Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a major global health problem. The most common cause of death in these patients is due to cardiovascular disorders. The aim of this study was to examine the effects of curcumin supplementation on cardiovascular risk factors in patients with NAFLD.
Methods and materials
In this randomized, placebo-controlled, clinical trial, fifty two patients with NAFLD were randomly assigned to receive life style recommendations plus either 1500 mg curcumin or placebo for 12 weeks. Anthropometric indices, blood lipid profile, insulin resistance, as well as hepatic steatosis and fibrosis scores were measured at the beginning and the end of the study, and compared between and within groups.
Results
Hepatic fibrosis, serum cholesterol, glucose and alanin aminotransferase (ALT) reduced significantly only in curcumin group (p < 0.05). Anthropometric indices, blood lipid profile, insulin resistance, and hepatic steatosis decreased significantly in both groups (p < 0.05), without any significant difference between two groups.
Conclusion
Our results showed that daily intake of 1500 mg curcumin plus weight loss is not superior to weight loss alone in amelioration of cardiovascular risk factors in patients with NAFLD. Further studies with different dosages of curcumin are needed to be able to conclude about the effects of this dietary supplement on cardiovascular risk factors and NAFLD characteristics.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the world that causes several morbidities and mortalities [1,2,3]. NAFLD encompasses a wide spectrum of disorders from simple steatosis to steatohepatitis, which can progress to life-threatening states including cirrhosis and hepatocellular carcinoma [4, 5]. The disease pathophysiology is related to some metabolic disorders such as obesity and type 2 diabetes [5, 6]. Currently, the multiple hit model is an accepted hypothesis in the pathophysiology of NAFLD, indicating that insulin resistance, obesity, environmental or nutritional factors, gut microbiota, genetic and epigenetic factors are involved in the development of this disease [7]. Today, a definitive pharmacological treatment has not yet been approved for the treatment of NAFLD. Neither anti-inflammatory nor anti-oxidants are yet the treatment of choice for NAFLD [8,9,10,11].
Curcumin is one of the active components of turmeric, which is isolated from the plant Curcuma longa Linn, and used as a spice and dye in food preparation [5, 12, 13]. In the late twentieth century, curcumin was recognized as an active agent for most of the biological activity of turmeric [14]. The World Health Organization (WHO) research committee approved it as an additive for daily intake of up to 3milligramsper kilogram of body weight [15].
Antioxidative and anti-inflammatory properties of this substance have been observed in a number of studies [16,17,18,19,20]. In vivo and in vitro studies have shown that curcumin inhibits the activation of hepatic stellate cells through blocking leptin signaling, regulating intracellular glucose and lipid metabolism, as well as balancing formation and degradation of the extracellular matrix of the liver [21]. It has been shown that curcumin has anti-inflammatory, anti-fibrotic, and anti-hyperlipidemic effects in experimental models of NAFLD [22,23,24].
Considering the small number of randomized clinical trial investigating the effects of supplementation with curcumin in patients with NAFLD [25,26,27], and limitations of these studies, we aimed to investigate the effects of curcumin supplementation on lipid profiles, liver enzymes and hepatic steatosis and fibrosis in patients with NAFLD in a randomized clinical trial.
Materials and methods
Ethical consideration
All participants signed the consent form after receiving an explanation of the study protocol. The research protocol was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute at Shahid Beheshti University of Medical Sciences (IR.SBMU.NNFTRI.1395.106).
Study design and participants
This study was a placebo-controlled, double-blind, randomized clinical trial to evaluate the effects of curcumin on NAFLD patients.
The sample size was calculated for the Fibroscan controlled attenuation parameter (CAP) score. Determining sample size for this study was based on detection of a 10 unit difference in the mean CAP score with a power of 80% (β = 20%), yielding a sample size of 21 for each group. Due to the potential loss of samples, 25 patients in each group were considered [8].
