Abstract
Epilepsy is a prevalent and severe neurological disorder and approximately 30% of patients are resistant to existing medications. It is of utmost importance to develop alternative therapies to treat epilepsy. Schisandrin B (SchB) is a major bioactive constituent of Schisandra chinensis (Turcz.) Baill and has multiple neuroprotective effects, sedative and hypnotic activities. In this study, we investigated the antiseizure effect of SchB in various mouse models of seizure and explored the underlying mechanisms. Pentylenetetrazole (PTZ), strychnine (STR), and pilocarpine-induced mouse seizure models were established. We showed that injection of SchB (10, 30, 60 mg/kg, i.p.) dose-dependently delayed the onset of generalized tonic-clonic seizures (GTCS), reduced the incidence of GTCS and mortality in PTZ and STR models. Meanwhile, injection of SchB (30 mg/kg, i.p.) exhibited therapeutic potential in pilocarpine-induced status epilepticus model, which was considered as a drug-resistant model. In whole-cell recording from CHO/HEK-239 cells stably expressing recombinant human GABAA receptors (GABAARs) and glycine receptors (GlyRs) and cultured hippocampal neurons, co-application of SchB dose-dependently enhanced GABA or glycine-induced current with EC50 values at around 5 μM, and application of SchB (10 μM) alone did not activate the channels in the absence of GABA or glycine. Furthermore, SchB (10 μM) eliminated both PTZ-induced inhibition on GABA-induced current (IGABA) and strychnine (STR)-induced inhibition on glycine-induced current (Iglycine). Moreover, SchB (10 μM) efficiently rescued the impaired GABAARs associated with genetic epilepsies. In addition, the homologous mutants in both GlyRs-α1(S267Q) and GABAARs-α1(S297Q)β2(N289S)γ2L receptors by site-directed mutagenesis tests abolished SchB-induced potentiation of IGABA and Iglycine. In conclusion, we have identified SchB as a natural positive allosteric modulator of GABAARs and GlyRs, supporting its potential as alternative therapies for epilepsy.
Similar content being viewed by others
Introduction
Epilepsy is a prevalent and severe neurological disorder that impacts almost 1% of the world’s population. Epilepsy is a condition characterized by recurrent seizures that occur without any apparent trigger. These seizures are usually caused by abnormal activity in the neurons of central nervous system, which is often the result of an imbalance between excitatory and inhibitory neurotransmission [1]. Despite the numerous antiseizure medications (ASMs) in recent decades, around 30% of patients are still not responsive to the treatments [2]. Furthermore, the treatment of epilepsy needs a longer-term utilization of ASMs, which leads to various adverse effects such as allergic reactions, hepatotoxicity, and excessive depression of the central nervous system (CNS) [3]. These reactions impose a significant burden on both patients and society. Therefore, it is of utmost importance to develop alternative therapies to treat epilepsy.
GABAA receptors (GABAARs), the major inhibitory receptors in the adult mammalian CNS, are important targets of epilepsy [4], and also play significant roles in the development of various neurological disorders, such as migraine, neuropathic pain, and depression [5, 6]. The majority of GABAARs consist of two α subunits, two β subunits, and either a γ or δ subunit. Among them, γ-containing GABAARs mainly mediate phasic inhibitory synaptic transmission, while δ-containing GABAARs mediate tonic extrasynaptic inhibition [7]. Upon activation, the influx of chloride ions into the neuron hyperpolarizes and/or stabilizes the membrane potential and thus inhibits the neuronal excitability [8]. Glycine receptors (GlyRs), the other major inhibitory receptors in CNS, play important roles in maintaining the normal balance between excitation and inhibition in the brain [9,10,11]. Activation of GlyRs suppresses neuronal excitation and seizure-like events in the entorhinal cortex and hippocampus of rats [9,10,11]. The antagonists of these receptors, pentylenetetrazole (PTZ, blocking GABAARs) or strychnine (STR, blocking GlyRs), have often been used to generate seizure models [12], further demonstrating the crucial role of GABAARs and GlyRs in the development and progression of epilepsy. Mutations that cause dysfunction of GABAARs and GlyRs also result in seizure-like symptoms [13,14,15].
Schisandra chinensis fructus, the dried ripe fruit of Schisandra chinensis (Turcz.) Baill, is a well-known traditional Chinese medicine [16]. The extracts from Schisandra chinensis fructus have been reported to possess a variety of pharmacological effects on CNS disorders. These effects include neuroprotection [17], improvement of learning and memory [17], sedative-hypnotic [18], anxiolytic [19], and antidepressant effects [20]. Schisandrin B (SchB) (chemical structure was shown in Fig. 1a) is the most abundant dibenzocyclooctadiene lignan from Schisandrae chinensis. Due to its high availability in the brain after administration [21, 22], SchB possessed multiple neuroprotective effects [23], sedative and hypnotic properties [24]. However, there have been no reports for the effects of SchB on epilepsy. Coincidently, the present study identified SchB as a natural positive allosteric modulator of GABAARs and GlyRs, which indicates its potential use in epilepsy. Thus, we investigated the antiseizure effects of SchB on various mouse models of seizure and explored the underlying mechanism.
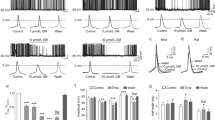
a Left, chemical structure of SchB; right, schematic diagram for patch-clamp recordings in GlyR-CHO cells. b Bar graphs illustrating SchB-induced potentiating effect on Iglycine. c Representative current traces for SchB-induced potentiation of Iglycine activated by EC10-20 concentrations of glycine (40 μM for α1 receptor, 80 μM for α2 receptor, and 80 μM for α3 receptor). d The concentration-response curves for SchB-induced potentiation of Iglycine in cells expressing different α subunits. e Strychnine (STR)-induced inhibition of Iglycine was reversed by SchB 10 μM in recombinant α1 glycine receptors.***P < 0.001, ns not significant (P > 0.05), Student’s paired t-test. All data are expressed as the mean ± SD, n = 5-6.
Materials and methods
Materials
Schizandrin B was purchased from Mreda Technology Inc. (Beijing, China). Pentylenetetrazole and etomidate were purchased from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). Strychnine hydrochloride was purchased from Shanghai Aladdin Biochemical Co., Ltd (Shanghai, China). Pilocarpine hydrochloride was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Diazepam, GABA, sodium valproate, pentobarbital, and Poly-D-lysine were purchased from Sigma-Aldrich (Shanghai, China). Atropine, glycine, and NMDA were purchased from Topscience Co., Ltd. (Shanghai, China). L-Glutamate was purchased from Abcam (Cambridge, UK).
Animals
In total, 80 male CD-1 mice (weighing 25–30 g and aged 4–5 weeks), 24 male C57BL/6 N mice (weighing 20–22 g and aged 6–8 weeks) and 6 Sprague–Dawley rat pups (postnatal day 1, P1) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animals were housed in plastic cages and given free access to standard food and water under laboratory conditions with a temperature range of 22–24 °C, humidity between 50% and 60%, and a 12 h light/dark cycle. Animal care and experimental procedures have been approved by the Institutional Animal Care and Welfare Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College. Animals were randomly assigned to treatment or control groups. All the behavioral experiments were performed by experimenters who were blinded to the groups and treatments.
