Abstract
In order to adapt ex vivo regional gene therapy for clinical applications in orthopaedic surgery, safety issues must be considered. In this study we developed a suicide approach using a dual gene expression two step transcriptional amplification lentiviral vector (LV-TSTA) encoding BMP-2 and an inducible caspase 9 (iC9) system that selectively induces apoptosis upon activation with a chemical inducer of dimerization (CID). Transduction of rat bone marrow stromal cells (RBMSCs) with LV-TSTA-iC9/BMP-2 led to abundant BMP-2 production (90.3 ± 7.9 ng/24 h/106 cells) in vitro and stimulated bone formation in a mouse muscle pouch in the absence of CID. Moreover it was shown that CID could be used to selectively induce apoptosis in iC9-transduced cells both in vitro and in vivo. Double exposure to serial dilutions of CID decreased in vitro production of BMP-2 by 85–87% and Luc activity by 97–99% in iC9/BMP-2 or iC9/Luc-transduced cells respectively. Early administration of CID (Days 0–1 post-op) in mice implanted with iC9/BMP-2-transduced RBMSCs was effective in blocking bone formation, indicating that CID was toxic to the transduced cells. In iC9/Luc-implanted mice, late administration of two doses of CID (Days 27–28 post-op) significantly reduced the luciferase signal. The current study provides proof of concept for the potential clinical application of regulated gene therapy to promote bone repair.
Similar content being viewed by others
Introduction
Multiple studies have demonstrated the potential of ex vivo regional gene therapy using a lentiviral vector encoding for bone morphogenetic protein-2 (BMP-2) to enhance osteogenesis in several animal models of bone repair [1, 2], suggesting a potential critical role in the future treatment of difficult bone repair scenarios. However, insertional mutagenesis and emergence of replication competent viral particles, though rare, remain areas of concern with respect to lentiviral vectors [3]. Moreover prolonged release of BMP-2 could be associated with potential adverse effects, such as heterotopic bone formation and soft tissue edema [4]. Since gene therapy would be used to treat non-lethal conditions, any increase in morbidity or mortality would not be acceptable. Thus, developing a system to regulate BMP-2 production could be a useful tool.
Caspase 9 is a late-stage apoptosis pathway molecule that selectively induces apoptosis in cells expressing iC9 upon activation with a chemical inducer of dimerization (CID) [5]. Inducible caspase-9 (iCasp9) is a system that has shown potential in both in vitro [6,7,8] and in vivo experiments [8,9,10] for different applications. This system is currently being used as a suicide gene therapy approach in multiple clinical studies for graft versus host disease (GVHD) [11].
In this study we aimed to develop a suicide approach using a dual gene expression lentiviral vector encoding BMP-2 or Luciferase (Luc) and iC9 to enhance the safety of ex vivo gene therapy for bone repair. We hypothesized that bone marrow cells transduced with this vector would produce sufficient BMP-2 to induce bone formation despite the presence of iC9. We also hypothesized that CID administration could regulate BMP production and would eradicate the implanted transduced cells once the bone is healed, thus minimizing the risks of excessive BMP-2 production and potential insertional mutagenesis associated with the use of lentiviral vectors.
Results
BMP-2 production by LV-TSTA-iC9/BMP-2 transduced RBMSCs
Rat bone marrow stromal cells were transduced with either the new lentiviral vector that concomitantly produces BMP-2 and the iC9 suicide gene (LV-TSTA-ic9/BMP-2), or our standard two-step transcriptional amplification lentiviral vector expressing BMP-2 alone (LV-TSTA-BMP-2) as previously described [12,13,14,15]. Transduction with LV-TSTA-IC9/BMP-2 led to in vitro BMP-2 production as seen by ELISA 48 h post-transduction. At an MOI of 25, 1 × 106 LV-TSTA-iC9/BMP-2 transduced RBMSCs produced 105.77 ± 17.22 ng of BMP-2 in a 24 h period, whereas the same number of LV-TSTA-BMP-2 transduced RBMSCs produced 252.47 ± 7.04 ng. Thus, the LV-TSTA-iC9/BMP-2 vector produced 42% less BMP-2 compared to the cells transduced with our regular TSTA vector under the same culture conditions (p < 0.05). Minimal BMP-2 production was noted in non-transduced cells (0.87 ± 0.68 ng BMP-2) (Fig. 1).
