Abstract
Non-alcoholic fatty liver disease (NAFLD) is a global health problem with increasing prevalence among overweight and obese patients. It is strongly associated with conditions of insulin resistance including type 2 diabetes mellitus (T2DM) and obesity. It has detrimental consequences ranged from simple steatosis to irreversible hepatic fibrosis and cirrhosis. Curcumin is a dietary polyphenol with potential effect in improving NAFLD. Therefore, the aim of this trial was to examine the effect of curcumin supplementation on various aspects of NAFLD. In this trial, a total number of 80 patients were randomised to receive either curcumin at 250 mg daily or placebo for 2 months. Lipid profiles, hepatic enzymes, anthropometric indices and hepatic fat mass were assessed at the baseline and the end of the trial, and compared within the groups. The grade of hepatic steatosis, and serum aspartate aminotransferase (AST) levels were significantly reduced in the curcumin group (p = 0.015 and p = 0.007, respectively) compared to the placebo. There was also a significant reduction in high density lipoprotein (HDL) levels and anthropometric indices in both groups with no significant differences between the two groups. Low dose phospholipid curcumin supplementation each day for 2 months showed significant reduction in hepatic steatosis and enzymes in patients with NAFLD compared to placebo. Further studies of longer duration and higher dosages are needed to assess its effect on other parameters of NAFLD including cardiovascular risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is a global health problem with increasing prevalence worldwide in parallel with obesity. It is a condition of excess hepatic fat accumulation in non-alcoholic subjects [1], associated with conditions of insulin resistance such as type 2 diabetes mellitus (T2DM) and obesity. Therefore, it is regarded as a hepatic manifestation of metabolic syndrome [2]. NAFLD has a wide spectrum of manifestations from simple steatosis with benign hepatic features to non-alcoholic steatohepatitis (NASH) and irreversible hepatic fibrosis [3]. NASH is a necroinflammatory process of the hepatic cells with a tendency to progress to liver cirrhosis and hepatocellular carcinoma [4]. The pathophysiology of NAFLD is associated with metabolic diseases such as insulin resistance and obesity [5]. It was initially hypothesised that the hepatic triglyceride accumulation is mediated by inflammatory reactions (cytokine/adipokines, oxidative stress and mitochondrial dysfunction) as the main driver for the underlying pathogenesis of steatohepatitis and fibrosis [6]. However, more recent hypotheses have proposed that the pathophysiology of NAFLD is driven by a combination of genetic, epigenetic, environmental and nutritional factors, as well as by obesity, hormone secretion from adipose tissue and insulin resistance [7].
Despite the recent advances in understanding of the pathological mechanism of NAFLD, effective therapeutic options are still limited. Currently, treatment options are primarily focused on improving metabolic parameters such as body weight, physical activity, insulin sensitivity, as well as lipid profiles and glycaemic control. Thus, insulin sensitising agents (e.g., metformin or pioglitazone), lipid lowering compounds (e.g. statins), weight loss medications (e.g., orlistat or sibutramine) and even bariatric surgery have been introduced as potential means for managing NAFLD [8]. There are also numerous new and emerging potential NASH therapeutic approaches including anti-oxidants such as vitamin C, vitamin E and anti-inflammatory agents [9, 10]. However, the challenge still remains to gain approval of these as a treatment approach in NAFLD patients. Within the past few years, curcumin popularity as a potential therapeutic option for treatment of NAFLD has increased. Traditionally, curcumin is in common use in Asian cooking, but also used as household triage for various diseases [11]. Its safety and therapeutic activities, including anti-inflammatory and antioxidant properties, have been reported in several previous studies [12,13,14,15,16,17,18,19,20,21]. It has been shown that curcumin prevents liver fibrosis and subsequent liver cirrhosis through its anti-inflammatory effects and suppression of the hepatic satellite cell (HSC) activity [22]. Furthermore, short term supplementation with curcumin has been demonstrated to improve anthropometric measures, hepatic enzymes and liver fat mass, as assessed by ultrasonography [23].