Construction of mutated receptor subunits
The following point mutations were introduced into human GABAA receptor cDNA α1 (gene accession number NM_000806.5): α1(H129R), α1(D219N), α1(G251S), and α1(S297Q); β2 (gene accession number NM_000813.3): β2(N289S); γ2L (gene accession number NM_198903.2): γ2L(R177G) and γ2L(P322A), and human glycine receptor cDNA α1 (gene accession number NM_000171.4): α1(S267Q), α1(S296A), α1(F380A) and α1(K385A). These mutations were generated using the Hieff MutTM Site-Directed Mutagenesis Kit (YEASEN Biotechnology Co., Ltd., Shanghai, China). The authenticity of the DNA sequence at the mutation sites was confirmed by Sanger sequencing (Genewiz, Tianjin, China).
Cell cultures and transfections
Cell culture of cell lines
T-REx™-CHO cells stably expressing human α1β2γ2L and α2β2γ2L GABAA receptors were cultured in DMEM/F12 nutrient mixture supplemented with 10% FBS, blasticidin (10 µg·ml−1), hygromycin (300 µg·ml−1), zeocin (100 µg·ml−1) and puromycin (1 µg·ml−1). Flp-In™ T-REx™ 293 cells stably expressing human α4β3δ and α6β3δ GABAA receptors were cultured in DMEM supplemented with 10% FBS, blasticidin 10 µg·ml−1, hygromycin 100 µg·ml−1, zeocin 100 µg·ml−1 and puromycin 0.2 µg·ml−1. Flp-In™-CHO cells stably expressing human α1, α2, and α3 glycine receptor subtypes were cultured in DMEM/F12 nutrient mixture supplemented with 10% FBS and 300 µg·ml−1 of hygromycin. T-REx™-CHO and Flp-In™-CHO cells were cultured in DMEM/F12 nutrient mixture supplemented with 10% FBS. These cells were utilized to generate recombinant mutant receptors (α1β2γ2L GABAARs and α1 GlyRs). HEK-293 cells were cultured in DMEM supplemented with 10% FBS and were used to express human AMPA (GluA1 and GluA2) and NMDA receptors (NR1/NR2A and NR1/NR2B).
Transfections
All transfections were performed by using Lipofectamine® LTX & Plus Reagent (Invitrogen). The enhanced green fluorescent protein (EGFP) was co-transfected with the genes of interest to facilitate the visualization of the transfected cells. The cDNA combinations were prepared as follows: for AMPA receptors, the ratio of GluA1 (or GluA2) to EGFP was 1:0.1; for NMDA receptors, the ratio of NR1: NR2A (or NR2B): EGFP was 1:1:0.2; for mutant α1β2γ2L GABAA receptors, the ratio of α1: β2: γ2L: EGFP was 1:1:3:0.5; for mutant α1 glycine receptors, the ratio of α1: EGFP was 1:0.1. Transfected cells were plated on Poly-D-Lysine-coated glass coverslips and were used for electrophysiological recordings 24–48 h after transfection.
Primary cultures of hippocampal neurons
Hippocampal neurons were extracted from Sprague-Dawley rat pups on postnatal day 1. Briefly, the rat pups were decapitated, and their hippocampi were dissected out and minced into pieces ~1 mm3 in size using scissors in HBSS on ice. The tissue was then digested in HBSS containing 0.125% trypsin (Gibco, USA) at 37 °C for 30 min. The cells were dissociated by undergoing three successive trituration and sedimentation steps in isolation buffer containing DNase I. The tissue debris was eliminated by filtering the cell suspension through a sterile cell strainer. The cell suspension was centrifuged at 1000 r/min for 5 min at room temperature. The pellet was then resuspended in plating medium, comprised of Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10% horse serum (HS), 1% penicillin/streptomycin (100 units·ml−1 and 100 μg·ml−1, respectively), and 2 mM L-glutamine (Sigma-Aldrich, USA). For whole-cell voltage-clamp recordings, hippocampal neurons were plated onto glass coverslips (12 mm) coated with 0.1 mg·ml−1 poly-D-lysine in 35-mm culture dishes at a density of 1 × 106 cells per dish. The cells were then incubated in a humidified 5% CO2 incubator at 37 °C. Four hours later, the plating media was replaced with maintenance media. The maintenance media was composed of Neurobasal medium (Gibco, USA) supplemented with 2% B27 (Gibco, USA) and 2 mM L-glutamine (Sigma-Aldrich, USA). The culture medium was replaced with half of maintenance media every three days. All the experiments were conducted between day 10 and day 14 in vitro.
Whole-cell voltage-clamp recordings
All the whole-cell patch clamp recordings were performed at a temperature of 24 ± 2 °C using a HEKA EPC-10 amplifier (HEKA Elektronik GmbH, Germany). The currents were filtered using a low-pass filter with a cutoff frequency of 2 kHz. The standard external solution contains (in mM): 140 NaCl, 3 KCl, 1.5 MgCl2, 2 CaCl2, 10 HEPES, and 10 Glucose, with a pH of 7.40 adjusted with NaOH. For GABA or glycine-evoked currents in recombinant GABAA and glycine receptors, the pipette solution contained (in mM): 145 KCl, 1 MgCl2, 5 EGTA, 5 Mg-ATP, 10 HEPES, adjusted to a pH of 7.3 with KOH. After filled with internal solution, the resistance of pipette was 1.5–2.5 MΩ. To measure GABA- or glycine-evoked currents in hippocampal neurons, the pipette solution contained the following components (in mM): 145 CsCl, 1 MgCl2, 5 EGTA, 5 Mg-ATP, and 10 HEPES, adjusted to a pH of 7.3 with CsOH, the resistances of pipette tip were 3.0–4.0 MΩ. For the current recording of AMPA receptors, the pipette solution contained (in mM): 140 CsF, 10 NaCl, 1 EGTA, and 10 HEPES, adjusted to a pH of 7.4 with NaOH. For the current recording of NMDA receptors, the Mg2+ free external solution was used (in mM): 150 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, and 10 glucose, adjusted to a pH of 7.4 with NaOH. The pipette solution contained (in mM): 145 KCl, 1 MgCl2, 5 EGTA, 5 Mg-ATP, and 10 HEPES, adjusted to a pH of 7.3 with KOH. To record the ligand-gated ion channels, membrane potential was held at −60 mV. GABA (or glycine) currents were elicited by applying GABA (or glycine) for 5 s to cells. AMPA currents were elicited by co-applying 1 mM glutamate and 100 μM cyclothiazide for 5 s to cells. NMDA currents were elicited by applying a solution containing 100 μM NMDA and 10 μM glycine for 5 s to cells.
Pentylenetetrazole (PTZ) -induced seizures
The animal model was adapted from the method reported by Mandhane et al. [25]. Male CD-1 mice (25–30 g; n = 8 per group) were treated with different drugs via intraperitoneal injections (i.p.). The animals were administered SchB (10, 30, or 60 mg·kg−1), sodium valproate (positive control, 300 mg·kg−1), or a vehicle solution (containing 30% HP-β-CD, 5% DMSO, and 1% Tween 80), 30 min prior to the subcutaneous (s.c.) injection of PTZ (85 mg·kg−1) dissolved in 0.9% sterile saline. After the injection of PTZ, the mice were closely monitored for 30 min via video recording. The latency for the onset of generalized tonic-clonic seizures (GTCS) and the mortality rate were recorded. GTCS are characterized by a rigid extension of all four limbs, exceeding a 90-degree angle with the body plane, lasting for over 10 s, followed by a loss of the righting reflex.