In vitro BMP-2 production over a 24-h period by RBMSCs transduced with LV-TSTA-iC9/BMP-2 or LV-TSTA-BMP-2 at an MOI of 25 (p < 0.05 between the two). The results are presented as ng of BMP-2/24 h per 1 × 106 cells. Non-transduced cells were used as negative control. Results given as mean ± SD. *p < 0.05
Detection of iC9 transgene in transduced RBMSCs
Analysis with quantitative RT-PCR successfully identified the iC9 transgene in the genome of LV-TSTA-iC9/BMP-2 and LV-TSTA-ic9/Luc transduced RBMSCs. The average number of viral copies per cell was 90.95 ± 13.94 copies/cell for iC9/BMP-2-transduced RBMSCs and 111.94 ± 5.62 copies/cell for iC9/Luc-transduced cells. In contrast, no iC9 copies were identified in non-transduced cells.
In vitro regulation of BMP-2 production
Exposure to one or two doses of CID, at dilutions ranging from 0.1 to 1000 nM, led to a decrease in the BMP-2 production in the treated iC9/BMP-2 transduced RBMSCs. (Fig. 2a) Exposure to a single dose of CID decreased the expression of BMP-2 by 48–58%, compared to 85–87% with 2 doses of CID. When comparing the administration of different doses of CID to each other, no dose seemed to be superior; all of the CID doses (0.1–1000 nM) were equally effective in reducing the BMP-2 production (p > 0.05).
In vitro regulation of BMP-2 production when administering 1 or 2 doses of CID on RBMSCs transduced with either LV-TSTA-ic9/BMP-2 (a) or LV-TSTA-BMP-2 (b). Serial dilutions (0–1000 nM) of CID were tested. (a) In LV-TSTA-iC9/BMP-2 transduced cells, a single dose of CID (0.1–1000 nM) decreased BMP-2 production by 48–58%. The addition of a second CID dose (0.1–1000 nM) resulted in a 85–87% decrease in BMP-2 production. (b) In cells transduced with our standard vector (LV-TSTA-BMP-2), 0.1–10 nM of CID had no effect on the viability of the cells , whereas 100–1000 nM led to a significant decrease in BMP-2 production. The results are presented as ng of BMP-2 per 1 × 105 cells. BMP-2 was measured 24 h after the last dose of CID
The same assays were done on RBMSCs transduced with our regular LV-TSTA-BMP-2 vector, to determine the impact of CID on cells that do not overexpress caspase 9. Both treatment plans and serial dilutions (0–1000 nM) of AP1903 were tested. 0.1–10 nM of CID (1 or 2 doses) had no effect on cells transduced with our regular vector. However, the higher doses (100 and 1000 nM) of CID led to a significant decrease in BMP-2 production compared to the cells that did not receive CID (single dose group, p < 0.001 and double dose group, p ≤ 0.01). (Fig. 2b) This finding suggests that high doses of CID may be toxic to RBMSCs.
In vitro effect of CID on LV-TSTA-iC9/Luc transduced cells
Similar results were obtained with the use of CID on cells transduced with the LV-TSTA-iC9/Luc vector. RBMSCs transduced with the LV-TSTA-iC9/Luc vector produced a Luc signal that declined with 1 or 2 doses of CID. Exposure to a single CID dose decreased luciferase expression by 93–97%, whereas double CID treatment led to a 97–99% decrease in the relative Luc activity. (Fig. 3)
Early administration of CID: In vivo inhibition of bone formation
The osteoinductive potential of the LV-TSTA-ic9/BMP-2 transduced cells and their ability to respond to CID in vivo was then tested in a severe combined immune deficient (SCID) mouse hind limb muscle pouch. Following implantation the mice were injected with either CID (Group I) or PBS (Group II) on post-op days 0–1 (Table 1). Since 2 doses of CID resulted in more significant decreases in BMP-2 production in vitro, we decided to test the double CID treatment plan in the animal model of heterotopic bone formation.
Robust heterotopic bone formation was seen 2 and 4 weeks post-operatively in the mice implanted with iC9/BMP-2 transduced cells and injected with PBS. In contrast minimal or no bone formation was noted in animals treated with iC9/BMP-2 and injected with CID right after the implantation (Fig. 4a). MicroCT imaging and subsequent analysis of newly formed bone within the muscle pouch revealed a significantly higher bone volume at 4 weeks in mice treated with PBS versus mice injected with CID (9.98 ± 4.9 vs 2.09 ± 1.7 mm3, p = 0.024) (Fig. 4b). This difference was also confirmed with histologic and histomorphometric analysis of Masson’s Trichrome stained slides (Fig. 5).