Given the limited number of clinical studies investigating the potential therapeutic effects of curcumin supplementation on various metabolic parameters in patients with NAFLD, we aimed to examine the therapeutic effect of low dose phospholipid curcumin on lipid profiles, hepatic enzymes and hepatic fat mass in patients with NAFLD in a randomised controlled clinical study. Previous studies have shown that this curcumin formulation drives higher systemic levels of curcumin compared to the non-formulated version, thereby increasing its bioavailability [24, 25].
3.2 Methods
3.2.1 Trial Design
This study was an 8-week, double-blind, placebo-controlled, parallel-group conducted in Neyshabur City in the northeast of Iran. The allocation ratio was 1:1 for two groups. The study was approved by the Institutional Review Board and the Ethical Committee of Neyshabur University of Medical Sciences (Code: IR.NUMS.REC.1394.18). Also, the study was registered in the Iranian Registry of Clinical Trials (http://www.irct.ir; IRCT registration number: IRCT2015052322381N1). All participants who were recruited signed a consent form before any trial-related procedures occurs.
3.2.2 Participants
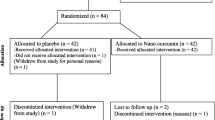
Eligible patients were all adults aged 18 to 65 years who met the eligibility criteria for NAFLD according to ultrasound examination and laboratory results. NAFLD was defined based on higher echogenicity of the liver compared with that of the renal parenchyma due to fatty infiltration. A normal liver was defined if the echogenicity of the liver parenchyma was equal to or only slightly higher than that of the renal parenchyma [26]. Eighty patients with NAFLD were recruited for the study and 8 of these dropped out (Fig. 3.1). Referred patients of the 22 Bahman Hospital (Neyshabur, Iran) from January 2017 to August 2017 were recruited. The exclusion criteria included females with pregnancy/lactation, the presence of alcoholic liver disease, severe heart or lung disease, or the taking of anti-inflammatory drugs such as corticosteroids and liver enzyme inducer drugs, acute or chronic liver disorders such as viral and autoimmune hepatitis, metabolic liver disorders including hemochromatosis and Wilson’s disease, Budd–Chiari syndrome, or other medical disorders such as hyper/hypothyroidism, alpha-1 antitrypsin deficiency, celiac disease or cancer .
3.2.3 Randomization
The subjects were randomly allocated to the curcumin or control group using a balanced block randomization technique. Accordingly, two letters were prepared and written on two sheets of paper with “A” for “curcumin” and “B” for “control.” The following quad blocks were possible: AABB, ABAB, ABBA, BBAA, BABA and BAAB. After this, the number was selected randomly using a table of random numbers. To ensure that implementation of the random allocation sequence occured without the knowledge of which patient will receive which treatment, the entire randomization process was concealed. To achieve this, the drugs had already been put in envelopes labelled a serial number from 1 to 80 and no one knew the nature of the envelopes apart from the coordinator of the trial.
3.2.4 Intervention
The patients in the treatment group received capsules of phospholipidated curcumin (250 mg/day, Meriva curcumin phytosome ; Indena SpA, Milan, Italy). Each capsule was composed of 250 mg curcumin phytosome powder, which was equivalent to 50 mg/day pure curcuminoids. The control group received matched placebo capsules at the same dose. The drug consumption route was oral for a period of 2 months. To keep track of the medication, the bottles of the drug were given to the subjects at the beginning and in the middle (after 1 month) of the interventions period.
3.2.5 Assessment of Outcomes
The primary and secondary outcomes were ultrasound examination and the anthropometric and clinical measurements, respectively.
3.2.5.1 Biochemical and Anthropometric Measurement
Venous blood samples were taken from each patient after an overnight fasting period before and after the intervention on days 0 and 60. This was carried out since the levels of biochemical measurements can be influenced by food intake and diurnal rhythms. For separation of serum, blood samples were centrifuged at 1000 x g for 10 min. Biochemical and lab measurements such as lipid variables, fasting blood glucose (FBG) and liver function tests were performed immediately after serum preparation via the BT-2000 Auto Analyzer machine (Biotechnica; Rome, Italy) using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran).