Strychnine (STR)-induced seizures
The animal model was adapted from the method reported by EI-Mowafy et al. [26]. Male CD-1 mice (weighing 25–30 g; n = 8 per group) were injected intraperitoneally with SchB (10, 30, or 60 mg·kg−1, i.p.), sodium valproate (positive control, 300 mg·kg−1, i.p.) or vehicle control solution containing 30% HP-β-CD, 5% DMSO, and 1% Tween 80, i.p.), 30 min prior to an administration of strychnine hydrochloride (0.75 mg·kg−1, s.c.) dissolved in 0.9% sterile saline. After the injection of STR, the mice were closely monitored for 30 min via video recording. The latency to the onset of GTCS and the mortality rate were recorded. GTCS are characterized as described in the PTZ model mentioned above.
Pilocarpine-induced status epilepticus
The animal model was adapted from the method by Gozzelino et al. [27]. Male C57BL/6 N mice (20–22 g; n = 8 per group) were administered SchB (30 mg·kg−1, i.p.) or vehicle control solution containing 30% HP-β-CD, 5% DMSO, and 1% Tween 80, i.p.), 30 min prior to an administration of pilocarpine hydrochloride (360 mg·kg−1, s.c.). At the time of compound injection, the animals were also administered 1 mg·kg−1 of atropine to block the peripheral cholinergic effects induced by pilocarpine. The animal behavior was closely monitored for 90 min through video recording and scored according to a modified Racine scale [28]: stage 0, normal activity; stage 1, freezing behavior; stage 2, tail extension, head bobbing; stage 3, continuous head bobbing and forepaw shaking; stage 4, forelimb clonus, rearing and falling; stage 5, repetitive stage 4 or big jumping; stage 6, death.
Data analysis and statistics
The behavioral data were analyzed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA). Electrophysiological data were collected and analyzed using Patchmaster and Fitmaster software (HEKA Electronics, Lambrecht, Germany), Igor Pro 6.0 (WaveMetrics, Portland, USA), GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA), and IBM SPSS Statistics Version 26 (IBM Corp., Armonk, NY, USA). Concentration-response curves were fitted using the logistic equation with four parameters: Y = Bottom + (Top-Bottom)/(1 + 10^((LogEC50 - X) * nH)), where Y represents the normalized peak current, X represents the concentration of compound, Bottom and Top are the minimum and maximum response, EC50 represents the half maximal effective concentration, and nH represents the Hill coefficient. All data are presented as mean ± SD, unless noted otherwise. The data were analyzed using paired or unpaired Student’s t tests, as well as one-way ANOVA followed by Dunnett’s post-hoc test. Statistical significance was determined at P < 0.05.
Results
SchB potentiated recombinant glycine receptors
In an earlier screening for potentiators of α1 glycine receptors based on compounds derived from Schisandra chinensis fructus, SchB was identified as a positive allosteric modulator of α1 GlyRs. Therefore, we further tested the effect of SchB on the three GlyRs (α1–α3). The EC50 concentration for glycine was determined for each subtype (Table 1). In the presence of glycine at its EC10–20 concentration, SchB-induced potentiation of glycine currents (Iglycine) was studied (Fig. 1a). SchB at 30 μM showed a more dramatic potentiation on α1 GlyRs with an Emax value of 5.32 ± 0.27-fold. It also displayed a 3.01 ± 0.44-fold potentiation on α2 GlyRs and a 2.86 ± 0.55-fold potentiation on α3 GlyRs. (Fig. 1b; Table 1). In the meanwhile, SchB enhanced the glycine currents (Iglycine) in a concentration-dependent manner (Fig. 1c, d) with EC50 values of 6.11 ± 0.75 μM, 5.11 ± 1.58 μM, and 4.93 ± 1.64 μM for α1, α2, and α3 GlyRs, respectively (Table 1). However, SchB alone did not induce any activation of GlyRs, which provides solid evidence that SchB is a positive modulator of GlyRs (Fig. S1). Due to the almost saturated effects of SchB at 10 μM, the concentration was used for the subsequent studies. Next, we examined whether SchB could reverse STR-induced antagonism of α1 GlyRs. As shown in Fig. 1e, STR (30 nM, ~IC50) significantly inhibited Iglycine in CHO cells expressing α1 GlyRs. Such inhibition of Iglycine was significantly eliminated by SchB (Fig. 1e). Meanwhile, we also investigated the impact of 10 μM SchB on the activation of glycine receptors (α1, α2, and α3 GlyRs). As shown in Fig. S2, SchB greatly enhanced the potencies of glycine but had no effects on the efficacies of glycine-induced activation on these receptors.
SchB potentiated recombinant synaptic and extrasynaptic GABAA receptors
Both GlyRs and GABAARs belong to Cys-loop receptors that form pentameric chloride channels with greater sequence homology [29]. Therefore, we assume that SchB might be also active on GABAA receptors. We successfully constructed stable cell lines expressing both synaptic and extrasynaptic GABAA receptors (Fig. S3). Diazepam (DZP) and delta selective compound 2 (DS2) were used to verify the existence of γ2 and δ subunits, respectively. Initially the effect of SchB on α1β2γ2 GABAARs was tested. In the presence of GABA at its EC10–20 concentration, the effect of SchB on GABA-elicited current (IGABA) was examined (Fig. 2a). Interestingly, SchB at 30 μM also showed potentiation on α1β2γ2 GABAA receptors with an Emax of 2.43 ± 0.36-fold (Fig. 2b). Then we further evaluated its action on the other subtypes, including α2β2γ2, α4β3δ, and α6β3δ GABAARs (Fig. 2b). These receptors represent the major synaptic and extrasynaptic GABAARs, respectively [7]. As shown in Fig. 2b, SchB also exhibited potentiation effects on the tested GABAARs. SchB at 30 μM enhanced GABA currents to 2.24 ± 0.26-fold, 3.55 ± 0.17-fold, and 2.43 ± 0.24-fold for α2β2γ2, α4β3δ, and α6β3δ GABAARs, respectively (Fig. 2b; Table 2). In the meanwhile, SchB potentiated IGABA of α1β2γ2, α2β2γ2, α4β3δ, and α6β3δ GABAARs in a concentration-dependent manner (Fig. 2c, d). The EC50 values were 4.03 ± 1.16 μM, 4.84 ± 0.95 μM, 4.93 ± 0.71 μM, and 1.51 ± 0.34 μM for α1β2γ2, α2β2γ2, α4β3δ, and α6β3δ GABAARs in the presence of EC10-20 concentrations of GABA, respectively. Based on the EC50 values and efficacies, apparently α4β3δ and α6β3δ GABAARs were more sensitive to SchB. However, SchB alone could not activate the channels in the absence of GABA, which further convinced that SchB is a positive modulator of GABAARs (Fig. S1). Then we also tested the effect of SchB on PTZ (0.5 mM, ~IC50)-induced antagonism of GABA currents (Fig. 2e). PTZ-induced inhibition of IGABA was significantly reversed by SchB (Fig. 2e). Next, we investigated the impact of SchB (10 μM) on GABA-induced activation of α1β2γ2L GABAARs. SchB not only enhanced the affinity of GABA, but also increased its efficacies (Fig. S4; Table 2).
a Schematic diagram for patch-clamp recordings in GABAAR-CHO/HEK-293 cells. b Bar graphs depicting the potentiating effect of SchB on IGABA. c Representative current traces for SchB-induced potentiation of GABA receptors. d Concentration-response curves illustrating the enhancement of IGABA by SchB on α1β2γ2L, α2β2γ2L, α4β3δ, and α6β3δ GABAARs. e SchB (10 μM) eliminated the pentylenetetrazole (PTZ)-induced inhibition of IGABA in recombinant α1β2γ2L GABAARs. ***P < 0.001, ns not significant (P > 0.05), Student’s paired t-test. All data are expressed as the mean ± SD, n = 5–6.