Imaging analysis in Group I and II animals. (a) X-rays. Robust heterotopic bone formation is seen in iC9/BMP-2 implanted mice injected with PBS, whereas minimal/no bone formation is noted in animals treated with iC9/BMP-2 and injected with CID at the time of implantation. (b) Micro-CT. A significantly higher bone volume was noted at 4 weeks in mice treated with PBS versus mice injected with CID, based on microCT volumetric analysis
Histologic analysis in Group I and II animals. Mason’s Trichrome stained sections show robust bone formation in animals treated with PBS immediately post-implantation versus no bone in the CID group (a). Histomorphometry confirmed the qualitative results, demonstrating a significantly higher BA and BA/TA in PBS vs CID treated animals (b). *p0.015, +p 0.002
The effect of CID treatment on bone formation
In clinical scenarios, CID could be given after bone repair had been completed. Thus it was important to determine whether administration of CID after bone formation would affect the quality of newly formed bone. In Group II, all mice demonstrated bone formation as seen in both X-rays and CT analysis, regardless of CID administration (Fig. 6). No differences with regards to bone formation were noted in the histologic and histomorphometric analyses (Fig. 7).
In vivo killing effect on ic9/Luc transduced cells by delayed CID administration
In iC9/Luc treated animals, in vivo bioluminescent imaging detected strong in vivo Luc expression 4 weeks following cell implantation. After confirmation of Luc expression in vivo, 2 injections of CID (8 uL) were given in 24 h intervals. Twenty-four hours after the second injection the mice were imaged again; a significant decrease of in vivo luciferase signal was noted after two injections of CID. In contrast no or minimal change in Luc expression was seen in control mice injected with PBS. (Fig. 8).
In vivo bioluminescence imaging of muscle pouch implanted with RBMSCs transduced with LV-TSTA-iC9/Luc. Strong in vivo Luc expression is seen 4 weeks following implantation. In vivo Luc signal is significantly decreased after 2 inj of CID (a), whereas no significant change is noted in Luc expression in control mice injected with PBS (b). The results were confirmed with quantification of the signal (c). *p < 0.05 compared to signal prior to injection, +p > 0.05 vs prior to injection
Discussion
The efficacy of lentivirally-mediated BMP delivery for pre-clinical bone repair scenarios has been well established in the literature by multiple pertinent animal studies from our laboratory and others [12, 16,17,18,19]. Although our lentiviral gene therapy strategy shows great promise for use in humans, concerns have been raised regarding the potential adverse effects of prolonged BMP production associated with the use of lentiviral vectors, including but not limited to heterotopic ossification, soft tissue edema, and wound healing issues [20, 21]. In addition, lentiviral vectors generally integrate into the host genome and are associated with a potential limited risk of insertional mutagenesis [3].
Because of these possible safety concerns, we decided to evaluate a suicide gene therapy strategy by adding the inducible caspase 9 gene, which activates the mitochondrial apoptotic pathway, to our lentiviral construct. To this end, we created a dual-gene expression lentiviral system that can concomitantly yet independently express BMP-2 and the suicide gene iC9 to enhance the safety of ex vivo regional gene therapy for bone repair applications. We demonstrated that RBMSCs transduced with LV-TSTA-iC9/BMP-2 could successfully produce BMP-2 in vitro and stimulate osteogenesis in a mouse muscle pouch model, in the absence of CID.
Ic9 was selected for use in our study due to its distinct mechanism and speed of action. Caspase 9 is a part of the intrinsic apoptotic pathway; damaged mitrochondria release cytochrome C, which in turn activates caspase 9 and triggers the apoptosis cascade [22]. As a suicide gene therapy strategy, this system uses a protein (iC9) and a ligand (CID) to selectively induce apoptosis in cells expressing iC9. In the absence of CID, iC9 is inactive. Administration of the ligand will activate apoptosis by binding to the CID binding domain of iC9 [23]. The utility of this system is that iC9 can be linked to other proteins, in this case BMP-2, in order to regulate their expression. Administration of CID allows for the targeted degradation of iC9 expressing cells. Furthermore, compared to other methods that interfere with DNA synthesis, CID has been shown to activate the endogenous apoptotic pathway within minutes following administration, thus leading to rapid cell death without the need for prolonged treatment [24].
