Abstract
In this study, we developed a lentiviral two-step transcriptional amplification (TSTA) system expressing bone morphogenetic protein-2 (BMP-2) under the control of a GAL4FF transactivator to enhance gene expression and limit toxicity for bone repair applications. To this end human MSCs, isolated from bone marrow or adipose tissue, were transduced overnight with a LV-TSTA system (GAL4FF or GAL4vp16) expressing BMP-2 or GFP and evaluated in vitro for transduction efficiency, mean fluorescence intensity, cell viability, and BMP-2 production. FACS analysis of GFP-transduced MSCs confirmed successful transduction with the GAL4FF+GFP vector. Moreover, ELISA demonstrated abundant BMP-2 production by GAL4FF+BMP2-transduced human MSCs over a period of 8 weeks, with minimal cytotoxicity at all time points. Compared to GAL4vp16, GAL4FF was superior with respect to BMP production at 1, 2, 4, 6, and 8 weeks in BMSCs. In ASCs, GAL4FF was still associated with higher BMP-2 production at weeks 2–8, but this difference was not as prominent as in BMSCs. To our knowledge, this is the first report of GAL4FF-mediated BMP-2 production by human BMSCs and ASCs. Compared to the standard GAL4vp16TSTA vector, GAL4FF was associated with lower cytotoxicity and higher in vitro gene expression in both BMSCs and ASCs.
Similar content being viewed by others
Introduction
Ex vivo regional gene therapy has been assessed pre-clinically by numerous independent laboratories for its potential to enhance bone healing, and improve the management of challenging bone repair scenarios for which there is no consistently effective solution [1,2,3]. The advantage of this gene therapy approach is that it combines an osteoinductive growth factor with osteoprogenitor cells, thus concurrently stimulating a reparative response from endogenous progenitor cells, while also having implanted cells differentiate into osteoblasts via an autocrine mechanism. This strategy allows for prolonged, localized excretion of the growth factor of interest, leading to longer half-life of the osteoinductive signal and thus a more robust osteogenic response compared to recombinant proteins [4,5,6,7].
Different viral vectors have been tested in an attempt to increase the efficiency of gene delivery for bone repair applications. The prevailing technology over the past two decades for transducing mesenchymal stem cells (MSCs) has been the use of integrating viral vectors, such as retroviruses and lentiviruses, or non-integrating vectors, such as adenoviruses and recombinant adeno-associated viruses, all of them with their own advantages and limitations. In our laboratory adenoviral vectors were initially used, but were subsequently replaced by lentiviral gene delivery due to concerns regarding transient gene expression and pronounced immunogenicity in immunocompetent animals and humans [8,9,10]. Lentiviral vectors can infect both dividing and non-dividing cells by incorporating into the host genome, thus allowing for sustained gene expression.
We have employed a lentiviral (LV) two-step transcriptional amplification (TSTA) system [11], that combines a transactivator vector with a transgene expression vector, to achieve enhanced gene expression and successfully promote bone repair [12,13,14]. Ex vivo regional gene therapy with LV-TSTA expressing bone morphogenetic protein-2 (BMP-2) under the control of the GAL4vp16 transactivator has been used successfully to induce osteogenesis in a rat critical-sized bone defect model [12], but GAL4vp16 has been shown to be cytotoxic when overexpressed in vitro. Expression of Gal4vp16 at high levels has been reported to cause inhibition of transcription of certain genes in mammalian cells [15, 16] and delayed or malformed embryogenesis in zebrafish [17].