SchB potentiated GABAA and glycine receptors in cultured hippocampal neurons
Both GlyRs and GABAARs are highly expressed in the central nervous system. Hippocampal neurons are responsible for seizure generation and propagation [30]. Thus, we further examined the effects of SchB on GABA- and glycine-induced currents in cultured hippocampal neurons (Fig. 3a). Consistent with the data from the recombinant cells, SchB also potentiated GABAARs and GlyRs in hippocampal neurons in a dose-dependent manner (Fig. 3b–e). The EC50 values were 4.47 ± 0.92 μM and 0.99 ± 0.12 μM for GABAARs and GlyRs, respectively. The Emax values were 2.43 ± 0.24-fold and 5.06 ± 0.43-fold for GABAARs and GlyRs, respectively. Meanwhile, SchB also potentiated the GABAARs-mediated tonic currents in cultured hippocampal neurons (Fig. S5). In addition, consistent with the results observed in the recombinant cells, after applying PTZ and STR at relatively higher doses (PTZ: 0.5 mM, ~IC50; STR: 30 nM, ~IC50), SchB was still capable of eliminating the antagonists-induced suppressions (Fig. 3f, g). These findings provide strong evidence that SchB is an efficacious positive allosteric modulator of GABAARs and GlyRs.
a The schematic diagram depicts the process of primary culture and patch-clamp recordings in hippocampal neurons. b, d Representative current traces for GABAA receptors (GABAARs) and glycine receptors (GlyRs) recorded in cultured hippocampal neurons in the absence and presence of SchB (pre-incubated for 1 min). c, e Concentration–response curves of SchB for GABAA and glycine receptors. Each data point represents the mean ± SD, n = 5. f SchB 10 μM reversed the inhibition effect of pentylenetetrazole (PTZ) (0.5 mM) on GABA-induced currents, and (g) of strychnine (STR) (30 nM) on glycine-induced currents. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s paired t test. Data are represented as mean ± SD, n = 5.
SchB alleviated PTZ-, STR-, and pilocarpine-induced seizures in mice
SchB-induced dual potentiation of both GABAARs and GlyRs prompted us to test the effect of this natural compound on preclinical seizure models. PTZ is a non-competitive antagonist of GABAARs [31]. It induces acute GTCS behaviors by reducing inhibitory synaptic transmission and enhancing neuron excitability. And PTZ-induced seizure model is a commonly used method for identifying potential antiseizure medications (ASMs) in preclinical studies. Therefore, the PTZ model was applied to assess the anticonvulsant activity of SchB. SchB was administered intraperitoneally at doses of 10, 30, or 60 mg·kg−1, 30 min prior to the PTZ injection. After administering PTZ (85 mg·kg−1, s.c.), the following parameters were measured for 30 min: the latency to the onset of GTCS, the incidence of GTCS, and mortality (Fig. 4a). As shown in Fig. 4b, SchB at doses of 30 and 60 mg·kg−1 significantly increased the latency to the onset of GTCS. However, no significant effect was observed at the lower dose of 10 mg·kg−1. Sodium valproate (VPA, 300 mg·kg−1, i.p.) was used as a positive control to verify the feasibility of the model, and significantly increased the latency to the onset of GTCS. What’s more, compared to the vehicle control group, SchB reduced the incidence of GTCS (vehicle group: 100%) and mortality (vehicle group: 50%) (Fig. 4c). After the treatment of SchB at 10, 30, and 60 mg·kg−1, the incidence of GTCS was 75%, 25%, and 37.5%, respectively, and the mortality rates were 25%, 12.5%, and 0%, respectively. There was no occurrence of GTCS or death in the VPA group (300 mg·kg−1, i.p.) (Fig. 4c).
a Schematic diagram illustrating PTZ-induced seizures in mice. b The statistical results for latency to the onset of generalized tonic-clonic seizures (GTCS) in the PTZ-induced seizure model as a result of SchB (30 and 60 mg·kg−1, i.p.) and positive drug sodium valproate (VPA, 300 mg·kg−1, i.p.) treatment. c Pie charts for the reduction in incidence of GTCS and mortality rates in the PTZ-induced seizure model after the treatment of SchB and VPA. d Schematic diagram illustrating STR-induced seizures in mice. e The statistical results for latency to onset of GTCS in the STR (0.75 mg·kg−1, s.c.)-induced seizure model in the absence and presence of SchB (30, 60 mg·kg−1, i.p.) and VPA (300 mg·kg−1, i.p.). f Pie charts for the reduction in mortality rates in the STR-induced seizure model in the absence and presence of SchB (10, 30, and 60 mg·kg−1, i.p.) and VPA (300 mg·kg−1, i.p.). g Schematic diagram for pilocarpine-induced seizures in mice. h Effects of SchB (30 mg·kg−1, i.p.) on Racine score after pilocarpine administration (360 mg·kg−1, s.c.). i Effects of SchB (30 mg·kg−1, i.p.) on the intensity of the seizure attack in mice. *P < 0.05, **P < 0.01 compared with the vehicle control group using one-way ANOVA followed by Dunnett’s multiple comparisons test. #P < 0.05, ##P < 0.01 compared with the vehicle control group using Wilcoxon rank-sum test. All data are expressed as the mean ± SEM, n = 8.
Strychnine (STR), the antagonist of GlyRs, could induce acute GTCS behaviors by reducing inhibitory synaptic transmission and further enhancing neuronal excitability. Since SchB potentiated glycine receptors, we wondered whether SchB was also effective in the STR-induced seizure model. To examine the antiseizure effects of SchB on STR model, we measured the latency to the onset of GTCS and mortality for 30 min after administering STR (0.75 mg·kg−1, s.c.). SchB was administered intraperitoneally at doses of 10, 30, and 60 mg·kg−1, 30 min prior to the STR injection (Fig. 4d). As expected, SchB at doses of 30 and 60 mg·kg−1 significantly increased the latency to the onset of GTCS (Fig. 4e). However, no significant effect was observed at the lower dose of 10 mg·kg−1. Compared to the vehicle control group, SchB decreased the mortality rate (vehicle group: 100%) to 75%, 62.5%, and 50% at doses of 10, 30, and 60 mg·kg−1, respectively. The positive control, sodium valproate (VPA, 300 mg·kg−1, i.p.), significantly increased the latency to the onset of GTCS and reduced the mortality rate to 12.5% (Fig. 4e, f).
