Abstract
Aim
Nuclear factor-erythroid 2-related factor-2 (Nrf2) acts as a defense system in the development of nonalcoholic steatohepatitis (NASH). Curcumin is a phenolic compound with lipid regulatory, anti-oxidative, anti-inflammatory and anti-tumorigenic properties that is beneficial in defending against NASH and was recently proved to be an Nrf2 activator. The aim of this study was to evaluate whether Nrf2 activation could be involved in NASH mitigation by curcumin.
Methods
Hepatic, metabolic, and inflammatory parameters, along with hepatic Nrf2 protein expression were explored in adult Sprague–Dawley rats developing high-fat-diet-induced NASH and submitted to curcumin gavage for 6 weeks.
Results
Curcumin administration led to lower degrees of hepatic steatosis and inflammation; lower levels of serum aminotransferases, lipids, and homeostasis model assessment of insulin resistance; and lower serum and hepatic contents of tumor necrosis factor-α (TNF-α), interleukin-6, and malondialdehyde. In contrast, higher hepatic contents of glutathione, heme oxygenase-1 and superoxide dismutase were observed in rats with curcumin. Moreover, Nrf2 expression in liver cell nuclei was significantly higher in rats with curcumin.
Conclusions
Curcumin can prevent and ameliorate NASH via lipid reduction, improve insulin resistance, improve anti-inflammatory, and have antioxidant effects, possibly related to its activation of Nrf2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease worldwide with a prevalence rate ranging from 6 to 35 % [1]. NAFLD represents a wide spectrum of liver diseases from simple steatosis to a more severe and treatment-resistant clinical entity characterized by the appearance of inflammation, termed nonalcoholic steatohepatitis (NASH), which may in turn progress to cirrhosis and hepatocellular carcinoma [2]. The pathogenesis of NASH involves numerous factors, such as changes in lipid metabolism, insulin resistance, inflammatory cytokines and oxidant stress [3]. Several recent studies suggest that nuclear factor-erythroid 2-related factor-2 (Nrf2), an important cytoprotective transcription factor, functions as a defense system in the development of NASH. Therefore, Nrf2 may be a novel therapeutic target for the prevention and treatment of fatty liver and NASH [4]. Curcumin is a phenolic compound found in the dietary spice turmeric, derived from the rhizome of Curcuma longa [5]. In addition, curcumin regulates lipid metabolism; has anti-inflammatory, anti-oxidation and anti-cancer effects; and has been recently proved to activate Nrf2 [6, 7]. Despite the large number of studies demonstrating the hepatoprotective effects of curcumin, which may attribute to its intrinsic properties [8–12], there are few reports on the effects of this polyphenolic compound on the NASH model induced by a high-fat diet under in vivo conditions, and on the possible molecular underlying mechanisms. Therefore, the aim of this study was to evaluate whether curcumin could attenuate or prevent high-fat diet-induced NASH in a rat model, and confirm whether Nrf2 activation is involved.
Materials and methods
Animals and diets
This study was performed in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (Guide for the Care and Use of Laboratory Animals, 1996). The Animal Research Committee of Shanghai Jiaotong University in China approved the protocol. Thirty-six male Sprague–Dawley rats with an average body weight of 190–210 g (B&K Universal Group Limited, Shanghai, China) were used in this study. Animals were housed six per cage at room temperature (20–22 °C) with a light/dark cycle of 12 h and free access to food and water. All rats were fed a standard laboratory diet for a week. They were then randomly divided into three groups of twelve rats each: normal group, model group and treatment group. Rats in the normal group were fed a standard diet, while those in the other two groups received a high-fat diet composed of 18.0 % protein, 45.0 % fat, and 37.0 % carbohydrates, and kept in darkness at 4 °C. Two rats from each group were euthanized at the end of the 6th week to detect pathological changes. Then, rats in the treatment group were gavaged with 50 mg/kg of curcumin (Sigma, USA) suspended in 0.5 % carboxymethyl cellulose (CMC) daily for 6 weeks. The normal group and model group received an equal volume of 0.5 % CMC (Songon, Shanghai, China) as controls.
Sample collection
Overnight food-deprived rats were anesthetized with ketamine (0.2 mL/100 g) at the end of the 12th week. Body weight was measured. Blood was collected from the heart of the rats into a tube, and serum was centrifuged at 2,500 rpm for 10 min. The liver was removed carefully and washed with physiological saline for weighing. Serum and liver tissues were stored at −20 and −80 °C, respectively, for further analyses.