Bodyweight and body mass were measured using the BPM040S12FXX 770 device (In Body; Seoul, South Korea), with an accuracy of 0.1 kg. According to the protocol of the device, all patients were barefoot with lightweight clothing during the measurements. Body mass index (BMI; kg/m2) and other anthropometric measurements were calculated using the device. Body height was measured by a BSM 370 digital stadiometer (InBody), with accuracy to the nearest 0.1 cm.
Due to the difference in the diet of patients and its possible impact on outcomes, the subjects reported a favourable response to the diet. All patients were asked to have an energy balanced diet according to the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults from the National Institutes of Health and the North American Association for the study of obesity. According to the guideline, the recommended diet consists of ≤30% fat (one-third saturated and two-thirds unsaturated fatty acids), 52–53% carbohydrates, 20–30 g/day fibre, < 300 mg/dL cholesterol and 15–18% protein (all percentages related to the total energy value). Also, all patients were advised to exercise three times each week for at least 30 min.
3.2.5.2 Statistics Analysis
Normal and non-normal distribution variables were presented as mean ± standard deviation (SD) and median (interquartile range (IQR)), respectively. The Kolmogorov-Smirnov test was used for assessing normality of the variables. To compare two related samples (before, after) for parametric and non-parametric variables, the dependent t-test and the Wilcoxon signed-rank test were used, respectively. For comparing characteristics of patients in the treatment and placebo groups, the independent T-test and the Mann-Whitney U test were performed for normal and non-normal distribution variables, respectively. Furthermore, categorical data such as sex and smoking were analyzed using chi-square and Fisher’s exact test.
3.3 Results
Out of 87 patients recruited with NAFLD, 7 subjects did not meet the inclusion criteria (Fig. 3.1). Thus, 80 patients were randomly allocated to the two groups (curcumin and control). After enrolment of all patients, 8 were lost during the follow up period due to curcumin side effects, discontinuation or forgetfulness regarding the intervention, or unavailability for other reasons.
3.3.1 Characteristics of Patients
The demographics and medical history of the patients with NAFLD are shown in Table 3.1. As can be seen, there was no significant difference between the curcumin and placebo groups apart for history of hypertension.
3.3.2 Anthropometric, Biochemical and Sonography Analyses
Anthropometric, biochemical and sonography data before and after intervention are given in Table 3.2. This showed that high-density lipoprotein cholesterol (HDL-C) was significantly increased in the placebo group, while this was decreased in the curcumin group (p < 0.05). Also, aspartate aminotransferase (AST) levels and NAFLD grade (based on sonography) were significantly decreased after the curcumin treatment (p < 0.05). However, there were no significant differences in these parameters in the placebo group. No other variables showed significant differences due to treatment.
3.3.3 Comparison of the Changes of Anthropometric, Biochemical and Sonography Data of Patients with NAFLD Between the Curcumin and Placebo Groups
The changes of anthropometric, biochemical andsonography data before and after intervention are represented in Table 3.3. The changes in each variable were obtained through data differences before and after the intervention. As can be seen in Table 3.3, AST and NAFLD grade were decreased significantly following treatment in the curcumin group compared to the effects on these same parameters in the placebo group (p < 0.05). The comparison of changes of other variables such as anthropometric, blood pressure and other biochemical data were not significantly different between the curcumin and placebo groups (p > 0.05).
3.4 Discussion
The present clinical study investigated the significant impact of low dose phospholipid curcumin supplementation on biochemical markers of NAFLD. In addition to abnormal liver enzymes and lipid profile, the sonographic features of hepatic steatosis (grades 1–3) were improved by the treatment. Consistent with current research findings, Rahmani et al. also showed the reduction of serum levels of AST and ALT as well as hepatic fat mass using bioavailability-enhanced curcumin in patients with NAFLD compared to the placebo group. The therapeutic properties of curcumin in improving liver steatosis and fibrosis have been previously reported [27, 28].