A different, GAL4 transcription activator, namely the GAL4FF, has been developed by Asakawa et al. [18], in an effort to improve the currently available GAL4 gene trap constructs and enable targeted gene expression in the desired cells in fish models. GAL4FF consists of the yeast Gal4 DNA-binding domain and two transcription activation modules from the herpes simplex VP16 [18]. To our knowledge, GAL4FF has never been tested for gene expression efficacy and toxicity in mammalian cells in vitro or in vivo. In this study, we aimed to develop a lentiviral GAL4FF-TSTA system overexpressing BMP-2 (LV-TSTA-BMP-2) (Fig. 1) that would enhance gene expression with minimal cytotoxicity when combined with human MSCs for use in ex vivo gene therapy for bone repair applications. Human MSCs isolated from bone marrow or adipose tissue were selected for evaluation for several reasons, including their ability to expand in tissue culture, osteogenic differentiation potential, and their clinical relevance.
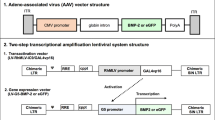
Schematics of the two-step transcriptional amplification lentiviral system containing the yeast Gal4 transcription activator (Gal4vp16 or Gal4FF). In the first vector/step, the Gal4vp16 or Gal4FF is expressed by a SIN18-based lentiviral vector controlled by the RhMLV promoter (LV-RhMLV-Gal4vp16 or LV-RhMLV-Gal4FF). The second vector/step consists of the Gal4 responsive G5 promoter (LV-G5-BMP2 or LV-G5-eGFP) that leads to transgene expression. RRE Rev-responsive element, cPPT central polyprine tract, LTR long terminal repeat, Ψ packaging signal, SIN self-inactivating
Results
Green fluorescent protein (GFP) expression in vitro
In order to determine optimal transduction conditions, ASCs and bone marrow stem cells (BMSCs) were transduced with LV-TSTA-GFP at MOIs of 1, 5, 25, and 50 and analyzed for transduction efficiency and mean fluorescence intensity (MFI). Strong eGFP expression was detected by fluorescence microscopy for both ASCs and BMSCs at 2 and 7 days post transduction (Fig. 2). Moreover, FACS analysis of GFP-transduced MSCs confirmed successful transduction with both TSTA-GFP vectors at MOIs of 1, 5, 25, and 50 at both time points. Based on the analysis of three lipoaspirate samples and three BM samples at 2 and 7 days post-transduction with LV-TSTA-GFP, there was no difference (p > 0.05) between GAL4FF+GFP and GAL4vp16+GFP with regards to transduction efficiency and MFI.
MOIs analysis showed a generally lower MFI and transduction efficiency for MOI = 1-transduced cells compared to the rest of the MOIs (Table 1, Fig. 3). For the GAL4FF vector, comparison of the four different MOIs in BMSC demonstrated that MOI = 1 exhibited a significantly lower transduction efficiency and MFI compared to MOI = 5 (p < 0.001 and p = 0.038, respectively), MOI = 25 (p < 0.001 and p = 0.020, respectively), and MOI = 50 (p < 0.001 and p = 0.005, respectively). In GAL4FF-GFP-transduced ASCs there was also a trend towards lower transduction efficiency in MOI of 1 compared to MOIs of 5 (p = 0.08), 25 (p = 0.047), and 50 (p = 0.026). Similar results were seen with the use of the GAL4vp16 vector (Table 1, Fig. 3). Thus, due to the low transduction achieved with the MOI of 1, MOI = 1 was excluded from further analysis. No differences were noted with regards to %GFP positive MSCs and mean GFP intensity between MOIs of 5, 25, and 50. However, MOI of 50 was associated with high viral toxicity-related cell death 7 days post transduction with LV-TSTA-GFP (>50% for ASC, 50–80% for BMSC). Based on cell viability, MOI = 50 was also eliminated. Therefore, MOIs of 5 and 25 and the combination thereof 5/25 were selected for further evaluation with the BMP-2-expressing vector.
