Abstract
Pilocarpine-induced status epilepticus (SE), which results in the development of spontaneous recurrent seizures (SRSs) activates glutamatergic receptors that contribute to seizure sustenance and neuronal cell death. In the current study, we evaluate whether the exposure to perampanel, an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor blocker, or amantadine, a N-methyl-d-aspartic acid (NMDA) receptor blocker would reduce the SE-induced long-term consequences. SE was induced in adult male Sprague Dawley rats with pilocarpine. Perampanel or amantadine was injected 10 or 60 min after SE onset. The efficacy of either, in overcoming pilocarpine-induced SE was assessed using electroencephalogram (EEG) recordings. In addition, alterations in cognitive function, development of spontaneous recurrent seizures (SRSs), and hippocampal damage that are generally encountered after SE were also assessed at 72 h and 5 weeks after the induction of SE. Our results indicate that both early and late treatment with perampanel but not amantadine significantly reduced seizure activity. Furthermore, perampanel but not amantadine, reversed the memory deficits in Y-maze and novel object recognition (NOR) tests and retarded the appearance of SRSs. Moreover, perampanel treatment led to reduced SE-induced caspase-3 activation in the hippocampal lysates. Taken together, the data obtained from the study reveals that blocking AMPA receptors by perampanel can modify SE-induced long-term consequences. Our results may provide a proof of principle for the potential therapeutic application of perampanel in clinical use for status epilepticus in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Status epilepticus (SE), characterized as a prolonged self-sustaining seizure, is associated with a significant high morbidity and mortality in people with diagnosed epilepsy or severe brain pathology [1, 2]. SE is a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms which lead to abnormally prolonged seizures [3]. A common characteristic feature of SE broadly includes the development of epileptic foci and injury primarily in the limbic region, followed by a latency period, during which epileptogenic process take place, which in turn lead to the development of spontaneous recurrent seizures (SRSs), i.e., the chronic epileptic phase. In addition to seizures, many patients after SE suffer from behavioral alterations and impairment of learning and memory, which appear to be progressive in a course of time [4, 5]. Post-mortem studies have revealed significant acute neuronal loss in the hippocampi of patients following convulsive SE. MRI studies have showed progressive atrophy of the hippocampus in people following SE [6,7,8,9]. Studies from SE animal models also showed that hippocampus is particularly vulnerable to damage by prolonged seizures [10]. The characteristics of hippocampal damage involve extensive loss of Cornu Ammonis 1 (CA1), Cornu Ammonis 3 (CA3) pyramidal cells, and mossy cells of the dentate hilus with relative sparing of dentate granule cells. In addition to neuronal damage, gliosis and mossy fiber sprouting is common and has been implicated to epileptogenesis [11, 12].
The current first-line therapy for SE is based on compounds that potentiate the inhibitory gamma-amino butyric acid A (GABAA) receptor complex. However, this first line treatment is effective in discontinuing SE in merely up to 35–65% of patients [13]. Time-dependent pharmaco-resistance is a major therapeutic problem in SE. As seizures continue, resistance to benzodiazepines develops progressively. The anti-convulsant potency of benzodiazepines can decrease 20-fold in 30 min of seizures [14]. In adult animals, diazepam was effective in controlling seizures when it was given 10 min after pilocarpine-induced SE but failed after 45 min [15]. Studies have revealed that GABAR-mediated inhibitory synaptic transmission is reduced in the hippocampi of animals in SE, due in part to the seizure-induced internalization of synaptic GABA receptors [16,17,18,19]. In contrast, as synaptic GABAA receptors are functionally inactivated, internal ionotropic glutamate receptors move to synaptic sites and become functionally active, which is believed to increase excitability and promote continued seizure activity [20, 21]. This might suggest that agents which block glutamate receptors could potentially be used in the treatment of SE. NMDA receptor antagonists such as ketamine were found to reduce neuronal degeneration when administered during early and prolonged status epilepticus [22, 23]. The non-competitive NMDA antagonist, MK801 was reported to reduce neuronal death after systemic injection of kainic acid [24]. Prolonged activation of AMPA receptors play a crucial role in the development and progression of epileptic seizures. In addition, altered expression in the different subunits of AMPA receptors have been reported in several animal models of SE [25, 26]. Moreover, seizures induce the expression of GluA2-lacking AMPA receptors which promote Ca2+ entry contributing to cell death [27, 28]. Competitive and non-competitive AMPA receptor antagonists are broad spectrum anticonvulsants in various SE animal models [29]. The role of AMPA receptors in SE has recently been reviewed by Leo et al. [27].