In our study we first assessed the impact of CID-activated iC9 in two in vitro experiments using RBMSCs transduced with the iC9/Luc or iC9/BMP LV. In both the Luc and BMP groups, double exposure to CID led to a significant decrease in the production of either Luc (98%) or BMP (87%) in vitro. Complete elimination of the iC9 transgene-expressing cells was not achieved. This finding is consistent with prior reports in the literature, with cell/signal elimination rates ranging from 80 to 96% even after higher doses of CID or repeated administration [23,24,25,26,27]. It has been hypothesized that there may be a small population of iC9 + cells that are resistant to CID. Factors that may be associated with this phenomenon include viral integration sites that favor low gene expression, transgene silencing by promoter hypermethylation, or sporadic nonsense mutations of the iC9 transgene [23, 27]. In any case, clinical trials using the iC9 system for GvHD have demonstrated prompt resolution and long-term lack of recurrence of GVHD, even in the presence of that residual resistant cell population [24,25,26].
In vivo, CID was also able to adequately eliminate the expression of the transgene. Early administration of CID in mice implanted with iC9/BMP-2-transduced RBMSCs was effective in blocking bone formation, indicating that CID induced apoptosis of the BMP-2 transduced cells. We also tested whether administration of CID after bone formation would affect the newly formed bone. This was done to mimic the use of the iC9 system in clinical scenarios, where CID would be given to selectively remove the implanted transduced cells after bone healing had been achieved. The RBMSCs transduced with the LV-TSTA-iC9/BMP-2 vector induced bone formation in the muscle pouch model at 2 weeks. Delayed CID administration had no impact on the quantity of bone formation at 4-weeks after cell implantation.
IC9/CID has been evaluated for clinical use in patients with acute GVHD following stem cell transplantation for hematologic malignancies and patients with solid tumors. Di Stasi et al. [24] and Zhou et al. [25] reported successful use of iC9 in children with recurrent acute leukemia or haploidentical transplants respectively. CID-induced activation of iC9 lead to ~90% eradication of iC9-expressing T cells in vivo within 2 h of administration, and controlled GVHD within 24–48h [28]. In addition, two phase 1 clinical trials in patients with sarcoma [29] or refractory neuroblastoma [30] are currently underway to assess the safety of transduced T-cells expressing GD-2 chimeric antigen receptor (CAR) and ic9.
However, limited studies have been done with regards to evaluating iC9 suicide gene therapy for tissue engineering applications. In the study by Ramos et al. [8], subcutaneously injected transduced MSCs were successfully selectively killed with CID (AP20187), without any adverse effects in a mouse model. In a more recent study, MSCs previously transduced with iC9, were genetically modified to express BMP-2 via an adenoviral vector [10]. Administration of CID led to a decrease in cell viability and BMP-2 production, but total eradication of the transduced cells was not achieved. Intraperitoneal injection of a single dose of CID effectively blocked osteogenesis when given early in the bone formation process. However, bone formation was only assessed at 10 days following implantation and thus it was not possible to determine whether osteogenesis in this model was truly eliminated or just delayed, especially since 25% of the transduced cells persisted following CID treatment in vitro. Furthermore, the percentage of residual transduced cells in that study [9] was higher compared to other studies that have utilized iC9. It is not clear whether the survival of this cell population was a result of low transduction efficiency associated with the use of Ad-iC9/BMP or inadequate administration of CID. Finally, due to the short duration of survival of adenovirally-transduced cells and associated gene expression (~2 weeks in vivo) [31] it is not clear why a suicide safety switch would be deemed necessary for an adenoviral gene therapy approach.
In our study, a TSTA lentiviral vector and double exposure to CID were used leading to a significant decrease in both Luc activity (98%) and BMP production (87%) in vitro. This decrease translated to complete elimination of the Luc signal and inhibition of bone formation in vivo as seen with imaging and histomorphometric analysis 4 weeks following implantation. To our knowledge this is the first study that attempts to insert the iC9 gene in a lentiviral vector for any gene therapy application. Since lentivirally-mediated BMP-2 delivery for bone repair is associated with a prolonged, stable production of BMP-2 for more than 12 weeks [31, 32] the use of the caspase 9 system could blunt the potential for BMP-2 associated adverse effects and eliminate any conerns for potential vector associated mutagenesis.