The mean fluorescence intensity (MFI) of GFP was determined in (a) BMSCs (three samples) and (b) ASCs (three samples) transduced with GAL4FF+G5-GFP or GAL4vp16+G5-GFP, 2 days post-transduction. MOI of 1 exhibited significantly lower MFI vs. MOIs of 5, 25, and 50 for both TSTA vectors. No differences were noted between GAL4FF+GFP and GAL4vp16+GFP with regards to MFI at all MOIs for both cell types. *p < 0.05 compared to MOIs of 5, 25, and 50 for both vectors
In vitro BMP-2 production
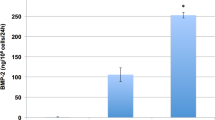
ELISA results confirmed abundant BMP-2 production by BMSCs and ASCs transduced with GAL4FF+BMP2 at MOIs of 5/5, 5/25, and 25/25, over a period of 8 weeks, with the peak BMP expression detected at 1 week (Fig. 4). In detail, BMSC transduced with GAL4FF+BMP-2 at MOIs of 5/5, 5/25, or 25/25 produced 180.97 ± 69.25, 454.5 ± 232.75, and 313.5 ± 128.4 ng, respectively 1 week post-transduction, vs. 83.67 ± 24.42, 285.03 ± 154.59, and 74.27 ± 18.87 ng produced by GAL4vp16+BMP-2-transduced BMSCs. High levels were also seen in ASCs transduced with GAL4FF+BMP-2 or GAL4vp16+BMP-2 at MOIs of 5/5 (52.37 ± 31.24 vs. 69.9 ± 40.1 ng, respectively), 5/25 (117.77 ± 26.28 vs. 130.4 ± 12.25 ng, respectively), and 25/25 (101.6 ± 29.16 vs. 103.53 ± 35.56 ng, respectively), 1 week post-transduction. In both cell types, there was a trend towards higher BMP-2 production at all time points when using MOI of 5/25 compared to MOIs of 5/5 and 25/25 (Fig. 4). BMP-2 production persisted for 8 weeks, albeit at decreasing levels. As expected, no or minimal (<1 ng/24 h/ml) BMP-2 production was seen in non-transduced cell cultures at all time points (p < 0.001, vs. GAL4FF+BMP-2-transduced cells at all MOIs).
In vitro BMP-2 production over an 8-week period by BMSCs and ASCs transduced with GAL4FF and G5-BMP-2 at MOIs of 5/5, 5/25, and 25/25, respectively. Peak BMP-2 levels were noted 1 week post-transduction, followed by gradually decreased yet sustained BMP-2 production. BMP-2 production for all MOIs at all time-points was significantly higher in BMP-2-transduced BMSCs and ASCs as compared to non-transduced cells (p < 0.05). Three samples per tissue type, each run in duplicate, were used for this aspect of the study
When comparing the two different transactivator vectors, GAL4vp16 was associated with a higher BMP-2 production at 2 days post-transduction in both ASCs and BMSCs. However, at the later time points (weeks 4–6), BMP-2 production from GAL4vp16 cells decreased significantly. In contrast, GAL4FF was associated with a more gradual decrease in BMP-2 levels. In BMSCs there was a trend towards higher BMP-2 production in GAL4FF-transduced cells vs. GAL4vp16 at all MOIs at 1–8 weeks post-transduction (Fig. 5a). In ASCs, the difference between the two vectors, though still present, was not as prominent as in BMSCs (Fig. 5b).
In vitro BMP-2 production by BMSCs (a) and ASCs (b) transduced with GAL4FF+G5-BMP-2 or GAL4vp16+G5-BMP-2 at MOIs of 5/5, 5/25, and 25/25. A. In BMSCs, GAL4FF was superior with respect to BMP production at 1, 2, 4, 6, and 8 weeks compared to GAL4vp16. B. In ASCs, GAL4FF was still associated with a higher BMP-2 production at the later time points (weeks 2–8), but this difference was not as prominent as in BMSCs. A total of three samples each run in duplicate. Results presented as mean ± SD. *p < 0.05 vs. GAL4vp16 at the given time point
Cell viability
GAL4FF was associated with minimal cytotoxicity at all time points in both cell types (p > 0.05 vs. non-transduced cells in both cell types and all MOIs).