In this study, adult subjects referring to the Taleghani Hospital Hepatology Clinic (THHC) with a diagnosis of NAFLD, including ultrasonographic findings of hepatic steatosis grade 2 or more were screened in terms of the study’s inclusion criteria. Fifty-two patients who met the inclusion criteria were enrolled in the study. Inclusion criteria included age of 18 years or older, and evidence of NAFLD in Fibroscan (CAP > 263 dB/m). Anyone with a history of alcohol use, diseases such as hepatitis, cirrhosis, biliary disorders, autoimmune disorders, malignancies, hypertension, diabetes, hypothyroidism, Cushing’s syndrome, respiratory, cardiovascular and renal diseases; use of metformin, vitamin E, ursodeoxycholic acid, phenytoin, tamoxifen, lithium, corticosteroids and methotrexate within three months of the study; weight loss or bariatric surgery in recent years; pregnancy, supplement intolerance and unexpected adverse effects was excluded from the study.
Randomization
Eligible patients were block randomized based on body mass index (BMI) and gender, then using a randomization number table, they were assigned to receive either curcumin or placebo capsules for 12 weeks.
Intervention
Patients in the curcumin group received three, 500 mg capsules of curcumin per day. Curcumin capsules were filled withBCM-95 (BIO-CURCUMIN®), a proprietary combination of 95% curcuminoids and essential oil of turmeric-ar-turmerone. Patients in the placebo group received three placebo capsules filled with maltodexterin. The placebo capsules were similar to the curcumin capsules in terms of the size, color, shape, and distribution bottles. Both Curcumin and placebo (Maltodexterin) capsules were produced by Arjuna Natural Extract, India, and labeled as A or B, so that both investigators and participants were unaware of the capsules’ contents. Patients were interviewed every 4 weeks. A 4-week supply of capsules was given to the patients at the beginning of the study and at the end of the fourth and eighth weeks. We asked patients to take three capsules with cold water every day, each capsule after a main meal. Compliance was assessed by counting the remaining capsules in each visit. Patients who had not consumed at least 90% of the expected capsules were excluded from the study.
Lifestyle modifications
An energy-balanced diet and physical activity recommendations were given to all participants based on the Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults from the National Institutes of Health and recommendations by the North American Association for the Study of Obesity [28]. The distribution of nutrients in relation to the total energy value was as follows: total fat, ≤30% total energy value, SFA, 10%; MUFA, 15%; PUFA, 5%; protein, 15–18%; carbohydrates, 52–55%; dietary cholesterol, <300 mg per day; and 20–30 g of fi The/d. In addition, they were advised to exercise at least for 30 min, three times per week. Dietary intakes and physical activity were assessed at the beginning and the end of the study using three 24-hour recalls (two week days and one weekend), and a metabolic equivalent of task (MET) questionnaire, respectively [29].
Clinical, para-clinical assessment
Weight was measured for all patients at the beginning of the study using a Squeal Scale to an accuracy of 100 g. Height was measured using a Seca scale without shoes and to a precision of 0.5 m. Waist circumference was measured using a tape with a precision of 0.5 cm at the narrowest part between the last rib and the iliac bone. Hip circumference was measured with the tape measure to a precision of 0.5 cm at the outermost part of the hip. BMI was calculated by dividing weight in kilograms to height in squared meters. Every 4 weeks, anthropometric measurements were re-performed.
Blood samples were collected at the beginning and end of the study, after 12–14 h of fasting. Serum concentration of triglyceride, total cholesterol, HDL cholesterol, glucose, aspartate amino transferase (AST), alanin amino transferase (ALT), and gamma glutamine tranferase (GGT) were assessed by enzymatic methods using Pars azmun kits (Parsazmun Co., Tehran, Iran). Serum concentration of LDL cholesterol was calculated using the Friedwald formula (LDL-C = TC – HDL – 0.16 (TG)) [30]. Serum Insulin was measured using the Monobind kit (Monobind, Inc., Lake FOREST, CA, USA, Catalog NO: 58K1L4). Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated by HOMA-IR = \(\frac{{{\rm{glucoseconcentration}}{\rm{(mg/dl)}} + {\rm{insulinconcentration}}{\rm{(\mu U/mL)}}}}{{405}}\) [31], and quantitative insulin check index (QUICKI) was calculated by QUICKI = \(\frac{1}{{\sqrt {{\rm{glucose}}} {\rm{concentration}}\left( {\frac{{{\rm{mg}}}}{{{\rm{d}}l}}} \right) + \sqrt {{\rm{insulin}}} {\rm{concentration}}(\mu {\rm{U/mL}})}}\) [32].