Serum parameters
Concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, serum triglyceride (TG), total cholesterol (TC), and fasting plasma glucose were determined by automatic biochemistry analyzer (Hitachi, Japan). Fasting insulin levels were measured by radioimmunoassay and insulin resistance was estimated based on the final blood glucose and insulin values using the homeostasis model assessment of insulin resistance (HOMA-IR) [13]. The concentrations of serum free fatty acids (FFA), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined using enzyme-linked immunosorbent assay (ELISA; BlueGene, Shanghai, China) in a 48-well plate.
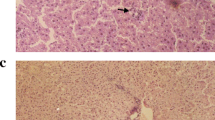
Histological analysis of hepatic lesions
Liver pathology was assessed by hematoxylin and eosin (H&E) and Oil Red O staining of liver sections, and scored via blinded samples by a board-certified pathologist. Histological lesions were evaluated by the improved grading and staying system proposed by Brunt et al. [14] as follows: (1) steatosis was graded S0–S3 based on the percentage of hepatocytes in the biopsy involved (S0 corresponded to none; S1 was <33 %; S2 was 33–66 %; S3 was >66 %); (2) intra-acinar (lobular) inflammation was graded L0–L3 based on inflammatory foci per 10× with 20× ocular (L0 corresponded to none; L1 was 1–2/10×; L2 was up to 4/10×; L3 was >4/10×); and (3) portal tract inflammation was graded as none, mild, moderate, and severe (P0–P3). With these data, the Necroinflammatory Grading System for Steatohepatitis (NASH grade) proposed by Brunt was applied: Grade 0 = S0 + L0 + P0; Grade 1 (mild) = S1–2 plus L1 plus P0–1; Grade 2 (moderate) = S2–3 plus L2 plus P1–2; or Grade 3 (severe) = S3 plus L3 plus P1–2.
Measurement of liver inflammatory cytokines
Liver levels of TNF-α and IL-6 were assayed using ELISA using BlueGene Kits according to the manufacturer’s instructions (BlueGene, Shanghai, China). Absorbance was measured at 450 nm using a Sunrise absorbance reader (UNICO, USA) and quantified relative to a standard row.
Liver contents of malondialdehyde (MDA), glutathione (GSH) and activity of superoxide dismutase (SOD)
Liver tissues were rinsed, weighed, resuspended at 1 g/9 mL in normal saline, and homogenized. After centrifugation at 2,500 rpm for 10 min at 4 °C, the supernatants were collected for protein quantitative assay (JianCheng, NanJing, China). The liver contents of MDA, GSH and the activity of SOD were analyzed by corresponding assay kits (BlueGene, Shanghai, China) in accordance with the manufacturer’s instructions.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted after homogenization in TRIzol regent (Invitrogen, USA) using an ultracentrifuge (BECKMAN, USA) according to the standard protocol. cDNA was synthesized by reverse transcription using total RNA (1 µg) as a template (Fermentas, Canada). Gene expression analysis was performed by RT-PCR (Funglyn Biotech, Canada). Primer sequences were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward) GACGCTTTGGTGAAGAAACTGA, (reverse) CACACGGCAATAAATGACATGAG; heme oxygenase-1 (HO-1) (forward) TTTCACCTTCCCGAGCATC, (reverse) TTAGCCTCTTCTGTCACCCTGT. RT-PCR monitoring with SYBR Green I was performed according to the following protocol: initial incubation at 95 °C for 30 s, followed by 40 cycles of denaturation for 5 s at 95 °C, and finally annealing/extension for 30 s at 60 °C repeated three times for each hole. Results were normalized to GAPDH mRNA as an internal control and shown as relative mRNA levels.
Western blot analysis for Nrf2 expression
Nuclear extracts were prepared as described previously [15]. Nuclear extracts (20 μg) were separated by SDS-PAGE gel, and proteins were transferred to a polyvinylidene difluoride membrane. Membranes were blocked for 1 h in 5 % nonfat dry milk and incubated with primary anti-Nrf2 (1:500) (BlueGene, Shanghai, China) in 5 % nonfat dry milk overnight at 4 °C. Antibodies to Lamin B were used as internal control. After being washed three times for 5 min in TBS-T, membranes were subsequently incubated with secondary antibodies appropriately diluted in TBS-T for 1 h at room temperature. The membrane was washed four times and detection was performed using an enhanced chemiluminescence system and exposed to X-ray film.