Finally, we investigated the antiseizure effects of SchB on the pilocarpine-induced status epilepticus (SE) model, which closely resembles clinical temporal lobe epilepsy. This model often results in resistance to various ASMs in patients [32]. To evaluate the antiseizure effects of SchB on pilocarpine-induced seizures, we measured the seizure scores within 90 min after administering pilocarpine (360 mg·kg−1, s.c.). SchB has shown almost equal activity at the dosage 30 and 60 mg·kg−1 in PTZ and STR models. Therefore, SchB was administered at 30 mg·kg−1 (Fig. 4g). As shown in Fig. 4h, SchB resulted in a significant decrease in seizure scores. The analysis of stage distribution (Fig. 4i) revealed that all of the mice treated with the vehicle developed stage 0–4, characterized by turning to a side position, while 87.5% of the mice reached stages 5–6. Among the mice treated with SchB, 62.5% exhibited stage 0–4, and 37.5% progressed to stages 5–6.
Overall, these results indicated that SchB could alleviate acute tonic-clonic seizures and status epilepticus.
SchB efficiently rescued the impaired GABAA receptors associated with genetic epilepsies
GABAARs are the primary inhibitory neurotransmitter-gated ion channels in the mammalian central nervous system. They play a crucial role in providing inhibitory tone to balance the tendency of hyperexcitability in excitatory neural circuits [33]. Several point mutations of GABAARs have been reported to be linked to various genetic epilepsies [13]. We selected four representative mutations of GABAARs, associated with severe genetic epilepsies, to investigate the modulation of SchB on these mutant GABAARs and explore its potential therapeutic value. These four mutations are α1(D219N, c.655G>A), α1(G251S, c.751G>A), γ2L(R177G, c.529C>G), and γ2L(P322A, c.964C>G) (Fig. 5a), closely associated with idiopathic generalized epilepsy [34], Dravet syndrome [35], febrile seizures [36], and early infantile epileptic encephalopathy, as well as the development of pharmacoresistance [37], respectively. Firstly, we evaluated the electrophysiological characteristics of these mutations. Notably, these mutations significantly decrease the sensitivity to GABA, suggesting a loss-of-function (LOF) effect for these mutations. The EC50 values of GABA for α1(D219N), α1(G251S), γ2L(R177G), and γ2L(P322A) were 8.74 ± 1.63 μM, 20.80 ± 3.36 μM, 9.04 ± 1.13 μM, and 6.60 ± 0.63 μM, respectively. The current densities of these mutations [α1(D219N), γ2L(R177G), and γ2L(P322A)] were significantly suppressed by ~60%–70% compared to the wild type of α1β2γ2L (144.2 ± 44.7 pA·pF−1), with values of 56.7 ± 22.8 pA·pF−1, 51.3 ± 28.1 pA·pF−1, and 33.8 ± 16.5 pA·pF−1, respectively. Additionally, the current density of α1(G251S) (10.9 ± 5.9 pA·pF−1) was suppressed by ~90% (Fig. 5b, d; Table 2). Despite the decreased expression level, SchB (10 μM) still showed greater potentiation of GABA-induced current in all the mutant receptors. The efficacies reached 2.31 ± 0.41-fold, 2.98 ± 0.84-fold and 3.48 ± 0.89-fold, and 2.62 ± 0.57-fold at 10 μM for α1(D219N), α1(G251S), γ2L(R177G), and γ2L(P322A), respectively (Fig. 5e, f; Table 2).
a Topology of GABAAR α1 and γ2. This diagram displays the four transmembrane segments, as well as the N-terminal and C-terminal domains. The solid circles highlighted in various colors indicate the locations of the mutations under investigation. b Representative traces for the responses to the application of 1 mM GABA were recorded from CHO cells expressing mutant α1β2γ2L GABAA receptors (α1: p.D219N, p.G251S; γ2L: p.R177G, p.P322A). c The average current density was obtained from CHO cells expressing wild-type α1β2γ2L (n = 8), p.D219N (n = 7), p.G251S (n = 7), p.R177G (n = 6), and p.P322A (n = 6) mutations. ***P < 0.001, one-way ANOVA followed by Dunnett’s multiple comparisons test, compared to the wild type. The data are expressed as mean ± SD. d The dose-response curve for GABA in wild-type α1β2γ2L (n = 8), p.D219N (n = 7), p.G251S (n = 7), p.R177G (n = 6), and p.P322A (n = 6) receptors was recorded. Statistically significant differences in the EC50 values were verified by ANOVA followed by Dunnett’s multiple comparisons test. **P < 0.01 for p.D219N, ***P < 0.001 for p.G251S, p.R177G, and p.P322A (in Table 1). e Representative current traces for GABA (EC10-20)-induced GABA currents in mutant α1β2γ2L GABAA receptors in the presence and absence of 10 μM SchB (pre-incubation for 1 min). f Summary data for the effects of SchB on wild-type and mutant α1β2γ2 GABAA receptors. All data are expressed as the mean ± SD, n = 5–6.
Collectively, our findings suggest that SchB shows great potential for treating severe genetic epilepsies caused by LOF effects of GABAARs.
Identification of amino acid residues critical for the actions of SchB in glycine receptors
A number of drugs have been reported as positive modulators of GlyRs, such as volatile anesthetics enflurane [38, 39], CBD/Δ9-THC [40], propofol [41], and endocannabinoids [42], respectively. Possible binding sites for these compounds to the GlyRs have been demonstrated. Therefore, to clarify the possible interaction sites of SchB in glycine receptors, we successfully constructed these reported mutations in mature GlyR α1, including α1S267Q (premature: S295Q, c.883A>C_884G>A_885C>G), α1S296A (premature: S324Q, c.970T>G), α1F380A (premature: F408A, c.1222 T > G_1223T > C), and α1K385A (premature: K413A, c.1237A>G_1238A>C) (diagram shown in Fig. 6a). These mutants were expressed in CHO cells and all showed similar functional activation to glycine except α1S267Q with a weaker potency (Fig. 6b; Table 1).
a Topology of GlyR α1 subunit showing the four transmembrane segments, N-terminal domain, and C-terminal domain. The solid circles highlighted in different colors indicate the location of the investigated mutations. b Concentration-response curves for glycine in wild-type and mutant α1 GlyRs. Responses at indicated concentrations in each cell were normalized to the maximum glycine-evoked peak current. Each data point represents the mean ± SD, n = 5–6. c Representative current traces activated by EC10-20 glycine of wild type and mutant α1 GlyRs before and after application of 10 μM SchB (pre-incubation 1 min). d Summary data for the effects of SchB on wild-type and mutant α1 GlyRs. ***P < 0.001, one-way ANOVA followed by Dunnett’s multiple comparisons test, compared to wild type. All data are expressed as the mean ± SD, n = 5.
As shown in Fig. 6c, d, SchB-induced potentiation (10 μM) was unaffected by the mutations S296A, F380A, and K385A. However, the mutation S267Q located in transmembrane domain 2 (TM2) almost completely abolished SchB-mediated potentiation (Fig. 6c, d). These results suggest that Ser267 in TM2 of GlyR α1 is critical for the actions of SchB.