In conclusion, the current study provides proof of concept for potential clinical applications of regulated gene therapy for bone repair. Using a lentiviral TSTA system we successfully introduced BMP-2 and inducible caspase 9 in rat bone marrow MSCs, leading to abundant expression of BMP-2 and iC9 as seen on ELISA and qPCR respectively. We also demonstrated that RBMSCs transduced with the BMP-2 containing vector retain their ability to stimulate bone formation in vivo in spite of caspase 9 co-transduction, in the absence of CID. Administration of 2 doses of CID was able to selectively induce apoptosis in cells expressing iC9/BMP-2 or iC9/Luc in vitro and in vivo. Introducing a “safety switch” to allow a treating physician to eradicate implanted cells once they are no longer needed could enhance the safety and feasibility of ex vivo gene therapy. Further experiments are needed to assess this strategy in a more stringent model of orthotopic bone formation.
Materials and methods
Vector construction
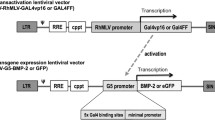
We constructed a dual gene expression two step transcriptional amplification lentiviral system (LV-TSTA) encoding BMP-2 or Luciferase (Luc) and inducible caspase 9 (iC9). A TSTA system was used since it has been shown to significantly increase gene expression [12, 13]. This system requires co-transduction with two different lentiviral vectors to lead to production of the protein of interest: the transactivator vector GAL4vp16 and the transgene expression vector, encoding the G5 promoter and the transgene (BMP-2 or Luc in this case). The iC9 transgene was inserted in both vectors.
In brief, the iC9 transgene was linked through a T2A peptide sequence with the GAL4 transcriptional activator, as well as the transgene BMP-2 or firefly luciferase (Luc). The iC9-T2A-GAL4 cDNA was created by overlap extension PCR using the pMSCV-F-del Casp9.IRES.GFP (Addgene, Cambridge, MA) and pLV-RhMLV-GAL4 plasmids as template. The PCR amplicom was cloned into a commercial T vector system, pGEM-T Easy (Promega, Madison, WI) for DNA sequencing. After confirmation of no point or frame shift mutations in the PCR amplicoms, the iC9-T2A-GAL4 cDNA fragment was isolated from pGEM-T-Easy by restriction enzymes and inserted into a lentiviral backbone plasmid containing the RhMLV promoter (named LV-RhMLV-iC9/GAL4). (Fig. 9) The iC9-T2A-BMP2 and iC9-T2A-Luc cDNAs were created in the same way. The plasmids of pcDNA3.1-BMP2 or pG5-luc (Promega) were used for overlap extension PCR instead of pLV-RhMLV-GAL4. The iC9-T2A-BMP2 or iC9-T2A-Luc were inserted downstream of the GAL4 responsive minimal promoter, namely G5, in the lentiviral backbone plasmid (named LV-G5-iC9/BMP2 or LV-G5-iC9/Luc).
All lentiviral vectors were generated by transfecting 293T cells (American Type Culture Collection, Manassas, VA), under conditions described on a previously established protocol [12,13,14]. The titers of LV-TSTA vectors were determined by quantifying p24 protein contents in vector solution by enzyme-linked immunosorbent assay (ELISA).
Cells and viral transduction
Rat bone marrow stromal cells (RBMSCs) were isolated from the intramedullary canal of femora and tibiae of 8-week-old male Lewis rats (Charles River, San Diego, CA). The whole bone marrow cell pellet was re-suspended with Iscove’s modified Dulbecco’s media (IMDM) (ThermoFisher Scientific, Walthman, MA, USA) supplemented with 15% Fetal Bovine Serum (Seradigm FBS, VWR Life Science, Radnor, PA, USA), penicillin (100 U/ml) and streptomycin (100 mg/ml), and plated on a 10-cm dish. RBMSCs were cultured for 2 weeks at 5% CO2, 37 °C until passage 3. Non-adherent cells were removed by aspiration when changing media. Passage 3 RBMSC were co-transduced overnight with LV-RhMLV-iC9/GAL4 and LV-G5-iC9/BMP2 or LV-G5-iC9/Luc at a multiplicity of infection of 25 for each vector based on a previously established protocol. All viral transductions were carried out at 37 °C, 5% CO2, in the presence of 8 μg/ml polybrene (Sigma, St. Louis, MO, USA).