Statistical analysis
All statistics were analyzed using SPSS17.0 (SPSS Inc., Chicago, IL, USA). All results are shown as the mean of three or more replicates. Data are presented as mean ± standard error (SE). The data of three groups were analyzed by one-way ANOVA followed by Tukey’s methods or the Kruskal–Wallis test. LSD t and Wilcoxon rank sum tests were used to evaluate the statistical significance of the results. P values <0.05 were considered significant.
Results
Effect of curcumin on morphological and functional liver parameters
Significant differences were observed for macroscopic alterations between model and control groups at the end of the 12th week. The livers of model group rats were enlarged and yellow with an irregular, partially nodular surface. Steatosis, ballooning, mixed acute and chronic lobular inflammation and focal necrosis were present in model group rats accompanied by elevated body weight, liver weight, and serum ALT and AST levels (P < 0.05). In the treatment group, the macroscopic appearance of the livers, the intensity of hepatic steatosis and inflammation were significantly alleviated, and a remarkable reduction (P < 0.05) was observed in body weight, liver weight and serum ALT and AST, as compared to model group (Fig. 1; Tables 1, 2).
Effect of curcumin on serum lipids and insulin resistance
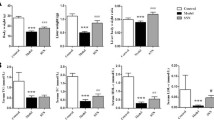
Rats fed on the high-fat diet for 12 weeks (model group) showed an increase (P < 0.05) in all lipid parameters (i.e., TG, TC, and FFA) in serum and insulin resistance when compared to those of rats fed the standard diet. Curcumin treatment resulted in significant reduction (P < 0.05) of these lipid parameters (Table 3).
Effect of curcumin on inflammatory cytokines
Rats in the model group had higher levels of serum TNF-α and IL-6 and higher liver contents of TNF-α and IL-6 than those in the normal group (P < 0.05). The administration of curcumin resulted in a remarkable reduction of these inflammatory cytokines (P < 0.05) (Table 4).
High-fat diet-induced oxidative stress was decreased by curcumin treatment
Administration of a high-fat diet to rats increased the liver protein MDA content by more than 200 % versus the model group, whereas curcumin treatment decreased the high-fat diet-induced protein MDA content to basal levels. Concerning the hepatic contents of GSH and activity of SOD, the model group exhibited the lowest values, significantly different (P < 0.05) from the normal and treatment groups. In relation to HO-1 protein, the treatment group showed the highest values, significantly different (P < 0.05) from the normal and model groups (Table 5; Fig. 2).
Effect of curcumin on Nrf2 expression
We examined whether 6-week treatment with curcumin affected the liver expression of Nrf2. There was a 160 % increase in liver nuclear Nrf2 protein in rats given curcumin; no differences were found between the normal and model groups (Fig. 3).
Discussion
The high-fat diet rat model closely resembles the pathophysiology observed in human NAFLD. By feeding rats a high-fat diet for 6 weeks, we reproduced the hepatic lesions of nonalcoholic simple fatty liver. Moreover, when dietary fat time was extended to 12 weeks, significant further increases in macro- and micro-steatosis, lobular inflammation and necrosis were observed, accompanied by elevated body weight, liver weight and serum ALT and AST levels, suggesting that we have reproduced the model of NASH [16].
Despite the fact that pathogenesis of steatosis and progression to steatohepatitis have not yet been fully elucidated, some factors, such as changes in lipid regulation, insulin resistance, inflammatory cytokines, and oxidative stress have been identified as factors in NAFLD [17]. These factors directly or indirectly induce and aggravate liver steatosis, trigger production of inflammatory cytokines (causing inflammation and fibrogenic response), and promote cell apoptosis and death [17–20]. This leads to development of NASH, which can ultimately result in end-stage liver disease. This study showed significant increases in serum levels of lipids, HOMA-IR, TNF-α and IL-6; increases in hepatic content of MDA; and reductions in hepatic GSH content and SOD activity, thus confirming the role of the above factors on the pathogenesis of NASH.