Hepatic fibrosis and steatosis were also assessed for all participants at the beginning and at the end of the study using FibroScan (Echosense, France). All measurements and recommendations were performed/given by the same individuals to limit intra-observer biases.
Primary and secondary outcomes
The primary outcome of the study was a significant reduction in hepatic steatosis scores through the Fibroscan measurements. Secondary outcomes were changes in serum concentrations of liver enzymes, lipids, glucose, insulin and insulin sensitivity indices as well as reduction in anthropometric measurements.
Statistical analyses
In this study, the analysis of 24-h food recall questionnaires was done using Nutritionist IV (N4) software and statistical analysis of data was done using SPSS software version 21. Normality was checked through the Kolmogorov–Smirnov test. To compare the categorical variables Χ2 test was performed. To compare mean of quantitative variables, ANCOVA, independent and paired t-tests, or Wilcoxon and Mann-Whitney were performed where applicable. A p value < 0.05 was considered significant.
Results
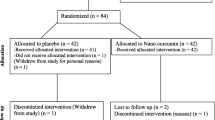
Fifty two patients were enrolled in this study (Fig. 1). Only two patients (8%) in the placebo group were excluded from the study due to personal reasons and hospitalization. The number of drop-outs did not differ between two groups (p = 0.161). Fifty patients were included in the study analysis. Participation rate in this study was 96%. Baseline characteristics of the study groups were comparable in terms of age, gender, physical activity, smoking, anthropometric indices, total energy, lipid profile, serum levels of glucose and insulin, liver enzymes and histology (p > 0.05) (Table 1). Both groups were similar in having insulin resistance and metabolic syndrome. Only one patient in each group did not have insulin resistance, while eight patients in each group had metabolic syndrome. Dietary intakes of patients before and after intervention are shown in Table 2. All dietary intakes reduced significantly in both groups; however, there was no significant difference between the two groups.
As shown in Table 3, weight, BMI, waist circumference, hip circumference, energy intake and physical activity decreased significantly in both groups (p < 0.05). Waist/hip ratio decreased significantly only in the curcumin group (p < 0.05). None of these changes were significantly different between the two groups.
Hepatic fibrosis score, and serum levels of cholesterol and glucose reduced significantly only in the curcumin group, while hepatic steatosis score, serum insulin concentration, and insulin resistance decreased significantly in both groups (p < 0.05). However, these changes were also not significantly different between the two groups (Table 4).
Discussion
To our knowledge, this is the first randomized, double blind, placebo controlled, clinical trial evaluating the effects of curcumin supplementation in NAFLD patients using FibroScan. Our results showed that consumption of 1500 mg per day curcumin beside lifestyle modification is not superior to lifestyle modification alone in amelioration of CVD risk factors in NAFLD patients.
Anti-inflammatory, anti-fibrotic, and anti-hyperlipidemic effects of curcumin have been shown in experimental models of NAFLD [22,23,24]. The most common dosages used for curcumin were 50–200 mg/kg body weight, which is more than what is used in clinical trials [33, 34]. Previous clinical trials used 1000 mg per day [25] or 500 mg per day for 8 weeks [26]. Due to poor absorption, bio-distribution, metabolism, and bioavailability of curcumin, as well as its maximum safe dose (3 mg/kg body weight) [15], we decided to use 1500 mg/day for 12 weeks.
