62:1870–80.
Yang Q, Liu Y, Liu D, Zhang Y, Mu K. Association of polymorphisms in the programmed cell death 1 (PD-1) and PD-1 ligand genes with ankylosing spondylitis in a Chinese population. Clin Exp Rheumatol. 2011;29:13–8.
Huang CH, Wong RH, Wei JC, Tsay MD, Chen WC, Chen HY, et al. Effects of genetic polymorphisms of programmed cell death 1 and its ligands on the development of ankylosing spondylitis. Rheumatology. 2011;50:1809–13.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81773514, 82073655) and the funds for academic and technical leaders in Anhui province (2017D140) and Clinical Medicine Discipline construction project of Anhui Medical University (2021lcxk043).
Author information
Authors and Affiliations
Contributions
YW: conceptualization, methodology, software, investigation, formal analysis, writing—original draft; YC: data curation, writing—original draft; XS: visualization, investigation; YD: resources, supervision; MN: software, validation; FP: conceptualization, funding acquisition, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethical Committee of Anhui Medical University (Hefei, China) and all procedures have complied with the Declaration of Helsinki.
Informed consent
All the subjects were given an informed consent and were well told of the study protocol.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Chen, Y., Sun, X. et al. DNA methylation and transcriptome signatures of the PDCD1 gene in ankylosing spondylitis. Genes Immun 24, 46–51 (2023). https://doi.org/10.1038/s41435-023-00196-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-023-00196-w
- Springer Nature Limited
This article is cited by
-
Methylation of T and B Lymphocytes in Autoimmune Rheumatic Diseases
Clinical Reviews in Allergy & Immunology (2024)
-
Aberrant DNA Methylation Profile of Dickkopf-1 in Ankylosing Spondylitis
Biochemical Genetics (2024)
-
Application of methylation in the diagnosis of ankylosing spondylitis
Clinical Rheumatology (2024)