As an important cytoprotective transcription factor, Nrf2 could be activated by electrophilic agents and play a central role in mediating a cytoprotective response against a wide variety of stress and toxic insults. Protection against oxidative/nitrative stress involves not only enhanced expression of Nrf2 but also the expression of Nrf2-regulated gene products including GSH, glutathione peroxidase, glutathione synthesis, HO-1, NAD(P)H quinine oxido-reductase 1, glutamate cysteine ligase and many other factors [21–23]. In fact, besides its role in regulating cellular anti-oxidative defense, Nrf2 has also been shown to attenuate insulin resistance and has anti-obesity and anti-inflammatory functions. As an example, the increased susceptibility of Nrf2-deficient mice to dextran sulfate sodium or carcinogen-induced colitis and colorectal cancer is associated with decreased expression of antioxidant/phase II detoxifying enzymes in parallel with up-regulation of pro-inflammatory cytokines, such as cyclooxygenase-2 and TNF-α [24, 25]. It was shown recently that Nrf2 inhibits lipid accumulation in mice fed a high-fat diet, and prevents the development of insulin insensitivity, obesity, and other related metabolic abnormalities [26–28]. Furthermore, diverse Nrf2 activators, such as phenethyl isothiocyanate, resveratrol and oltipraz, can attenuate lipopolysaccharide-induced nuclear factor-κB activation, insulin resistance, and obesity that have been induced by a high-fat diet [29–31]. These findings suggest that Nrf2 deficiency, and the resulting impaired antioxidant activity, are important for the susceptibility to obesity-related metabolic, inflammatory and oxidative stress diseases.
Interestingly, it has been demonstrated that Nrf2 expression increases during the development of NAFLD and NASH in mice and that the increase reflects the severity of disease [4]. Although no significant differences were found between normal and model groups with respect to Nrf2 expression (data not shown) in our study, Nrf2 protein expression presented a rising trend in our model group rats. This suggests that Nrf2 functions as a defense system in the development of NASH. In fact, accumulating evidence has demonstrated hepatoprotective effects of Nrf2 [32]. In addition, Chowdhry and colleagues found that Nrf2−/− mice were considerably more sensitive to NASH on the methionine- and choline-deficient diet. The Nrf2−/− livers suffered more oxidative stress than their wild-type counterparts as assessed by a significant depletion of reduced glutathione that was coupled with increases in oxidized glutathione, MDA, and inflammation [33]. The above findings suggest that Nrf2 associates with NASH and may be a novel therapeutic target for the prevention and treatment of fatty liver disease.
Curcumin, a natural phenolic compound found in the dietary spice turmeric, derived from the rhizome of Curcuma longa, regulates lipid metabolism, and exerts anti-inflammatory, anti-oxidation and anti-cancer effects in a variety of pathological conditions including cancer, inflammation, obesity and cardiovascular disease [8–12]. Recently, several lines of evidence have suggested that curcumin inhibits oxidative stress and inflammation by triggering Nrf2 signaling. For instance, dietary curcumin increases Nrf2 expression at the transcriptional and translational levels, suggesting that Nrf2–Keap1 interaction enables Nrf2 to translocate to the nucleus [6, 7]. Activated Nrf2 binds to the antioxidant-responsive element and initiates the transcription of genes coding antioxidants against oxidative/nitrative stress and inflammation. Curcumin can also elicit its prostate cancer chemopreventive effect in TRAMP C1 cells, potentially through epigenetic modification of the Nrf2 gene with its subsequent induction of the Nrf2-mediated anti-oxidative stress cellular defense pathway [34].
Although numerous studies confirm its potential beneficial role, the mechanisms whereby curcumin might be effective at mitigating NASH remain unclear. In the present study, NAFLD rats induced by high-fat diet, showed marked reduction of steatosis and inflammation after being treated with 50 mg/kg curcumin for 6 weeks. These data agree with the efficacy of curcumin in NASH rabbits as reported by Ramirez-Tortosa et al. [35], who also showed that curcumin supplementation lowered the aminotransferase activity and the levels of TNF-α protein. Serum TG, TC and FFA were reduced in our study. However, reports about the influences of curcumin on lipids are contradictory. While some reported data and our results showed the hypolipidemic activity of curcumin [36–38], several reports indicate that plasma lipid levels are not affected by curcumin supplementation [35, 39]. This discrepancy may be related to diet composition, concentration of curcumin, method of supplementation, and duration of treatment. Our results showed for the first time that curcumin could improve insulin resistance; reduce liver MDA levels; and increase hepatic GSH content, SOD activity and HO-1 expression, thus confirming the antioxidant effect of curcumin in NASH model rats induced by a high-fat diet. Moreover, we demonstrated for the first time that expressions of Nrf2 and its target genes were enhanced in our rat liver model of NASH, and that this Nrf2 induction by the polyphenolic compound may be involved in its preventive and therapeutic effects.