In this study, we advised both groups to follow an energy-balanced diet and perform physical activity, and both groups followed the recommendations. Thus, the energy intakes, and body weight reduced significantly in both groups. Therefore, we could not find any significant difference between the two groups in cardiovascular risk factors and NAFLD characteristics. This finding confirms the pivotal role of weight loss in amelioration of cardiovascular risk factors and NAFLD characteristics, which had been shown previously [35]. Furthermore, it indicates that 1500 mg per day curcumin consumption plus weight loss is not superior to weight loss alone in reduction of cardiovascular risk factors in NAFLD patients.
Previous clinical trials on NAFLD patients had evaluated the effects of lower doses (500–1000 mg/day) of curcumin with shorter study durations (8 weeks) [25, 26]. Although these trials had not evaluated hepatic steatosis and fibrosis by a reliable method such as Fibroscan, they observed improvements in Ultrasound examination of the liver. Fibroscan (ultrasonic elastography) measures the stiffness of the liver non-invasively, measuring it in volumes equal to one centimeter in diameter and 5 centimeter in length, which is almost 100 times the volume of liver biopsy. Therefore, less sampling error is one of its advantages compared to ultrasonography [36]. Fibroscan has been approved for NAFLD detection with a sensitivity of 0.94 and specificity of 0.95 [37].
All participants had normal hepatic enzymes at the baseline, which indicates that they were in initial stages of NAFLD. This might be another reason for the non-significant difference observed in the liver enzymes between two groups.
This study has several strengths; the stratified block randomization design, using Fibroscan for hepatic steatosis and fibrosis measurement, and the inclusion of patients with newly diagnosed NAFLD who had not yet received any treatment. All of these strengths are unique in comparison with few other clinical trials that have evaluated the effects of curcumin alone on NAFLD.
The limitation of this study is that we used a single dosage of curcumin, which makes it hard to conclude about the effects of curcumin on the study outcomes. Moreover, we considered only the natural form of curcumin, which has low bioavailability. Since previous studies have shown that the micronized and liquid micelles of curcumin have the optimum bioavailablity [38], it would be beneficial if we could compare these forms of curcumin with the natural type, in addition to the different dosage. Furthermore, since the study was conducted in a single center, the generalizability of results should be examined in further studies.
Conclusion
In conclusion, this double-blind, placebo-controlled clinical trial showed that the daily intake of 1500 mg curcumin plus weight loss is not superior to weight loss alone in amelioration of cardiovascular risk factors in patients with NAFLD. Further studies with different dosages of curcumin are needed to be able to conclude about the effects of this dietary supplement on cardiovascular risk factors and NAFLD characteristics.
References
Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–43.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64.
Khoshbaten M, Hekmatdoost A, Ghasemi H, Entezariasl M. Prevalence of gastrointestinal symptoms and signs in northwestern Tabriz, Iran. Indian J Gastroenterol. 2004;23:168–70.
Yilmaz Y. is non‐alcoholic fatty liver disease a spectrum, or are steatosis and non‐alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–23.
Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R, Badhe B. Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high-fructose-fed male Wistar rats. Pharm Biol. 2016;54:2857–63.
Morisco F, Vitaglione P, Amoruso D, Russo B, Fogliano V, Caporaso N. Foods and liver health. Mol Asp Med. 2008;29:144–50.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48.
Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study–. Am J Clin Nutr. 2014;99:535–42.
Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014;34:143–8.
Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;114:796–803.
Yari Z, Rahimlou M, Eslamparast T, Ebrahimi-Daryani N, Poustchi H, Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int J Food Sci Nutr. 2016;67:461–9.
Curcumin BengmarkS. An atoxic antioxidant and natural NFκB, cyclooxygenase‐2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enter Nutr. 2006;30:45–51.
Nabavi SF, Daglia M, Moghaddam AH, Habtemariam S, Nabavi SM. Curcumin and liver disease: from chemistry to medicine. Compr Rev Food Sci Food Saf. 2014;13:62–77.
Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int: Off J Int Assoc Study Liver. 2009;29:1457–66.
Dattani J, Rajput D, Moid N, Highland H, George L, Desai K. Ameliorative effect of curcumin on hepatotoxicity induced by chloroquine phosphate. Environ Toxicol Pharmacol. 2010;30:103–9.