SchB potentiated GABA-elicited currents not through diazepam- or etomidate-binding sites
To gain a deeper understanding of the molecular mechanisms underlying the modulation of GABAARs by SchB, we conducted a series of experiments to differentiate the action site of SchB from those of DZP and etomidate (ETO). DZP and ETO are classified as classical benzenediazenes (BDZs) and general anesthetics, respectively, and both act as positive modulators of GABAARs. To investigate whether SchB binds to the DZP-binding site, we firstly examined whether the activity of SchB could be eliminated by flumazenil, an antagonist that binds to the high-affinity site of BDZs [43]. In the presence of flumazenil (10 μM), SchB still kept its potentiation effect on α1β2γ2L (Fig. 7a). It was known that a histidine at position 129 (human) or 101 (murine) in α1 subunit is essential for the binding of classical BDZs [44]. To confirm the observation from the flumazenil test, we introduced the H129R (c.386A>G) mutation to the α1 subunit. As anticipated, it did not exhibit any response to 1 μM DZP (Fig. 7b). By contrast, SchB (10 μM) still potentiated GABA-elicted currents in this mutant (Fig. 7b; Table 2). These results indicated that the pontentiation mechanism of SchB on α1β2γ2L is different from that of BDZs.
a Left, representative current traces for the potentiation of IGABA by SchB in the absence and presence of flumazenil (Flu) (10 μM) in α1β2γ2L. Right, summary for SchB-induced potentiation in the absence and presence of Flu; ns not significant (P > 0.05), Student’s paired t-test. b Left, examples of current traces for the potentiation of IGABA by SchB (10 μM) and DZP (1 μM) in the mutant channel α1(H129R)β2γ2L. Right, summary for the potentiation induced by SchB and DZP. ***P < 0.001, ns = not significant (P > 0.05), Student’s paired t-test. c Current traces for the potentiation of EC10-20 GABA-elicited currents by SchB (10 μM) and ETO (10 μM) in the wild-type (WT) and mutant channel α1β2(N289S)γ2L. d Summary data for the potentiation induced by SchB (10 μM) and ETO (10 μM) in the WT and mutant channel α1β2(N289S)γ2L; ***P < 0.001, ns not significant (P > 0.05), Student’s unpaired t-test, compared to WT; **P < 0.01, Student’s paired t-test, compared to WT. All data are expressed as the mean ± SD, n = 5.
To investigate whether SchB binds to the ETO-binding site, we conducted the following experiments. Firstly, we did a co-application test to see whether SchB and ETO show any competitive effects on GABA-elicited currents. As shown in Fig. 7c, d, the co-stimulation by both SchB (10 μM) and ETO (10 μM) resulted in a supra-additive effect, rather than competitive effects, suggesting that the action site for SchB in GABAARs is different from that of ETO. To confirm this, we constructed the mutation β2N289S (c.866A>G), which is known to be able to abolish the potentiation of ETO [45]. The mutant channel α1β2(N289S)γ2L was functionally expressed in CHO cells (Table 2). As expected, the potentiation induced by SchB (10 μM) was not affected, but ETO (10 μM)-induced potentiation was significantly reduced (Fig. 7d).
Taken together, these results demonstrate that SchB might potentiate GABAARs through a site distinct from the classical DZP- and ETO-binding sites.
The residues α1S297 and β2N289 located in the β+/α- subunit interfaces of TM2 in GABAA (α1β2γ2L), homologous to Ser267 in GlyR α1, are crucial for SchB-induced potentiation of GABA-elicited currents
GABAARs and GlyRs belong to Cys-loop receptors that form pentameric chloride channels with greater sequence homology [29]. Now that the mutation S267Q in GlyRs significantly reduced the activity of SchB, we would expect that the mutations from the homologous site in GABAARs might exhibit a similar effect as GlyRs. Thus, we generated mutants in the homologous positions in the GABAAR α1 and β2 subunits. By aligning the amino acid sequences between GlyR α1, GABAAR α1, and β2 subunits, Ser297 in GABAAR α1 and Asn289 in GABAAR β2 subunits were identified as the homologous sites as Ser267 in GlyR α1 (Fig. 8a). Previous studies have found that the residues α1S297 and β2N289, located in the β+/α- subunit interfaces, are critical for the potentiation of GABA-elicited currents by pentobarbital, ethanol, and volatile anesthetic enflurane in recombinant α1β2γ2L receptors [38, 46]. Before making these mutations, we did the co-application test to see whether SchB and pentobarbital show any competitive or additive effects on GABAA1 receptors. As shown in Fig. 8b, c, the co-application of SchB (10 μM) and PB (100 μM) did not produce any additive effects on α1β2γ2L GABAARs instead of keeping the potentiating effect at the similar level as pentobarbital. These data mostly indicated a competitive effect, which suggested that the two compounds may share same or similar sites in GABAARs. We then constructed mutations α1S297Q (c.889A>C_890G>A_891C>G) and β2N289S (c.866A>G). These mutant GABAARs, including α1(S297Q)β2γ2L, α1β2(N289S)γ2L, and α1(S297Q)β2(N289S)γ2L, were functionally expressed in CHO cells (Table 2). As shown in Fig. 8b, c, the mutation S297Q in the α1 subunit significantly decreased SchB- and PB-induced potentiation in α1(S297Q)β2γ2L GABAARs. However, SchB- and PB-induced potentiation remained unaffected in mutant α1β2(N289S)γ2L GABAARs. By contrast, when using the combined mutant channels (α1(S297Q)β2(N289S)γ2L GABAARs), SchB- and PB-induced potentiation was almost completely lost. Overall, similar to PB, the results indicated that the residues α1S297 and β2N289, located in the interfaces of the β+/α- subunits, are also crucial for SchB to potentiate the activity of GABAA receptors in recombinant α1β2γ2L GABAARs. The conservation of the sequence between GlyRs (Ser267) and GABAARs (Ser297) is very critical for the action of SchB (Fig. 8d). This finding provides a solid explanation for the reason that SchB affects both GABAARs and GlyRs.
a Sequence alignment of the transmembrane domains 2 (TM2) of human GlyR α1, GABAAR α1, and β2 subunits. b Representative traces depicting EC10-20-induced GABA currents in the absence and presence of 10 μM SchB, 100 μM pentobarbital (PB) or the co-application of the two compounds in wild type (WT), α1(S297Q)β2γ2L, α1β2(N289S)γ2L, and α1(S297Q)β2(N289S)γ2L GABAARs. c Summary data for the effects of SchB and PB on wild-type and mutant α1β2γ2 GABAARs. ***P < 0.001, ###P < 0.001, ns = not significant (P > 0.05), one-way ANOVA followed by Dunnett’s multiple comparisons test, compared to the wild type. All the data are expressed as the mean ± SD, n = 5. d α1β2γ2L GABAA receptor is composed of two α, two β and one γ2L subunit. Two GABA-binding sites are located at the interfaces of the two β+/α- subunits. The scheme illustrates the localization of the point mutations α1 S297 and β2 N289 in TM2. “+“ and “−“ indicate the positive and negative sides of the subunits. The red stars represent possible binding sites for SchB and PB.
Discussion
Both GlyRs and GABAARs are the major inhibitory receptors in the central nervous system and play important roles in maintaining the excitatory-inhibitory balance. Clinical and genetic evidences have demonstrated their advantages and potentials as targets for epilepsy treatment. Currently in drug discovery field, the strategy “one gene, one disease” for drug development has become increasingly inefficient. The situation also occurs in the field of epilepsy. A drug by acting on multiple targets within the disease network would be more preferable.