In BMSCs cultures, an average of 10–34% of cells died 1 week following transduction with different MOIs of GAL4FF+G5-BMP-2. No further decrease in cell numbers was noted at the later time points (2–8 weeks post-transduction). In contrast, GAL4vp16 was associated with high toxicity 1 week post-transduction as compared to both non-transduced (p = 0.017 at MOI = 5/5, p = 0.002 at MOI = 5/25, p < 0.001 at MOI = 25/25) and GAL4FF-transduced cells (p ≤ 0.04, at MOIs of 5/25 and 25/25) leading to 30–58% cell death. The cells eventually recovered from transduction and started proliferating again at the 4-week time point, essentially ending up matching the non-transduced and GAL4FF cell numbers at 8 weeks (Fig. 6a).
Average cell viability in BMSCs (a) and ASCs (b) transduced with GAL4FF+G5-BMP or GAL4vp16+G5-BMP at MOIs of 5/5, 5/25, and 25/25 over 8 weeks. Three samples per tissue type per MOI were used for this aspect of the study. Each sample was run in duplicate. Non-transduced human MSCs were used as a control. GAL4FF was associated with minimal cytotoxicity at all time points in both cell types (p > 0.05 vs. non-transduced cells in both cell types and all MOIs). *p < 0.05 vs. non-transduced, +p < 0.05 vs. GAL4FF
In ASCs, GAL4FF did not cause any virus-associated toxicity at all MOIs. The cells continued to proliferate for the duration of the experiment, despite the presence of the vector. Similar results were seen in the GAL4vp16-transduced ASCs, though the cell proliferation was slower and yielded fewer cells at all time points and all MOIs compared to GAL4FF (Fig. 6b).
Discussion
In vivo and ex vivo gene therapy strategies using viral vectors are currently being investigated for use in bone repair applications in orthopedic surgery. In vivo gene therapy approaches target the host’s osteoprogenitors using live viruses carrying the cDNA for osteoinductive growth factors. This strategy is considered less invasive, not as technically challenging, and potentially cheaper, as it accomplishes gene transfer in a one-step approach [2, 19, 20]. However, the greatest limitation of in vivo strategies is that they require endogenous mesenchymal stem/progenitor cells to populate the injury site and serve as local bioreactors, which may not be possible in large bone defects with compromised biology [21, 22]. Moreover, there are safety concerns due to the direct viral inoculation of the host associated with this technique. Due to the limitations of in vivo gene therapy we decided to pursue an ex vivo gene therapy approach as one aspect of a comprehensive tissue engineering strategy to enhance bone repair. The advantage of regional ex vivo gene therapy is that it allows for a specific cell type to be harvested from the patient, transduced in vitro to express the desired osteoinductive growth factor, and then re-implanted in the patient at the area of interest [3, 22]. These transduced cells will not only produce the growth factor that will signal the host’s osteoprogenitors to migrate to the preferred site, but may also differentiate into an osteogenic phenotype through an autocrine effect and participate in bone healing themselves.
A lentivirus was selected in this study for its ability to transduce both dividing and non-dividing cells, the prolonged gene expression and the minimal immunogenicity [4,5,6]. Prior experiments in our laboratory have clearly established the potential of a lentiviral vector carrying the gene for BMP-2 to transduce rodent BMSCs and lead to successful osteogenesis in animal models of ectopic (hind limb muscle pouch) and orthotopic (critical-sized femoral defect, spinal fusion) bone formation [4,5,6, 23,24,25,26]. In order to boost gene expression we started using a lentivirus-based gene delivery system that concurrently employs two different lentiviral vectors: a GAL4 transactivator vector and a G5 transgene expression vector [12, 13]. This TSTA system, under the control of the GAL4vp16 transactivator, allows for transduction of both cultured and freshly isolated rodent [12] and human cells [14], with the levels of BMP-2 produced being 10 times higher compared to the regular LV-BMP-2 vector (725.4 vs. 64 ng/day/mg, respectively, in rat bone marrow cells) [12].