The current findings are consistent with those of a previous trial with a high bioavailability curcumin-phosphatidylcholine complex that was administered at a higher dose (1000 mg/day) [23]. Most of the previous studies which used higher doses of curcumin used concentrations ranging from 500 mg to 1000 mg per day [29, 30], as a means of maximizing therapeutic effects. However, in this trial we managed to demonstrate the efficacy of curcumin in improving metabolic parameters at an even lower dose (250 mg per day for 8 weeks). Similar results were reported in previous animal studies where curcumin consumption dosages ranged from 50 mg to 200 mg a day, and these demonstrated significant improvement in insulin resistance, hepatic fat levels and it attenuated liver injury [21, 32].
These results can be explained by hormetic effect of curcumin. For example, low-dose curcumin administration could have antioxidant characteristics and high dose may induce autophagy and apoptosis. The observed biphasic dose–response potential of curcumin on cells showed the stronger effect of low dose administration than at higher dosages [33].
Curcumin modulates some metabolic risk factors such as inflammation, along with lipid, glycemic and oxidative pathways in NAFLD [34]. Regarding these positive effects and the lack of approved medications for NAFLD, it would be valuable to investigate the hepatoprotective effect of phospholipid-curcumin in NAFLD patients. Thus, curcumin may be able to slow down the initiation of the “first hit” in development of hepatic steatosis as well as significantly reduce the pro-inflammatory cytokines triggering the “second hit” of NAFLD pathogenesis [23].
Lifestyle changes through increasing the adherence to a well-established diet and optimal physical activity are considered as an initial step in the prevention and treatment of NAFLD [35, 36]. In this trial, all participants were instructed to follow energy balanced diets according to the current clinical guidelines for management of overweight and obesity. This is the likely reason why we did not find significant differences in terms of weight reduction and glycaemic control between the groups. The enhancement of physical performance and physiological fatigue reduction following curcumin supplementation might contribute to BMI reduction and other indices of NAFLD [37].
This study has several strengths. These include the balanced block randomisation design, rigorous inclusion and exclusion criteria, a lengthy (8 week) follow up period, and the direct comparison of curcumin and placebo effects. Moreover, we used phospholipid-curcumin which has optimal bioavailability unlike the natural form of curcumin used in previous studies which has a lower bioavailability [38].
There are also limitations of this trial that should be considered in interpretation of the results. First, we used ultrasonography to assess hepatic steatosis instead of other modalities such as elastography or histopathology. In addition, it was a single centre trial which could jeopardise its generalizability.
3.5 Conclusions and Future Perspectives
In conclusion, the findings of the present trial suggest a hepatoprotective effect of low dose phospholipid-curcumin supplementation associated with disease severity alterations in patients with NAFLD. While no pharmacological therapy has yet been approved for NAFLD, supplementation with curcumin may provide a safe and viable approach for patients and suppress the progression of NAFLD. However, further trials over longer durations and which assess various dosages of curcumin and its effects on the metabolic parameters in patients with NAFLD are needed.
References
Abdelmalek MF, Diehl AM (2007) Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am 91(6):1125–1149. ix
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R et al (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37(4):917–923
Yilmaz Y (2012) Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther 36(9):815–823
Farrell GC, Larter CZ (2006) Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43(2 Suppl 1):S99–S112
Morisco F, Vitaglione P, Amoruso D, Russo B, Fogliano V, Caporaso N (2008) Foods and liver health. Mol Asp Med 29(1–2):144–150
Day CP, James OF (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114(4):842–845
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8):1038–1048
Lam B, Younossi ZM (2010) Treatment options for nonalcoholic fatty liver disease. Ther Adv Gastroenterol 3(2):121–137
Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ (2003) Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 38(2):413–419
Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF (2016) Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS One 11(1):e0147985. https://doi.org/10.1371/journal.pone.0147985
Noorafshan A, Asadi-Golshan R, Karbalay-Doust S, Abdollahifar MA, Rashidiani-Rashidabadi A (2013) Curcumin, the main part of turmeric, prevents learning and memory changes induced by sodium metabisulfite, a preservative agent, in rats. Exp Neurobiol 22(1):23–30