In this study, we found that SchB, a major bioactive component of Schisandra chinensis (Turcz.) Baill, significantly potentiated GABA- and glycine-induced currents in a dose-dependent manner in both neuronal and recombinant receptors. Importantly, SchB showed anti-seizure effects in various experimental seizure models. The primary mechanism of action for SchB in seizures appears to be related to its potentiating effect on GABAA and glycine receptors.
SchB exerted significant potentiating effects on recombinant (α1–α3) and neuronal GlyRs. Additionally, SchB effectively eliminated STR-mediated inhibition of GlyRs. These findings suggest that SchB-induced enhancement of GlyRs may contribute to its anti-seizure effect in STR-induced seizures. Compared to the potentiating effects on recombinant homologous α1-α3 GlyRs, SchB was found to be more effective on neuronal GlyRs. It is very possible that the compositions of native GlyRs receptors, especially the presence of some other auxiliary subunits, such as β-subunit, may enhance the activity of SchB.
GABAARs play significant roles in the development of epilepsy. Recent studies have found that the surface expression of the GABAARs β2/3 and γ2 subunits was frequently reduced during status epilepticus (SE) and temporal lobe epilepsy. However, the surface expression of the δ subunit remained unchanged [47, 48]. BZDs only affect the activity of γ-containing GABAARs, but have no effect on δ-containing GABAARs [49]. This may partially explain the pharmacoresistance to BZDs during prolonged SE [50]. Therefore, δ-containing GABAARs become attractive targets for anti-seizure therapies. Encouragingly, SchB significantly potentiated both γ- and δ-containing GABAARs, with higher activity on the latter. SchB could also decrease the severity of seizures in pilocarpine-induced SE. Thus, these results indicate that compounds like SchB might have significant advantages in the treatment of epilepsy, especially a potential as an adjunctive agent with DZP for the treatment of the later stages of SE. Mutations in genes encoding subunits of GABAARs (GABRA1, GABRB2/B3, GABRG2, and GABRD) have been linked to several types of genetic epilepsy [13]. Our results demonstrated that SchB effectively restored the function of those mutated GABAARs. This suggests that SchB has a therapeutic potential for severe genetic epilepsies caused by the loss-of-function mutations of GABAARs. However, in vivo experiments are necessary to validate this effect in the future. Previous studies showed that α1-containing GABAARs are associated with sedation [51]. α2-containing GABAARs have been linked to anxiolysis [52]. Antidepressant effects of neuroactive steroids are mainly dependent on δ-containing GABAARs in the postpartum period [53], such as the recent approved drugs, brexanolone injection (ZULRESSO™), a mixture of allopregnanolone, an endogenous inhibitory pregnane neurosteroid, and sulfobutylether-beta-cyclodextrin [54]. In the present study, we found SchB significantly potentiated both α1β2γ2L, α2β2γ2L, α4β3δ, and α6β3δ GABAARs, which may account for those reported effects, such as sedative-hypnotic [18], anxiolytic [19], and antidepressant effects [20].
Finally, we thoroughly investigated the potentiation mechanism of SchB on GABAARs and GlyRs. The data suggest that SchB potentiated glycine-elicited currents through the sites different from the classical positive allosteric modulators such as CBD/Δ9-THC-, propofol-, and endocannabinoid-binding sites. Nevertheless, the mutation S267Q in TM2 of GlyR α1 almost completely eliminated SchB-mediated potentiation. Coincidentally, the mutation has also been found to eliminate ethanol- (or anesthetics such as enflurane)-induced function enhancement of GlyRs [38].
Meanwhile, the subunit dependence of SchB in GABAARs is distinct from that of DZP, indicating that SchB might function at a different site from DZP. We showed that the potentiation effect of SchB on αβ2γ2L is not interfered by flumazenil or mutant α1(H129R)β2γ2L GABAARs. Furthermore, the co-stimulation of SchB and ETO resulted in a synergistic effect. The mutation β2(N289S), which eliminated ETO-induced potentiation, did not affect SchB-induced potentiation. These results suggest that the action site of SchB is independent of those of DZP and ETO. Based on the fact that GABAARs and GlyRs belong to Cys-loop receptors that form pentameric ion channels [29], we investigated whether the residues in GABAARs homologous to Ser267 (premature: Ser295) in GlyR α1 subunit were crucial for the effect of SchB on GABAARs. Notably, SchB-induced potentiation was significantly reduced on α1(S297Q)β2γ2L, but not changed on α1β2(N289S)γ2L. Furthermore, the combined mutant constructs of α1(S297Q)β2(N289S)γ2L almost abolished SchB-induced potentiation. Coincidently, the sites happened to be able to diminish pentobarbital (PB)-induced potentiation of α1β2γ2L GABAARs [46]. The ability of PB to directly activate GABAA receptor may contribute to its anesthetic and sedative actions at higher doses [55, 56]. However, unlike barbiturates, SchB did not directly activate GABAARs. Barbiturates and SchB also differ in their activity on other targets. For example, barbiturates have been shown to block AMPA receptors [57], whereas our findings indicated that SchB did not show any effects on either AMPA or NMDA receptors (Fig. S6). These effects distinguished SchB from classical positive allosteric modulators of GABAARs and GlyRs, thereby demonstrating the unique pharmacological profile of SchB.
In conclusion, our results demonstrated, for the first time, that SchB is a dual positive allosteric modulator of GABAARs and GlyRs, alleviates the seizures in multiple mouse models. However, it cannot be excluded that other mechanisms might be involved, and additional studies will be necessary to be explored for the actions of SchB in CNS. Meanwhile, for the goal of creating innovative candidates with enhanced bioavailability and permeability through the blood–brain barrier, this study may provide a valuable scaffold for further structural design.
References
Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, et al. Epilepsy. Nat Rev Dis Prim. 2018;4:18024.
Loscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72:606–38.
Ko Y, Lee C, Lee Y, Lee JS. Systematic approach for drug repositioning of anti-epileptic drugs. Diagnostics. 2019;9:208.
Greenfield LJ Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure. 2013;22:589–600.
Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–97.
Sieghart W, Savic MM. International Union of Basic and Clinical Pharmacology CVI: GABAA receptor subtype- and function-selective ligands: key issues in translation to humans. Pharmacol Rev. 2018;70:836–78.
Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34.
Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–60.
Chattipakorn SC, McMahon LL. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J Neurophysiol. 2003;89:1339–42.
Kirchner A, Breustedt J, Rosche B, Heinemann UF, Schmieden V. Effects of taurine and glycine on epileptiform activity induced by removal of Mg2+ in combined rat entorhinal cortex–hippocampal slices. Epilepsia. 2003;44:1145–52.
Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–93.
Kaputlu İ, Uzbay T. L-NAME inhibits pentylenetetrazole and strychnine-induced seizures in mice. Brain Res. 1997;753:98–101.
Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–9.
Chung SK, Vanbellinghen JF, Mullins JG, Robinson A, Hantke J, Hammond CL, et al. Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J Neurosci. 2010;30:9612–20.
Paucar M, Waldthaler J, Svenningsson P. GLRA1 mutation and long-term follow-up of the first hyperekplexia family. Neurol Genet. 2018;4:e259.