However, Gal4-VP16 has been associated with cell toxicity. In zebrafish studies, high doses of GAL4vp16 were associated with developmental delay or malformation [17, 27, 28]. Moreover, long-term expression of Gal4vp16 at high levels has been reported to cause nonspecific promoter squelching [16, 17], and thus inhibition of certain genes’ transcription due to the unusual avidity with which vp16 interacts with certain components of the transcriptional apparatus [15]. In our study, GAL4-VP16 at MOI of 50 caused significant cell toxicity (cell death: >50% for ASCs, 50–80% for BMSCs) few days post-transduction. Lower MOIs were still associated with viral-induced cell death in GAL4vp16-transduced BMSC, with MOI = 5 killing 15–47% cells and MOI = 25 killing 52–71% cells 7 days post-transduction. Cell death due to viral transduction may hinder long-term transgene expression and could compromise bone repair in challenging large bone defects or biologically stringent environments. Although in our pre-clinical studies we have used the GAL4vp16 vector to successfully heal critical sized femoral defects, it is still critical to address any virus-mediated cytotoxicity.
To overcome the cytotoxic effects associated with GAL4vp16, we assessed a modified, less toxic version of the Gal4 yeast transcription activator, the GAL4FF, as part of a similar TSTA lentiviral system to induce BMP-2 production. As seen by flow cytometric analysis, transduction of BMSCs and ASCs with LV-TSTA-GFP under the control of GAL4FF was associated with high transduction efficiency and high survival rates at MOIs of 5 and 25. Moreover, GAL4FF-mediated lentiviral gene therapy led to long-term BMP-2 production for up to 8 weeks in vitro, with minimal cell death at all time points. Compared to the standard GAL4vp16 vector, GAL4FF was superior with regards to cytotoxicity and overall in vitro gene expression in both BMSCs and ASCs. In BMSC there was a trend towards higher BMP production at 1, 2, 4, 6, and 8 weeks and higher cell viability at all time points compared to GAL4vp16. In ASCs, GAL4FF was still associated with higher BMP-2 production at the later time points (weeks 2–8) and faster cell recovery and proliferation following transduction, but this difference was not as prominent as in BMSCs. This finding suggests that ASCs may be more resistant to the toxic effects of GAL4vp16.
The transduction efficiency and levels of BMP-2 seen in our study when transducing human BMSC and ASCs with the LV-TSTA-GFP or BMP-2 vector under the control of GAL4FF were superior compared to the rates observed with other gene therapy approaches. Adenoviral-mediated ex vivo gene therapy in prior studies was associated with a transduction efficiency of 35% for BMSC/Ad-Lac-Z and 55% for ASC/Ad-Lac-Z at MOI = 25 [29]. Higher BMP-2 production was also noted 48 h following transduction with LV-TSTA-BMP-2 at all MOIs compared to Ad-BMP-2 gene therapy in both human BMSCs and ASCs (reported by Dragoo et al. as 25.5 and 32.7 ng, respectively [29]). Stender et al. described transduction efficiencies ranging from 1% for MOI 1 to 50% for MOI 10,000 in human BMSCs transduced with an AAV2 vector 2 days following transduction [30]. A peak in percentage of GFP-positive cells was observed at 4 days post transduction for MOI ≥ 100, with a dramatic decrease in transgene expression 2 weeks following transduction. This decline of GFP-positive MSCs over time can be explained by the replication deficiency of the AAV vector and by the fact that AAV does not integrate in the host cellular chromosome, which prohibits vertical vector transmission in dividing cell cultures. Prior experiments with double-stranded AAV-BMP-2 and AAV-GFP in our lab also showed the relative inefficiency of the scAAV2 in transducing human bone marrow MSCs and producing any BMP-2 in vitro [31].