In the present study, we used two glutamate receptor antagonists that are currently used in patients for indications other than SE. For example, amantadine which inhibits NMDA receptors by accelerating channel closure during channel block [30]. Amantadine is routinely used in humans for the treatment of the Parkinson’s disease [31]. It has recently been found to improve cognitive outcomes and neuronal survival after traumatic brain injury in rats [32]. Furthermore, amantadine was found to be effective as adjuvant therapy for refractory absence epilepsy [33,34,35]. Perampanel is a noncompetitive AMPA receptor antagonist that has been approved as adjuvant treatment for partial seizures [36] as well as primary generalized tonic clonic seizures [37]. In previous reports, perampanel-protected mice form 6-Hz electroshock-induced seizures and increased the after-discharge threshold (ADT) in amygdala-kindled rats [38]. In addition, perampanel provided efficacy in terminating benzodiazepines resistant SE [39].
This study was designed to investigate whether perampanel or amantadine could suppress seizure activity and attenuate the long-term memory impairment and neuronal loss in an in vivo pilocarpine rat model of SE. In addition, we monitored the occurrence of SRSs after the prophylactic treatment with perampanel or amantadine. Overall, our study provides evidence and underlines mechanisms by which glutamate receptor antagonists exert therapeutic efficacy in the treatment of SE.
Materials and Methods
Animals
Adult male Sprague-Dawley (SD) rats weighing 250–300 g (with only two rats weighed around 400 g in the perampanel control group) were used in the study. Animal care protocols and guidelines were approved by the University of Saskatchewan Animal Research Ethics Board, following the Canadian Council on Animal Care. Rats were housed two per cage in standard polypropylene cages in a temperature controlled (21 °C) colony room on a 12/12-h light/dark cycle. Experimental procedures were carried out during the light phase. Rats were divided into six groups: group 1: vehicle control (n = 23), group 2: perampanel control (n = 8), group 3: amantadine control (n = 8), group 4: pilocarpine + vehicle (n = 24), group 5: pilocarpine + perampanel (n = 38), and group 6: pilocarpine + amantadine (n = 38).
Electrode Implantation and Electroencephalography
All surgeries were performed as described before with some modifications [40]. The animal was anesthetized using 5% isoflurane and positioned in a Kopf stereotaxic instrument. Anesthesia was maintained throughout the surgery with isoflurane gas (2% isoflurane delivered in O2). The incisor bar was adjusted until bregma was leveled with lambda. One unipolar stainless-steel depth electrodes (E363-1-SPC stainless steel electrode, bare diameter 0.25 mm, insulated diameter 0.28 mm, Plastics One, Roanoke, VA) was introduced into the brain parenchyma to record intrahippocampal electroencephalogram (EEG) activity. The stereotaxic coordinates relative to bregma according to the atlas of Paxinos and Watson were as follows: anterior-posterior (AP) = −3.5 mm, medial-lateral (ML) = 2.4 mm, and distal ventral (DV) = −3.5 mm. Another unipolar electrode was implanted into the cortex (AP = 0.5 mm, ML = 4.0 mm, DV = − 1.2 mm). A third depth electrode was positioned in the white matter of the cerebellum (AP = − 11 mm, ML = 5.3 mm, DV = −5.6 mm) to serve as the reference. A fourth screw electrode was positioned in the occipital bone to serve as the ground. The other end of the electrodes was inserted into a plastic pedestal (plastics one) and the entire setup was secured by acrylic adhesive. The wound was closed with surgical sutures, and Anafen was given on the surgery site for post-operative analgesia as follows: one dose 30 min before surgery (5 mg/kg, sc) The same dose was repeated for 3 days after surgery. Animals were allowed to recover for a period of 1 week.