Liao J, Zang J, Yuan F, Liu S, Zhang Y, Li H, et al. Identification and analysis of anthocyanin components in fruit color variation in Schisandra chinensis. J Sci Food Agric. 2016;9:3213–9.
Cai N-N, Wang Z-Z, Zhu X-C, Jiang Y, Zhu W-Q, Yang R, et al. Schisandrin A and B enhance the dentate gyrus neurogenesis in mouse hippocampus. J Chem Neuroanat. 2020;105:101751.
Zhang C, Zhao X, Mao X, Liu A, Liu Z, Li X, et al. Pharmacological evaluation of sedative and hypnotic effects of schizandrin through the modification of pentobarbital-induced sleep behaviors in mice. Eur J Pharmacol. 2014;744:157–63.
Chen WW, He RR, Li YF, Li SB, Tsoi B, Kurihara H. Pharmacological studies on the anxiolytic effect of standardized Schisandra lignans extract on restraint-stressed mice. Phytomedicine. 2011;18:1144–7.
Yan T, Wang N, Liu B, Wu B, Xiao F, He B, et al. Schisandra chinensis ameliorates depressive‐like behaviors by regulating microbiota‐gut‐brain axis via its anti‐inflammation activity. Phytother Res. 2021;35:289–96.
Wang Z, You L, Cheng Y, Hu K, Wang Z, Cheng Y, et al. Investigation of pharmacokinetics, tissue distribution and excretion of schisandrin B in rats by HPLC-MS/MS. Biomed Chromatogr. 2018;32:e4069.
Ma C, Sheng N, Li Y, Zheng H, Wang Z, Zhang J. A comprehensive perspective on the disposition, metabolism, and pharmacokinetics of representative multi-components of Dengzhan Shengmai in rats with chronic cerebral hypoperfusion after oral administration. J Ethnopharmacol. 2023;307:116212.
Lee TH, Jung CH, Lee DH. Neuroprotective effects of Schisandrin B against transient focal cerebral ischemia in Sprague-Dawley rats. Food Chem Toxicol. 2012;50:4239–45.
Li N, Liu J, Wang M, Yu Z, Zhu K, Gao J, et al. Sedative and hypnotic effects of Schisandrin B through increasing GABA/Glu ratio and upregulating the expression of GABAA in mice and rats. Biomed Pharmacother. 2018;103:509–16.
Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: a comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44.
El-Mowafy AM, Abdel-Dayem MA. Novel protection by omega-3-FAs against strychnine-induced tonic-convulsion in mice: synergy with carbamazepine. J Food Sci Nutr Res. 2021;4:227–39.
Gozzelino L, Kochlamazashvili G, Baldassari S, Mackintosh AI, Licchetta L, Iovino E, et al. Defective lipid signalling caused by mutations in PIK3C2B underlies focal epilepsy. Brain. 2022;145:2313–31.
Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94.
Lemoine D, Jiang R, Taly A, Chataigneau T, Specht A, Grutter T. Ligand-gated ion channels: new insights into neurological disorders and ligand recognition. Chem Rev. 2012;112:6285–318.
Derchansky M, Rokni D, Rick J, Wennberg R, Bardakjian B, Zhang L, et al. Bidirectional multisite seizure propagation in the intact isolated hippocampus: the multifocality of the seizure “focus. Neurobiol Dis. 2006;23:312–28.
Ramanjaneyulu R, Ticku MK. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur J Pharmacol. 1984;98:337–45.
Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57.
Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–9.
Han DY, Guan BJ, Wang YJ, Hatzoglou M, Mu TW. L-type calcium channel blockers enhance trafficking and function of epilepsy-associated alpha1(D219N) subunits of GABAA receptors. ACS Chem Biol. 2015;10:2135–48.
Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Moller RS, Hjalgrim H, et al. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology. 2014;82:1245–53.
Audenaert D, Schwartz E, Claeys K, Claes L, Deprez L, Suls A, et al. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–90.
Maillard PY, Baer S, Schaefer E, Desnous B, Villeneuve N, Lepine A, et al. Molecular and clinical descriptions of patients with GABAA receptor gene variants (GABRA1, GABRB2, GABRB3, GABRG2): A cohort study, review of literature, and genotype-phenotype correlation. Epilepsia. 2022;63:2519–33.
Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–9.
Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther. 2010;127:53–65.
Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol. 2011;7:296–303.
Moraga-Cid G, Yevenes GE, Schmalzing G, Peoples RW, Aguayo LG. A Single phenylalanine residue in the main intracellular loop of alpha1 gamma-aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology. 2011;115:464–73.
Yevenes GE, Zeilhofer HU. Molecular sites for the positive allosteric modulation of glycine receptors by endocannabinoids. PLoS One. 2011;6:e23886.
Hall BJ, Chebib M, Hanrahan JR, Johnston GA. Flumazenil-independent positive modulation of gamma-aminobutyric acid action by 6-methylflavone at human recombinant alpha1beta2gamma2L and alpha1beta2 GABAA receptors. Eur J Pharmacol. 2004;491:1–8.
Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–9.
Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–6.
Maldifassi MC, Baur R, Sigel E. Functional sites involved in modulation of the GABAA receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology. 2016;105:207–14.
Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28:2527–38.
Pavlov I, Walker MC. Tonic GABAA receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology. 2013;69:55–61.
Sigel E, Ernst M. The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol Sci. 2018;39:659–71.
Goodkin HP, Kapur J. The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009;50:2011–8.
McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nat Neurosci. 2000;3:587–92.
Engin E, Liu J, Rudolph U. alpha2-containing GABAA receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol Ther. 2012;136:142–52.
Melon L, Hammond R, Lewis M, Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front Endocrinol. 2018;9:703.
Scott LJ. Brexanolone: first global approval. Drugs. 2019;79:779–83.
Schulz DW, MacDonald RL. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res. 1981;209:177–88.
Ziemba AM, Forman SA. Correction for inhibition leads to an allosteric co-agonist model for pentobarbital modulation and activation of alpha1beta3gamma2L GABAA receptors. PLoS One. 2016;11:e0154031.
Taverna FA, Cameron B-R, Hampson DL, Wang LY, MacDonald JF. Sensitivity of AMPA receptors to pentobarbital. Eur J Pharmacol. 1994;267:R3–R5.
Acknowledgements
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS, no. 2021-I2M-1-029); Major Science and Technology Special Program of Yunnan Science and Technology Department (grant number 202102AA100018); Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (grant number: BZ0150).
Author information
Authors and Affiliations
Contributions
JW: Investigation, Methodology, Formal analysis, and Writing-original draft; MZ, YCJ, ML, and KXY: Investigation, Methodology, and contributing reagents. HBY: Investigation, Methodology, Writing-review & editing, Funding acquisition, Supervision, and Project administration. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All the experimental procedures on animals have been proved by the Institutional Animal Care and Welfare Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Zhao, M., Jin, Yc. et al. Schisandrin B, a dual positive allosteric modulator of GABAA and glycine receptors, alleviates seizures in multiple mouse models. Acta Pharmacol Sin 45, 465–479 (2024). https://doi.org/10.1038/s41401-023-01195-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01195-3
- Springer Nature Singapore Pte Ltd.