To our knowledge, this is the first report of GAL4FF-mediated BMP-2 production by human BMSCs and ASCs. Asakawa et al. [18], were the first to develop and successfully test GAL4FF to improve the currently available GAL4 gene trap constructs in zebrafish. Since then, other laboratories have evaluated GAL4FF for efficacy of gene expression and toxicity in fish models. In brief, transgenic zebrafish and/or medaka embryos have been created through the GAL4FF system to image specific neuronal circuits [32], evaluate the effects of oestrogens in fish early life stages [33], and assess toxicity and teratogenic effects of the bisphenols BPA, BPS, BPF, and BPAF [34].
This study establishes the feasibility of transducing human MSCs with a LV-TSTA system under the control of GAL4FF to enhance BMP-2 production while also minimizing cytotoxicity. Transduction of BMSCs and ASCs with GAL4FF+BMP-2 was associated with long-term transgene expression in vitro with minimal cell death at all time points. Further experiments are needed to evaluate the in vivo bone induction capacity of the GAL4FF+BMP-2 vector and assess whether the superiority of GAL4FF with regards to overall BMP-2 production in vitro translates to more robust bone formation in vivo.
Material and methods
Construction of lentiviral vectors
The LV-TSTA system consists of two different lentiviral vectors: the transactivator vector and the transgene expression vector. Two separate transactivators were constructed for use in this study, (a) GAL4vp16, which is based on the yeast GAL4-DNA-binding domain fused with the herpes simplex virus transcriptional activation domain VP16, and (b) GAL4FF, a fusion of the yeast GAL4 protein and two transcription activation modules (2xPADALDDFDLDML) from VP16. The plasmid for the lentiviral vector containing Gal4vp16 was constructed as previously described [17]. To create pLV-RhMLV-Gal4FF, the Gal4FF gene was amplified by polymerase chain reaction (PCR) from pT2KhspGGFF [18] using a forward primer (5′-GGA TCC GCC ACC ATG AAG CTA CTG TCT TCT AT-3′) and a reverse primer (5′-GTC GAC TTA GTT ACC CGG GAG CAT ATC -3′). The resultant DNA was ligated into pGEM®-T Easy Vectors (Promega, Madison, USA) and the inserted sequence was confirmed. The Gal4vp16 gene was isolated from pLV-RhMLV-Gal4vp16 by BamHI and SalI enzymes, then replaced with Gal4FF. The transgene expression vector, encoding the G5 promoter and BMP-2 or GFP cDNA, was then constructed (LV-G5-BMP2 or LV-G5-GFP) (Fig. 1). GAL4 activates the G5 promoter in the transgene expression vector to amplify the expression of BMP-2 or GFP. Both constructs contain the Rev-responsive element (RRE) and the central polyprine tract (cPPT), which enhance the efficiency of gene expression.
All lentiviral vectors were generated by transfecting 293T cells (American Type Culture Collection, Manassas, VA), as previously described [11, 12]. The titers of both LV-TSTA vectors were determined by quantifying p24 protein concentration in vector supernatant by enzyme-linked immunosorbent assay (ELISA, Quantikine, R&D Systems, Minneapolis, MN, USA).