Induction of Seizure and Treatment Protocol
Animals were injected with scopolamine methyl bromide (1 mg/kg, sc) 15 min before pilocarpine injection to minimize peripheral cholinergic effects. Pilocarpine (380 mg/kg, ip) was dissolved freshly in 0.9% saline. The beginning of SE was considered when the animal suffered a stage 4–5 motor seizure in Racine’s scale and high-frequency spikes on EEG. One hour after the development of SE, rats were given pentobarbital injection (25 mg/kg, ip). Animals in the vehicle control and SE groups received the same dose of pentobarbital. Rats in the pilocarpine + perampanel and perampanel control groups have not received pentobarbital. In the pilocarpine + amantadine group, we found that a dose of 10 mg/kg of pentobarbital given 2 h after the amantadine dose have reduced the mortality rate in this group; rats in the amantadine drug control group received the same dose of pentobarbital. Seizures were monitored for 2–2.5 h by recording EEG. Perampanel (8 mg/kg, ip; dissolved fresh in 1:1:1 (v/v) distilled water, dimethyl sulfoxide, and polyethylene glycol 300) or amantadine (45 mg/kg ip, dissolved fresh in saline) was administered 10 or 60 min after the onset of seizures. Two hours after pentobarbital injection, rats were given subcutaneous injections of 5% dextrose and 0.9% saline (2 ml/rat) for hydration and were monitored daily for adequate food and water intake by measuring body weight. Following the day of induction of SE, the dose of perampanel was given in tapering down plan (4 mg/kg for week I, 2 mg/kg for week II, and 0.5 mg/kg for week III). As the chance of post-SE seizures is higher and comparable with the time of SE, higher doses of perampanel early on makes more sense to save neurons. Further, the treatment was planned for tapering down dosage as the half-life of perampanel was long (70 h), and accumulation of high dose may lead to toxic effects in rats. In addition, rapid withdrawal of perampanel, as an antiseizure medication, may result in recurrence of status epilepticus or seizure clusters. Amantadine (45 mg/kg) was given twice daily and stopped 2 weeks before the behavioral assessment. Using this dosing protocol, rats were sacrificed either 72 h after the induction of SE in the acute study or after 5 weeks in the long-term study. In the 5-week study, medications were stopped for the last 2 weeks in order to give the rats a chance to wash out the medications. As we needed the rats to be free of medications that may interfere with the development of SRSs (Fig. 1).
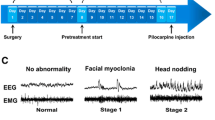
Schematic illustration of the pilocarpine induction and treatment protocol to investigate the effects of perampanel and amantadine in a pilocarpine-induced rat model of status epilepticus. Animals were implanted with electrodes, 1 week before SE induction. Status epilepticus was induced by injection of a high dose of pilocarpine (380 mg/kg) following pretreatment with scopolamine methyl bromide. Drugs or vehicle were injected 10 or 60 min after seizure onset. Rats were tested with Y-maze and NOR 72 h or 5 weeks after SE onset and then sacrificed to assess neuronal injury. In long-term study, rats from different treatment groups were monitored for the detection of spontaneous recurrent seizures 3 weeks after SE induction
Assessment of Behavioral Seizures
Following pilocarpine injection, the animals were observed for seizure scoring according to Racine criteria with slight modification [41]. The seizure scoring was as follows: stage 1, immobilization, eye blinking, twitching of vibrissae, and mouth movements; stage 2, head nodding, often accompanied by severe facial clonus, piloerection; stage 3, straub tail, forelimb clonus; stage 4, rearing; stage 5, rearing, falling, and generalized convulsions.