In vitro assessment of gene expression
Two treatment plans (single or double dose) and serial concentrations (0.1, 1, 10, 100 or 1000 nM) of CID (AP1903, APEX BIO, Houston, TX) were tested. The first dose of CID was administered right after the overnight viral transduction. The second dose was given 24 h following the first one. Assessment of BMP-2 production or luciferase expression was carried out 24 h after the addition of CID.
Luciferase assay
The luciferase activity was measured by a Luciferase Assay System (Promega, Madison, WI), according to the manufacturer’s protocol. In brief, after overnight viral transduction, the cells were split onto a 96-well assay plate (flat clear bottom black plate, Corning Inc., Corning, NY) at a density of 5x103 cells per well. The transduced cells were incubated at 37 °C for 24 h in media containing serial concentrations (0.1, 1, 10, 100 or 1000 nM) of the CID. Half of the cells were used for the luciferase assay (single dose group). The rest of the cells were treated with a second CID dose (0.1, 1, 10, 100 or 1000 nM) after the initial incubation, then cultured for another 24 h before the luciferase assay (double dose group). Luminescence was analyzed using a microplate reader (Synergy H1 Multi-Mode Reader, BioTek, Winooski, VT). Relative luciferase activity was expressed as the ratio of luminescence of AP1903 treated cells to that of untreated cells.
Quantification of in vitro BMP-2 production
RBMSCs transduced with LV-TSTA-ic9/BMP-2 were incubated with fresh media (with and without CID) in a 6 well plate for a 24 h period following overnight transduction. After 24 h, the culture supernatant was harvested for evaluation of BMP-2 production and a second dose of fresh medium (with and without CID) was added to the cells for an extra 24 h incubation. Each sample was run in triplicate. Non-transduced cells and cells transduced with the regular LV-TSTA-BMP-2 were used as controls. The BMP-2 levels in culture supernatant were quantified by a commercially available ELISA kit (BMP-2 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The assay results were standardized by cell number and reported as nanograms of BMP-2/day per 1 × 106 cells.
Detection of iC9 transgene
Genomic DNA was extracted from a known number of RBMSCs transduced with either LV-TSTA-ic9/BMP-2 or LV-TSTA-ic9/Luc, 48 h post-transduction. Real-time polymerase-chain-reaction (PCR) was then performed to detect and quantify the number of copies of the iC9 transgene in each cell, as previously described [24, 25]. Non-transduced RBMSCs and cells transduced with LV-TSTA-BMP-2 were used as negative control.
Animal model
After approval from the Institutional Animal Care and Use Committee (IACUC), a hind limb muscle pouch was created unilaterally on 8–10 week old, female, NOD scid gamma mice (NSG, Jackson laboratory, Bar Harbor, ME). Briefly, after inhalation induction with isoflurane, the left hind limbs of mice were shaved and aseptically prepared for surgery. An incision was created on the posteromedial aspect of the thigh, and an intermuscular plane was defined between the hamstring muscles using blunt dissection. A collagen sponge (HELISTAT® Absorbable Collagen Hemostatic Sponge, Integra Lifesciences CO, Plainsboro, NJ USA) loaded with 2 × 106 RBMSCs transduced with LV-TSTA-ic9/BMP-2 or LV-TSTA-ic9/Luc was implanted in the muscle pouch. Following the surgery, the mice received proper analgesia subcutaneously, antibiotics in drinking water and were allowed to walk, eat and drink ad libitum.
A total of 30 mice were randomized into 6 treatment groups (Table 1). All of the animals received two intraperitoneal (ip) injections of 8 μL CID (0.4 mg/kg) or PBS. Group I and II animals were injected right after cell implantation (Days 0–1) to assess CID’s ability to block bone formation in vivo. Group III and IV animals received the injections 2 weeks after the surgical procedure (Days 14–15) to evaluate the effects of CID on the quality of newly formed bone. Finally, Groups V and VI were used as a surrogate to assess late administration of CID or PBS (Days 27–28) and the transduced cells’ ability to respond to CID in vivo.