Cell culture and transduction with lentiviral vectors
After Institutional Review Board approval, de-identified bone marrow and adipose tissue samples, which are normally discarded during elective surgeries, were harvested for use in our study (coded data/specimens study). Bone marrow was acquired from 7 healthy patients (2 female, 5 male), aged 58.9 ± 12.2 years undergoing primary total hip arthroplasty for osteoarthritis of the hip at our institution. Adipose tissue was harvested from 7 healthy patients (6 female, 1 male), aged 47 ± 10.1, following tumescent liposuction of the abdomen, buttock and/or thigh for cosmetic purposes. The samples were processed within 4 h of collection. Standard protocols were used to obtain the mononuclear cell fraction from bone marrow samples and the stromal vascular fraction (SVF) from lipoaspirates [14, 35, 36]. Following isolation, both cell types were expanded in culture for three passages. Briefly, cells were counted using trypan blue and an automated cell counter (BioRad, Hercules, CA). They were then plated in 10 cm plates in DMEM + 10% FBS and maintained in 5% CO2 at 37 °C for the duration of the experiment. Culture medium was replaced every 3–4 days. When adherent cells reached 90–100% confluence, they were passaged at a density of 0.8–1.0 × 106 cells per plate.
After culture-expansion, adipose-derived stem cells (ASCs) and BMSCs were transduced overnight with the LV-TSTA system overexpressing BMP-2 or GFP, under the control of GAL4vp16 or GAL4FF. Transduction with the GFP vectors was used to determine transduction efficiency. Transduction with LV-TSTA-BMP2 was then performed to compare in vitro cell viability and BMP production between the two TSTA-BMP vectors. Transductions were carried out in the presence of 8 μg/ml polybrene at MOIs of 1, 5, 25, and 50 for LV-TSTA-GFP (GAL4FF+G5-GFP or GAL4vp16+G5-GFP) and at MOIs of 5 and 25 for LV-TSTA-BMP-2 (GAL4FF+G5-BMP-2 or GAL4vp16+G5-BMP-2). The cells were washed 24 h after transduction, to remove any extracellular viruses.
GFP expression
In order to determine optimal transduction conditions, ASCs and BMSCs from three different patients (per tissue type) were expanded in culture until they reached passage 3. At that point 10 × 106 cells per donor per cell type were collected for further processing; 8 × 106 cells were transduced with the LV-TSTA-GFP vector (GAL4FF+G5-GFP or GAL4vp16+G5-GFP) at MOIs of 1, 5, 25, or 50, whereas the rest were used as negative control (non-transduced). Following overnight transduction, the cells were washed to remove extracellular viruses and then re-plated in fresh media for additional 24 h. Each well was imaged using a Revolve R4 inverted microscope (Echo, San Diego, CA) to confirm GFP expression. Half of the cells were then trypsinized, counted and resuspended in PBS + 1%FBS at a concentration of 1 × 106 cells/ml and used for fluorescence-activated cell sorting (FACS) analysis (Day 2 post-transduction). The remaining cells were kept in culture for one week and then processed for FACS (Day 7 post-transduction). FACS was performed using a BD LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ) to determine transduction efficiency and MFI of GFP, 2 and 7 days post transduction. Analysis of the raw data was done with Flowjo software (FlowJo LLC, Ashland, OR).
Quantification of BMP-2 production in vitro
Transduction with LV-TSTA-BMP-2 (GAL4FF or GAL4vp16) was used to compare in vitro cell viability and BMP production between the two TSTA-BMP-2 vectors. Three samples per tissue type were used for this aspect of the study. Each sample was run in duplicate. In vitro BMP-2 production over a 24 h period was quantified in culture medium at 2 days, and at 1, 2, 4, 6 and 8 weeks post-transduction using an ELISA assay (Quantikine, R&D Systems, Minneapolis, MN, USA). The amount of BMP-2 produced was reported as ng BMP-2/24 h/ml. Cell numbers were determined at all time points using staining with trypan blue and an automated cell counter. Non-transduced cells were used as control.
Statistical analysis
Statistical analysis was performed using the SPSS statistics software. The distribution of all data was assessed for normality using Shapiro–Wilk test. Equality of variances was also confirmed before performing any further analysis. One-way ANOVA and post hoc analysis with Tukey’s range test were used for between-MOIs comparisons in GFP and BMP-2 transductions and comparisons between non-transduced, GAL4FF-transduced and GAL4vp16-transduced cells. Independent-samples Student t-test was used to compare transduction efficiency, MFI and BMP-2 production between the two vectors. All data are reported as mean ± standard deviation. Significance level was set at 0.05.