Spatial Memory Test
Y-maze apparatus with three enclosed arms (60 cm length × 16 cm width × 30 cm height) was used for spatial memory as described previously with some modifications [42]. Visual cues outside but around the maze were used to assess hippocampal-dependent spatial recognition memory. The test consisted of two trials with a 90 min interval in between. Rats were transported to the behavioral testing room in their home cages at least 1 h before testing. In the first training (acquisition) trial, rats were placed in the maze facing the end of a randomly chosen arm (start arm) and allowed to explore the maze for 15 min with one arm closed (novel arm). Rats were returned to their home cages until the second (retrieval) trial, during which they could explore freely all three arms of the maze. The time spent in each arm was measured using video recordings. Rats entering an arm with all four paws was counted as an entry. Data were presented as the time spent in the novel arm to the total time in all three arms during the 5-min retrieval trial. The maze was cleaned with 40% ethanol between trials to ensure that animal’s behavior was not guided by odor cues.
Novel Object Recognition Test
The NOR task was used to evaluate recognition memory as descried previously with some modifications [43]. This task consisted of two phases, a learning phase and a memory phase. During the learning phase, rats were placed into the behavioral arena for 15 min and allowed to explore two identical stimulus objects before being placed back into the home cage. After a 90-min delay, rats were placed back into the arena where one of the two identical objects were replaced by an entirely new stimulus object. The recognition index (RI, representing the time spent investigating the novel object (T novel) relative to the total object investigation) was used as the main index of retention, which was calculated according to the following formula: RI = T novel / (T novel + T familiar). The arena and objects were cleaned with 40% ethanol between the trials to prevent the existence of olfactory cues.
Recording for SRSs
Arida et al. and Hoexter et al. previously demonstrated that the average latency onset to SRSs in rats treated with the pilocarpine protocol was 11–18 days [44, 45]. In the present study, rats were observed for behavioral and electrographic seizure for 8–10 h/day for 2 weeks starting 3 weeks after the induction of SE. Because the frequency of SRSs in rats after pilocarpine-induced SE is much higher during the light (diurnal) compared with the dark (nocturnal) period [46, 47], all recordings for spontaneous seizures were done during the light period (7 a.m.–7 p.m.). Electrographic seizures were analyzed offline and seizure was confirmed by manual review of the tracing morphology of EEG recording and the taped videos. Since most SRSs following pilocarpine-induced SE are generalized [48], only the occurrence of class 4/5 behavioral seizures was included in the spontaneous seizure analysis. A rat was considered epileptic after exhibiting one or more SRSs. Outcome measures were the percentage of animals that developed SRSs and the number of SRSs recorded per week.
Fluoro-Jade C Staining
To examine the dying neurons in the rat brains, the fixed brain samples were cut into 30 μm thick sections. To visualizing the degenerative neurons, fluoro-jade C staining (FJC) staining was carried out following the standard procedures suggested by the manufacturer (Millipore Sigma, cat No. AG325). FJC-positive staining exhibited bright green color visualized on a fluorescence microscope (Olympus, BX-60). Three sections from each brain and three to five rats in each group were used for analysis.
NeuN and GFAP Immunohistochemistry
Immunohistochemistry on PFA fixed free-floating sections was performed on brains sectioned at a thickness of 30 μm. Briefly, the sections were treated with 0.1 M Tris buffer (TB) containing 1% hydrogen peroxide for 30 min. The slices were washed in phosphate-buffered saline solution (PBS 0.1 M, pH 7.4) containing 0.1% Triton X-100. Then incubated in blocking solution (0.5% Triton X-100, 10% bovine serum albumin for 1 h). Sections were incubated overnight at 4 °C in the primary antibody diluted in 0.1% Triton X-100 and 2% bovine serum albumin. The antibodies used were as follows: rabbit anti-glial fibrillary acidic protein (GFAP) (1:200, Thermofisher) and mouse anti-neuron-specific nuclear protein (NeuN) (1:500, Chemicon). Biotinylated secondary antibodies (goat anti-rabbit, goat anti-mouse, all from Vector Laboratories, Burlingame, CA), diluted at 1:200 for 2 h, followed by standard avidin-biotin complex (ABC; Vector). The tissue-bound peroxidase was then developed using 3,3-diaminobenzidine (DAB) visualization procedure (1–3 min). The sections were mounted on slides and cover slipped with DPX. Images were taken using a × 4 lens of a light microscope. The numbers of positive cells were manually counted. The data were presented as mean ± standard deviation. All measurements were repeated three to five times, and the mean value was used.