In conclusion, curcumin treatment attenuated insulin resistance, serum lipids, oxidative stress, and liver inflammation in rats with NASH induced by high-fat diet. All the above results suggest that the beneficial effect of curcumin occurred at least in part through activation and modulation of the Nrf2–Keap1 signaling pathway. Based on the findings of the present study, and due to the safety profile as well as low cost of curcumin, we believe that these studies might facilitate future clinical trials with curcumin in the treatment of NASH.
References
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34(3):274–285
Pinzani M (2011) Pathophysiology of non-alcoholic steatohepatitis and basis for treatment. Dig Dis 29(2):243–248
Greenfield V, Cheung O, Sanyal AJ (2008) Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol 24(3):320–327
Xu W, Shao L, Zhou C, Wang H, Guo J (2011) Upregulation of Nrf2 expression in non-alcoholic fatty liver and steatohepatitis. Hepatogastroenterology 58(112):2077–2080
Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75
Carmona-Ramírez I, Santamaría A, Tobón-Velasco JC et al (2013) Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J Nutr Biochem 24(1):14–24
Charoensuk L, Pinlaor P, Prakobwong S et al (2011) Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int J Parasitol 41(6):615–626
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50(3):151–161
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153
Vera-Ramirez L, Pérez-Lopez P, Varela-Lopez A et al (2013) Curcumin and liver disease. BioFactors 39(1):88–100
Zingg JM, Hasan ST, Meydani M (2013) Molecular mechanisms of hypolipidemic effects of curcumin. BioFactors 39(1):101–121
García-Niño WR, Pedraza-Chaverrí J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69:182–201
Mlinar B, Marc J, Janez A, Pfeifer M (2007) Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta 375(1–2):20–35
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94(9):2467–2474
Singh S, Aggarwal BB (1995) Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 270(42):24995–25000
Neuschwander-Tetri BA, Clark JM, Bass NM, NASH Clinical Research Network et al (2010) Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 52(3):913–924
Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C (2008) Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14(2):72–81
Wieckowska A, Papouchado BG, Li Z et al (2008) Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 103(6):1372–1379
Lin FL, Hsu JL, Chou CH et al (2011) Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-α and p53 pathways. Eur J Pharmacol 650(1):120–129
Koek GH, Liedorp PR, Bast A (2011) The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta 412(15–16):1297–1305
Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278(14):12029–12038
Hayes JD, McMahon M (2009) NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34(4):176–188
Harvey CJ, Thimmulappa RK, Singh A et al (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46(4):443–453
Khor TO, Huang MT, Kwon KH et al (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66(24):11580–11584
Cheung KL, Lee JH, Khor TO et al (2014) Nrf2 knockout enhances intestinal tumorigenesis in Apc (min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol Carcinog 53:77–84
Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D (2011) The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care 14(1):41–48
Tanaka Y, Aleksunes LM, Yeager RL et al (2008) NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther 325(2):655–664
Yates MS, Tran QT, Dolan PM et al (2009) Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30(6):1024–1031
Jeong WS, Kim IW, Hu R, Kong AN (2004) Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 21(4):661–670
Cheng AS, Cheng YH, Chiou CH, Chang TL (2012) Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem 60(36):9180–9187
Yu Z, Shao W, Chiang Y et al (2011) Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54(4):922–934
Zhang YK, Yeager RL, Tanaka Y, Klaassen CD (2010) Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol 245(3):326–334
Chowdhry S, Nazmy MH, Meakin PJ et al (2010) Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med 48(2):357–371
Khor TO, Huang Y, Wu TY et al (2011) Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol 82(9):1073–1078
Ramirez-Tortosa MC, Ramirez-Tortosa CL, Mesa MD et al (2009) Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic Biol Med 47(7):924–931
Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M (1970) Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr 100(11):1307–1315
Hu GX, Lin H, Lian QQ et al (2013) Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: improving lipid profiles in high-fat-diet-treated rats. PLoS ONE 8(3):e49976
Shin SK, Ha TY, McGregor RA, Choi MS (2011) Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol Nutr Food Res 55(12):1829–1840
Asai A, Miyazawa T (2001) Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J Nutr 131(11):2932–2935
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Wang, L., Lu, Q. et al. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect?. Ir J Med Sci 185, 93–100 (2016). https://doi.org/10.1007/s11845-014-1226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-014-1226-9