Alm-Eldeen AA, Mona MH, Shati AA, El-Mekkawy HI. Synergistic effect of black tea and curcumin in improving the hepatotoxicity induced by aflatoxin B1 in rats. Toxicol Ind Health. 2015;31:1269–80.
Coban D, Milenkovic D, Chanet A, Khallou‐Laschet J, Sabbe L, Palagani A, et al. Dietary curcumin inhibits atherosclerosis by affecting the expression of genes involved in leukocyte adhesion and transendothelial migration. Mol Nutr Food Res. 2012;56:1270–81.
Jang E-M, Choi M-S, Jung UJ, Kim M-J, Kim H-J, Jeon S-M, et al. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat–fed hamsters. Metab-Clin Exp. 2008;57:1576–83.
Tang Y, Zheng S, Chen A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology. 2009;150:3011–20.
Tang Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo. Dig Dis Sci. 2015;60:1554–64.
Rivera‐Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009;29:1457–66.
Vizzutti F, Provenzano A, Galastri S, Milani S, Delogu W, Novo E, et al. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest. 2010;90:104.
Ramirez-Tortosa MC, Ramirez-Tortosa CL, Mesa MD, Granados S, Gil Á, Quiles JL. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-α. Free Radic Biol Med. 2009;47:924–31.
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol. 2016;68:223–9.
Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, et al. Treatment of Non‐alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo‐controlled Trial. Phytother Res. 2016;30:1540–8.
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg). 2017;67:244–51.
National Heart, Lung, and Blood Institute. The Practical Guide: identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute, NHLBI Obesity Education Initiative, North American Association for the Study of Obesity, 2000. https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf
Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN Study. Bull World Health Organ. 2007;85:19–26.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Vanhala P, Vanhala M, Kumpusalo E, Keinanen-Kiukaanniemi S. The quantitative insulin sensitivity check index QUICKI predicts the onset of type 2 diabetes better than fasting plasma insulin in obese subjects: a 5-year follow-up study. J Clin Endocrinol Metab. 2002;87:5834–7.
Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci (1971). 2016;185:93–100.
Zhao NJ, Liao MJ, Wu JJ, Chu KX. Curcumin suppresses Notch1 signaling: Improvements in fatty liver and insulin resistance in rats. Mol Med Rep. 2018;17:819–26.
Ghaemi A, Taleban FA, Hekmatdoost A, Rafiei A, Hosseini V, Amiri Z, et al. How much weight loss is effective on nonalcoholic fatty liver disease? Hepatitis Mon. 2013;13:e15227.
Vizzutti F, Arena U, Marra F, Pinzani M. Elastography for the non-invasive assessment of liver disease: limitations and future developments. Gut. 2009;58:157–60.
Yilmaz Y, Yesil A, Gerin F, Ergelen R, Akin H, Celikel ÇA, et al. Detection of hepatic steatosis using the controlled attenuation parameter: a comparative study with liver biopsy. Scand J Gastroenterol. 2014;49:611–6.
Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014;58:516–27.
Acknowledgements
We would like to thank all participants without whom this study was impossible. The study was financially supported by a grant from Shahid Beheshti University of Medical Sciences to AH.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saadati, S., Hatami, B., Yari, Z. et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur J Clin Nutr 73, 441–449 (2019). https://doi.org/10.1038/s41430-018-0382-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0382-9
- Springer Nature Limited
This article is cited by
-
Prepuberty is a window period for curcumin to prevent obesity in postnatal overfed rats
Pediatric Research (2024)
-
Defining the mechanisms behind the hepatoprotective properties of curcumin
Archives of Toxicology (2024)
-
Natural compounds proposed for the management of non-alcoholic fatty liver disease
Natural Products and Bioprospecting (2024)
-
An updated meta-analysis of effects of curcumin on metabolic dysfunction-associated fatty liver disease based on available evidence from Iran and Thailand
Scientific Reports (2023)
-
Targeting HSP47 and HSP70: promising therapeutic approaches in liver fibrosis management
Journal of Translational Medicine (2022)