Biotinylation
Surface expression of the GluA2 subunit of the AMPAR was studied in the hippocampal slices obtained from control (n = 4), perampanel control (n = 4), SE (n = 4), and SE + perampanel animals (n = 4) using a biotinylation assay as described previously with some modifications [16, 49]. For this experiment, the rats were monitored for the occurrence of the first grade 5 seizures, which corresponded to the beginning of SE. Perampanel (8 mg/kg) was given 10 min post-SE. Animals were sacrificed 20 min after the first tonic-clonic seizures. Hippocampal slices (300 μm) were prepared with a vibratome using ice-cold oxygenated dissection buffer (4 °C, 95% O2, 5% CO2) containing 65.5 mM NaCl, 2 mM KCl, 5 mM MgSO4, 1.1 mM KH2PO4, 1 mM CaCl2, 10 mM dextrose, and 113 mM sucrose. The slices were then placed in an oxygenated artificial CSF (aCSF) at room temperature and allowed to equilibrate for 30 min. The aCSF contained 124 mM NaCl, 4 mM KCl, 1 mM MgCl2, 25.7 mM NaHCO3, 1.1 mM KH2PO4, 10 mM dextrose, and 2.5 mM CaCl2. Hippocampal slices were biotinylated by incubating them in ice-cold aCSF containing 1 mg/ml biotin for 30 min at 4 °C with gentle shaking. The unbound biotin was removed by washing the slices three times with Tris-buffered saline containing 25 mM Tris, pH 7.4, 137 mM NaCl, 5 mM KCl, 2.3 mM CaCl2, and 0.5 mM MgCl2. Slices were lysed in RIPA lysis buffer supplemented with 1 mm sodium orthovanadate and a protease inhibitor mixture. The insoluble fraction was removed after centrifugation of the lysate at 14,000×g for 15 min at 4 °C. Protein concentration was measured, and 45 μg of total protein was used for assay of total protein expression. To separate the biotin-tagged proteins, lysates containing 250 μg of protein were incubated with 100 μl of neutravidin-agarose beads overnight at 4 °C followed by extensive washing of the beads with a RIPA-lysis buffer and elution of the biotin-tagged protein in a non-reducing sample buffer for 5 min at 95 °C. Proteins were subjected to 8% SDS-PAGE as described below. The antibodies used in this experiment include anti-GluA2 antibody (1:1000). The absence of contaminating cytoplasmic proteins in the biotinylated samples was confirmed by re-probing blots with cytoplasmic protein 14-3-3 (Abcam, 1:1000). β-Actin was found to be attached to plasma membranes [50, 51]. The signal intensity was quantified using image J; the total expression of the GluA2 subunit was normalized with β-actin expression and the ratio of surface/total protein was also calculated.
Western Blotting
Hippocampal tissues were homogenized in lysis buffer (25 mM Tris, 150 mM NaCl, 0.1% sodium deodecyl sulphate, 0.5% sodium deoxycholate, and 1% Triton X-100, pH 7–8). The homogenates were kept on ice for 15 min and centrifuged at 15,000 rcf for 10 min at 4 °C. The supernatant was collected and used for western blot. Samples consisting of the same amount of total proteins were separated on 12% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were incubated with 5% fat-free milk for 1 h at room temperature to block nonspecific background. The target proteins were immunoblotted with primary antibodies against caspase-3 (cell signaling, 1:1000) overnight at 4 °C and then with corresponding HRP-conjugated secondary antibody for 1 h at room temperature. Membranes were re-probed with β-tubulin (Santa Cruz Biotech. Inc., 1:500) on the same blot to verify consistency of protein loading. Protein bands of interest were analyzed using NIH ImageJ software and data were expressed as the percentage of the intensity of target protein to that of corresponding to the loading control.
Statistical Analyses
Significance was set at p < 0.05 and assessed by one-way ANOVA with post hoc analyses relying on Tukey’s test (GraphPad Prism 5.0). Data are represented as mean ± standard deviation (SD).