References
Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012;64:1331–40.
Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42.
Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des. 2013;19:3466–73.
Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–8.
Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21.
Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–31.
Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. RhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Jt Surg Am. 2010;92:411–26.
Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–10.
Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vector containing factor IX gene: tolerization of factor IX and vector antigens allows for longterm expression. Proc Natl Acad Sci USA. 1995;92:1401–5.
Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–7.
Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–14600.
Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–8.
Alaee F, Bartholomae C, Sugiyama O, Virk MS, Drissi H, Wu Q, et al. Biodistribution of LV-TSTA transduced rat bone marrow cells used for “ex-vivo” regional gene therapy for bone repair. Curr Gene Ther. 2015;15:481–91.
Bougioukli S, Sugiyama O, Pannell W, Ortega BA, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of BMP-2 transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29:507–19.
Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–4.
Gill G, Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–4.
Köster RW, Fraser SE. Tracing transgene expression in living zebrafish embryos. Dev Biol. 2001;233:329–46.
Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA. 2008;105:1255–60.
Franceschi RT, Yang S, Rutherford RB, Krebsbach PH, Zhao M, Wang D. Gene therapy approaches for bone regeneration. Cells Tissues Organs. 2004;176:95–108.
Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–29.
Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Jt Surg Am. 2008;90:99–110.
Oakes DA, Lieberman JR. Osteoinductive applications of regional gene therapy: ex vivo gene transfer. Clin Orthop Relat Res. 2000;379:S101–112.
Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–8.
Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84:93–103.
Pensak M, Hong S, Dukas A, Tinsley B, Drissi H, Tang A, et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther. 2015;22:467–75.
Miyazaki M, Sugiyama O, Tow B, Zou J, Morishita Y, Wei F, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372–9.
Argenton F, Arava Y, Aronheim A, Walker MD. An activation domain of the helix-loop-helix transcription factor E2A shows cell type preference in vivo in microinjected zebrafish embryos. Mol Cell Biol. 1996;16:1714–21.
Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, et al. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–6.
Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–9.
Stender S, Murphy M, O’Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, et al. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–9.
Alaee F, Sugiyama O, Virk MS, Tang Y, Wang B, Lieberman JR. In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing. Genet Vaccin Ther. 2011;9:4.
Muto A, Kawakami K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun Integr Biol. 2011;4:566–8.
Lee O, Tyler CR, Kudoh T. Development of a transient expression assay for detecting environmental oestrogens in zebrafish and medaka embryos. BMC Biotechnol. 2012;12:32.
Moreman J, Lee O, Trznadel M, David A, Kudoh T, Tyler CR. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ Sci Technol. 2017;51:12796–805.
Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;79:e50585.
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9.
Acknowledgements
This work was supported by a National Institutes of Health grant to J.R.L. [R01AR057076]. The GAL4FF plasmid was kindly provided by Dr. Koichi Kawakami, National Institute of Genetics, Japan. The authors would also like to thank Frank Gonsalves of the Keck Hospital of USC and Judy Yoho of Dr. Yoho’s Cosmetic surgery practice for their valuable assistance in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.B., O.S., R.K.A., R.Y., and D.A.O. have no conflicts to report. J.R.L. has received royalties and has served as a paid consultant for Depuy, is a shareholder in Hip Innovation Technologies, Inc. and has received royalties, financial or material support from Elsevier.
Rights and permissions
About this article
Cite this article
Bougioukli, S., Sugiyama, O., Alluri, R.K. et al. In vitro evaluation of a lentiviral two-step transcriptional amplification system using GAL4FF transactivator for gene therapy applications in bone repair. Gene Ther 25, 260–268 (2018). https://doi.org/10.1038/s41434-018-0024-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-018-0024-9
- Springer Nature Limited