Dattani JJ, Rajput DK, Moid N, Highland HN, George LB, Desai KR (2010) Ameliorative effect of curcumin on hepatotoxicity induced by chloroquine phosphate. Environ Toxicol Pharmacol 30(2):103–109
Soleimani V, Sahebkar A, Hosseinzadeh H (2018) Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res 32(6):985–995
Saberi-Karimian M, Keshvari M, Ghayour-Mobarhan M, Salehizadeh L, Rahmani S, Behnam B, et al (2020) Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial (2020) Complement Ther Med 49:102322. https://doi.org/10.1016/j.phrs.2020.104921
Wu C, Qiu Y, Sun X, Chen D, Wu Y, Pang Q (2019) Effects of curcumin on liver fibrosis induced by cholestasis in mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 35(5):468–472
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A (2019) Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr 59(1):89–101
Ghandadi M, Sahebkar A (2017) Curcumin: An effective inhibitor of interleukin-6. Curr Pharm Des 23(6):921–931
Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar A (2016) Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther 20(4):335–345
Teymouri M, Pirro M, Johnston TP, Sahebkar A (2017) Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: a review of chemistry, cellular, molecular, and preclinical features. Biofactors 43(3):331–346
Panahi Y, Ahmadi Y, Teymouri M, Johnston TP, Sahebkar A (2018) Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J Cell Physiol 233(1):141–152
Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H (2009) Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev 18(5):412–415
Kyung EJ, Kim HB, Hwang ES, Lee S, Choi BK, Kim JW et al (2018) Evaluation of Hepatoprotective effect of curcumin on liver cirrhosis using a combination of biochemical analysis and magnetic resonance-based electrical conductivity imaging. Mediat Inflamm 2018:5491797–5491799. https://doi.org/10.1155/2018/5491797
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendia LE, Sahebkar A (2017) Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg) 67(4):244–251
Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ (2007) Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 60(2):171–177
Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A (2017) Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother 85:102–112
Stadlmayr A, Aigner E, Steger B, Scharinger L, Lederer D, Mayr A et al (2011) Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 270(1):41–49
Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, Sahebkar A (2016) Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res 30(9):1540–1548
Panahi Y, Valizadegan G, Ahamdi N, Ganjali S, Majeed M, Sahebkar A (2019) Curcuminoids plus piperine improve nonalcoholic fatty liver disease: a clinical trial. J Cell Biochem 120(9):15989–15996
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendia LE, Sahebkar A (2016) Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol 68(3):223–229
Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A et al (2016) Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res 30(9):1540–1548
Li B, Wang L, Lu Q, Da W (2016) Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci 185(1):93–100
Zhao NJ, Liao MJ, Wu JJ, Chu KX (2018) Curcumin suppresses Notch1 signaling: improvements in fatty liver and insulin resistance in rats. Mol Med Rep 17(1):819–826
Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A (2019) Hormetic effects of curcumin: what is the evidence? J Cell Physiol 234(7):10060–10071
Amel Zabihi N, Pirro M, P Johnston T, Sahebkar A (2017) Is there a role for curcumin supplementation in the treatment of non-alcoholic fatty liver disease? The data suggest yes. Curr Pharm Des 23(7):969–982
Nseir W, Hellou E, Assy N (2014) Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol 20(28):9338–9344
Finelli C, Tarantino G (2012) Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease? World J Gastroenterol 18(46):6790–6800
Huang W-C, Chiu WC, Chuang HL, Tang DW, Lee ZM, Wei L et al (2015) Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 7(2):905–921
Saadati S, Hatami B, Yari Z, Shahrbaf MA, Eghtesad S, Mansour A et al (2019) The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur J Clin Nutr 73(3):441–449
Acknowledgments
We are thankful for the financial support from the Neyshabur University of Medical Sciences (NUMS), Neyshabur, Iran. The authors are grateful for the supports provided by Indena SpA (Milan, Italy) for conducting this study.
Conflict of Interests
None.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mirhafez, S.R. et al. (2021). The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. In: Barreto, G.E., Sahebkar, A. (eds) Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health. Advances in Experimental Medicine and Biology, vol 1308. Springer, Cham. https://doi.org/10.1007/978-3-030-64872-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-64872-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64871-8
Online ISBN: 978-3-030-64872-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)