Results
Perampanel, Not Amantadine Terminated Pilocarpine-Induced Status Epilepticus in Rats
In order to quantitatively compare the responses with the different treatments administered after the development of status epilepticus, the seizure duration was monitored based on the EEG recordings (Fig. 2). Figure 2 illustrates a typical EEG recording from vehicle or perampanel- or amantadine-treated rats in which status epilepticus had been induced by ip injection of a 380-mg/kg dose of pilocarpine. Treatments were initiated 10 min after induction of seizure. Perampanel (8 mg/kg) caused a cessation of seizure behavior rapidly with sustained suppression of electrographic seizures, as illustrated in Fig. 2. The latency of seizure termination in the perampanel-treated group was 9.5 ± 4.3 min. In contrast, amantadine (45 mg/kg) showed no anti-seizure effect. Conversely, amantadine-treated animals showed very intense behavioral seizure with hyperlocomotion, jumping, rearing and falling and more intense seizure on EEG recording. In a pilot experiment (n = 4), amantadine alone failed to stop or reduce the intensity of seizure, and all rats were found dead the next day. In order to reduce the mortality rate in this group, a dose of 10 mg/kg of pentobarbital was given 2 h after the amantadine dose was given. In a separate series of experiments, the treatments were administered 60 min after continuous electrographic seizure activity. Such late administration of perampanel still presented high efficiency in terminating electrographic status epilepticus (latency, 18.2 ± 6.1 min) with only minimal recurrence of seizure. Late administration of amantadine alone was not effective to terminate electrographic status epilepticus (latency, 166.3 ± 17.9 min). Overall, when administered early or late, perampanel was more effective than amantadine in suppressing seizure activity.
Effect of perampanel or amantadine on terminating ongoing seizures. a Representative EEG recording from hippocampal and cortical electrodes. Left, the compressed EEG from SE, perampanel, and amantadine animals up to 60 min following treatment. Right, the magnified 6 s prior to SE, during SE, 15 min post-treatment and 1-h post-treatment. EEG traces prior to SE or following to SE (marked by vertical lines a–c at 0.5 mV, horizontal bar = 1 s). b Graph shows the effect of early and late treatment (10 and 60 min after onset of status epilepticus) with perampanel and amantadine on the duration of EEG seizure activity. Values were expressed in mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0. **p < 0.01 and ***p < 0.001 indicate comparisons with SE group
Perampanel, Not Amantadine Attenuated Cognitive Deficits in SE Rats
In order to examine the efficiency of perampanel or amantadine on attenuation of cognitive impairment induced by SE, Y-maze, and NOR were performed in rats. Figure 3a shows that the performance in the Y-maze was significantly impaired in the SE group compared with the control at 72 h and 1 month after SE initiation. When rats were treated with perampanel (10 min post-SE), their performance was improved significantly after 72 h (p < 0.05) or 5 weeks (p < 0.01) of treatment compared with the SE group. In contrast, SE rats treated with amantadine did not show any improvement on exploring the novel arm in 72 h and 5 weeks after SE. Similarly, in the NOR test, rats in perampanel-treated group spent more time in exploring novel object than the SE rats as shown in Fig. 3b (p < 0.05). Again, rats with amantadine treatment did not show any improvement on NOR performance. Together, these observations indicate that perampanel prevented both short and long-term memory deficit in pilocarpine-induced SE rats. Treatment with perampanel (n = 8) or amantadine (n = 8) alone did not significantly affect the behavior of control rats in the Y-maze or the NOR test.