Imaging analysis
Bone formation was evaluated with plain radiographs and microcomputed tomography (microCT). Radiographs were taken at 2 and 4 weeks post-operatively, using Ultrafocus60 (Faxitron Bioptics, Tucson, AZ), with animals anesthetized with 2% isoflurane and oxygen. MicroCT scans were performed post-euthanasia (4 weeks post-op) for Groups I-IV, on the Inveon Multimodality System (Siemens Medical Imaging, USA, Knoxville, TN). Acquisition and reconstruction were performed using the Inveon Acquisition Workplace software (Siemens Medical Imaging, USA, Knoxville, TN). The variable-focus source was set to 80kVp, 500uA with 800 ms exposures and a 0.5 mm aluminum filter. The 3072 × 2048 pixel detector was set to full CCD, binx2, medium magnification with 720 projections covering 360 degrees to scan an axial length of 35 mm. Scans were reconstructed with mouse beam hardening correction to produce 53 micron isotropic voxel resolution images. MicroCT data was visualized, segmented and quantified using Amira software (FEI Company). Volumes of interest were drawn throughout the muscle pouch region using the Image Segmentation Editor. Using a histogram based bone threshold within the muscle pouch volume, bone tissue was segmented and bone tissue volumes (BV) reported.
In vivo Luc expression was detected using in vivo bioluminescent imaging with a Xenogen-IVIS CCCD optical system (Xenogen IVIS, Alameda, CA, USA). The assay was performed twice for each mouse; once at Day 27 to confirm Luc expression and the second time at day 29, following two injections of CID or PBS. The mice were anesthetized with 2% isoflurane for the duration of the assay, and were injected with luciferin i.v. (15 mg/kg) 1 min before the imaging itself.
Histologic and histomorphometric analyses
Following euthanasia and imaging analysis, the muscle pouches were harvested for further processing for histology and histomorphometric analysis. The specimens were fixed in 10% formalin for 3–4 days at 4 °C, and then decalcified in Ethylenediaminetetraacetic acid (EDTA) for 2 weeks. Finally they were embedded in paraffin, then cut and loaded on glass slides. Slides were stained with either Hematoxylin and eosin or Masson’s Trichrome stain and imaged with AxioImager A2 (Carl Zeiss Microscopy, LLC, Thornwood, NY). Quantitative histomorphometric analysis was performed on Masson’s trichrome sections at ×1magnification with the Bioquant software (Bioquant Image Analysis, Nashville, TN). The region of interest was selected to include the whole implant in the muscle pouch and represented the tissue area (TA). The amount of new bone formed within this area was then quantified (Bone area-BA), and the BA/TA ratio calculated. All techs performing microCT and post-imaging analysis, histomorphometry and in vivo luciferase imaging were completely blinded to which group was being assessed.
Statistical analysis
A power analysis was first performed. The power analysis was designed to detect differences in bone volume between animals implanted with iC9/BMP-2 transduced cells and injected with PBS or CID. Assuming a pooled standard deviation of 4 units, the study would require a sample size of 5 for each group to achieve a power of 80% and a level of significance of 5% (two sided), for detecting a true difference in means between the test and the reference group of 7.5 units.
Data were assessed for normality using Shapiro-Wilk test. Equality of variances was also confirmed. One-way ANOVA and post hoc analysis with Tukey’s range test were used to compare the effect of the different doses of CID in the in vitro production of BMP or Luc. Independent-samples student t-test was performed to compare bone formation in CID versus PBS treated animals in groups I and II,. Paired samples t test was used to compare Luciferase expression in Group III. Statistical analysis was performed with SPSS Statistics 22. The significance was set at p < 0.05 for all comparisons. Results are reported as mean ± standard deviation.
References
Bougioukli S, Evans C, Alluri R, Ghivizzani S, Lieberman J. Gene therapy to enhance bone and cartilage repair in orthopaedic surgery. Curr Gene Ther. 2018;18:154–70.
Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42.
Bokhoven MC. Measurement of insertional mutagenesis by retroviral and lentiviral vectors. Doctoral thesis. University of London; 2008.
Garrison KR, Shemilt I, Donell S, Ryder JJ, Mugford M, Harvey I, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950.
Fan L, Freeman KW, Khan T, Pham E, Spencer DM. Improved artificial death switches based on caspases and FADD. Hum Gene Ther. 1999;10:2273–85.
Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–54.
Yagyu S, Hoyos V, Del Bufalo F, Brenner MK. An inducible caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol Ther. 2015;23:1475–85.
Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–15.
Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–24.
Alvarez-Urena P, Zhu B, Henslee G, Sonnet C, Davis E, Sevick-Muraca E, et al. Development of a cell-based gene therapy approach to selectively turn off bone formation. J Cell Biochem. 2017;118:3627–34.
Zhou X, Di Stasi A, Brenner MK. iCaspase 9 suicide gene system. Methods Mol Biol. 2015;1317:87–105.
Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–8.
Bougioukli S, Sugiyama O, Pannell W, Ortega B, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of BMP-2 transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29:507–19.
Alaee F, Bartholomae C, Sugiyama O, Virk MS, Drissi H, Wu Q, et al. Biodistribution of LV-TSTA transduced rat bone marrow cells used for “ex-vivo” regional gene therapy for bone repair. Curr Gene Ther. 2015;15:481–91.
Vakhshori V, Bougioukli S, Sugiyama O, Tang A, Yoho R, Lieberman JR. Cryopreservation of human adipose-derived stem cells for use in ex vivo regional gene therapy for bone repair. Hum Gene Ther Methods. 2018 Oct 25. https://doi.org/10.1089/hgtb.2018.191. [Epub ahead of print]
Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–8.
Pensak M, Hong S, Dukas A, Tinsley B, Drissi H, Tang A, et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther. 2015;22:467–75.
Guan J, Zhang J, Zhu Z, Niu X, Guo S, Wang Y, et al. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther. 2015;6:5.
Qing W, Guang-Xing C, Lin G, Liu Y. The osteogenic study of tissue engineering bone with BMP2 and BMP7 gene-modified rat adipose-derived stem cell. J Biomed Biotechnol. 2012;2012:410879.
Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91.
Woo EJ. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin Orthop Relat Res. 2013;471:1707–1011.
Chen M, Orozco A, Spencer DM, Wang J. Activation of initiator caspases through a stable dimeric intermediate. J Biol Chem. 2002;277:50761–7.
Zhou X, Brenner MK. Improving the safety of T-Cell therapies using an inducible caspase-9 gene. Exp Hematol. 2016;44:1013–9.
Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83.
Zhou X, Di Stasi A, Tey SK, Krance RA. Martinez C1, Leung KS et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood. 2014;123:3895–905.
Zhou X, Dotti G, Krance RA, Martinez CA, Naik S, Kamble RT, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125:4103–13.
Zhou X, Naik S, Dakhova O, Dotti G, Heslop HE, Brenner MK. Serial Activation of the inducible caspase 9 safety switch after human stem cell transplantation. Mol Ther. 2016;24:823–31.
Iuliucci JD1, Oliver SD, Morley S, Ward C, Ward J, Dalgarno D, et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol. 2001;41:870–9.
Kaplan RN. National Cancer Institute (NCI). A phase I trial of T cells expressing an anti-GD2 chimeric antigen receptor in children and young adults with GD2+Solid tumors. https://clinicaltrials.gov/ct2/show/NCT02107963?term=Caspase&cond=Sarcoma&rank=1. Accessed 17 Jun 2018.
Heczey A. 3rd generation GD-2 chimeric antigen receptor and icaspase suicide safety switch, neuroblastoma, GRAIN (GRAIN). https://clinicaltrials.gov/ct2/show/NCT01822652. Accessed 17 Jun 2018.
Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21.
Sugiyama O1, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–8.
Acknowledgements
This work was supported by a National Institutes of Health grant to JRL [R01AR057076]. The authors would like to thank Ryan Park and Ivetta Vorobyova of the USC Molecular Imaging Center for their invaluable contribution in microCT and in vivo bioluminescent imaging. We would also like to thank Amy Tang for the histology sections and staining. Finally, special thanks to Dr. David Spencer for his guidance on the iC9/CID system.
Author information
Authors and Affiliations
Contributions
SB participated in project development, and conducted the animal surgeries, plain radiographs, histomorphometry, data analysis and interpretation, and manuscript preparation. VV participated in project development, assisted in animal surgeries and imaging, data interpretation, and manuscript editing. BO participated in project development, generated the vectors, performed the in vitro experiments and edited the final manuscript. OS designed and generated the vectors, performed the transductions and in-vitro experiments, and edited the final manuscript. JRL designed and supervised the study, interpreted the results and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JRL has received royalties and has served as a paid consultant for Depuy, is a shareholder in Hip Innovation Technologies, Inc and has received royalties, financial or material support from Elsevier. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bougioukli, S., Vakhshori, V., Ortega, B. et al. Regulated ex vivo regional gene therapy for bone repair using an inducible caspase-9 suicide gene system. Gene Ther 26, 230–239 (2019). https://doi.org/10.1038/s41434-019-0069-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0069-4
- Springer Nature Limited