Effect of perampanel or amantadine on cognitive function in pilocarpine-induced status epilepticus rat model using Y-maze and NOR. a Cartoon represents Y-maze, and graph represents the time spent in novel arm (seconds) during retrieval trial at 72 h and 5 weeks after initiation of SE. Groups include control (Ctl), status epilepticus (SE), perampanel drug control, perampanel + SE, amantadine drug control, and amantadine + SE. b Cartoon represents NOR and graph represents the recognition index of NOR test from the above-mentioned groups. [The recognition index (RI), representing the time spent investigating the novel object (T novel) relative to the total object investigation]; RI = T novel / (T novel + T familiar): A, 72-h group (treatment started 10 min post-SE); B, 72-h group (treatment started 60 min post-SE); C, 5 weeks group (treatment started 10 min post-SE); D, 5 weeks group (treatment started 60 min post-SE). Values were expressed as mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by post hoc test. #p < 0.05 and ##p < 0.01 vs. control; *p < 0.05 and **p < 0.01 vs. seizure group
Perampanel, Not Amantadine Retarded Epileptogenesis
We then examined whether perampanel and amantadine treatment has a long-term effect on the development of SRSs. Neither of rats treated with perampanel 10 min after SE developed SRSs. However, when given 60 min after SE, 30% of the treated rats developed SRSs after stopping the treatment.
During the 2-week observation for SRSs, spontaneous seizures were noted in 66% of vehicle-treated SE rats and 54% and 62% of amantadine-treated rats 10 and 60 min post-SE, respectively. (Fig. 4). The frequency of seizure was not significantly different in the two groups in that no less frequent seizure were observed in the amantadine-treated groups during the monitoring period. Thus, under our experimental conditions, the prophylactic treatment with amantadine during or after SE exerted no effect in retarding the development of spontaneous seizures.
Effects of perampanel or amantadine on epileptogesesis in pilocarpine-induced status epilepticus rat model. a Representative recording of SRSs. Top, the compressed 60 s. EEG of SRSs. Bottom, the magnified 6 s. EEG recording marked by horizontal lines a and b. Vertical bar = 0.5 mV, horizontal bar = 1 s. b Graph showing the number of SRSs recorded per week. Values were expressed as mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0. *p < 0.005 and ***p < 0.001 vs. the SE group. c The percentage of rats that developed SRSs during the seizure monitoring period in the different treatment groups
Perampanel, Not Amantadine Inhibited SE-Induced Neuronal Loss in SE Rats
We examined the distribution of neuronal injury in the hippocampal regions from each group by FJC and NeuN immunohistochemistry analysis. Visual inspection of FJC-stained sections indicated severe neuronal degeneration in the CA1, CA3, and hilar regions of rat brains 72 h and 5 weeks after initiation of SE (Fig. 5). In addition, NeuN immunohistochemistry revealed neuronal loss in these parts of the hippocampus (Fig. 6). In contrast, there was no obvious neuronal damage in the hippocampal formation of perampanel-treated rats when given 10 min after SE (p < 0.001). When given 60 min after SE onset, the protective effect of perampanel was deceased only within the CA1.
Effects of perampanel or amantadine on neuronal survival in pilocarpine-induced SE rat model. Top, the Fluorojade C (FJC)-positive staining in bright green color, indicating degenerated neurons. Representative images of FJC-stained hippocampus in control, SE, and perampanel (Per.)- and amantadine (Aman.)-treated rats at (a) 72 h and (b) 5 weeks after SE induction. Bottom, the number of FJC-positive cells in the CA1 and CA3 regions: (1) 72-h group (treatment started 10 min post-SE), (2) 72-h group (treatment started 60 min post-SE), (3) 5 weeks group (treatment started 10 min post-SE), and (4) 5 weeks group (treatment started 60 min post-SE). Values are expressed as mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0. ###p < 0.001 vs. control. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. SE group
Effects of perampanel or amantadine treatment on neuronal survival after the induction of SE. Top, NeuN immunohistochemistry of the CA1, CA3, and DG regions of the hippocampus in control, SE, and perampanel (Per.)- and amantadine (Aman.)-treated rats at a 72 h and b 5 weeks after SE induction. c Bottom, the number of NeuN immune-positive cells in the CA1 and CA3 regions: a 72-h group (treatment started 10 min post-SE); b 72-h group (treatment started 60 min post-SE); c 5 weeks group (treatment started 10 min post-SE); and d 5 weeks group (treatment started 60 min post-SE) Values are expressed mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0. ###p < 0.001 vs. control group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. SE group
Amantadine, in contrast, showed a different profile in hippocampal cell death in SE rats. When amantadine was given 10 min post-SE, 75% of treated rats showed degenerating neurons in the CA1, CA3, and the hilar region. The degree of damaged neurons was more profound 5 weeks after SE in the amantadine treated group. Treatment of rats with amantadine 60 min post-SE did not exert obvious neuroprotective effect at all sections examined (p > 0.05). No cell loss was seen in brain sections from rats treated with perampanel or amantadine alone without the induction of seizure.
Effect of Perampanel and Amantadine Treatments on the Activation of Astrocytes
GFAP is regarded as a marker of reactive gliosis. It is well known that following brain lesions, astrocytes become reactive and release numerous proinflammatory cytokines that play an important part in secondary injury. Control rats showed few GFAP-positive cells in the CA1, CA3, and the dentate hilar regions (Fig. 7), and these cells had a typical morphology of resting astrocytes. At 72 h after the induction of SE, the pilocarpine-treated seizure group showed profound gliosis demonstrated by higher number of GFAP immunoreactivity. GFAP-positive astrocytes showed enlarged soma size (hypertrophy) and longer projections together with increased GFAP expression. Treatment with perampanel or amantadine alone without the induction of SE did not change GFAP expression in any of the treated rats. Compared with the SE group, the SE + perampanel group (10 min group) had significantly less GFAP-positive astrocytes while amantadine treatment 10 min failed to significantly suppress the SE-induced gliosis (p > 0.05). Administration of perampanel 60 min after SE reduced gliosis from the CA3 and the hilar region but not from the CA1 area. Compared with the SE group, amantadine treatment 60 min post-SE was not effective in reducing the degree of gliosis (p > 0.05).
Effects of perampanel or amantadine on astrocyte activation in hippocampus of pilocarpine-induced status epilepticus (SE) rat model at 72 h and 5 weeks after SE. a Representative images of GFAP immunohistochemistry of lower magnification images (× 4) showing the astrogliosis in the entire hippocampus in control, SE, and perampanel (Per.)- and amantadine (Aman.)-treated rats at 72 h and 5 weeks after SE induction. b Graphs showing number of GFAP immune-positive cells in the CA1 and CA3 regions: a 72-h group (treatment started 10 min post-SE); b 72-h group (treatment started 60 min post-SE); c 5 weeks group (treatment started 10 min post-SE); d 5 weeks group (treatment started 60 min post-SE). Values are expressed mean ± SD. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison in GraphPad prism 5.0. ##p value < 0.01 and ###p value < 0.00 vs. control; **p value < 0.05 and **p value < 0.01 vs. the SE group
Perampanel, Not Amantadine Inhibited SE-Induced Caspase-3 Activation
In order to explore the underlined mechanism exerted by perampanel, western blot analysis was applied to detect the activated caspase-3 levels in hippocampal region from all treatment groups. The immunoreactivity of caspase-3 cleaved bands, was increased in hippocampal regions from SE rat brains (p < 0.01); this enhanced expression was significantly reduced by early administration of perampanel (p < 0.05), not amantadine treatment (Fig. 8).
Effects of perampanel or amantadine on caspase-3 activation in pilocarpine-induced status epilepticus (SE) rat model. Representative western blot showing the expression levels of the total and active caspase-3 in control, vehicle SE, and perampanel- and amantadine-treated rats (drugs given 10 min after SE onset) at 72 h and 5 weeks after the induction of seizure. Graphs show the changes of active/full-length caspase-3 ratio in different treatment groups. Data are expressed as mean ± SD, control = 3, SE = 3, Per. = 3, Aman. = 3. The experimental groups were compared and analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0. #p < 0.05 and ##p < 0.01 vs. control group; *p < 0.05 and **p < 0.01 vs. the SE group
Perampanel Treatment Partially Reduced the Trafficking of GluA2 Subunit of AMPARs During SE
The surface expression of the GluA2 subunit were determined using a biotinylation assay. Representative western blot (Fig. 9) demonstrates reduced surface membrane expression of the GluA2 subunit in hippocampal slices from SE rats compared with controls. Surface GluA2 signal was normalized to the total expression, and the expression ratio in refractory SE hippocampi was less than that in control hippocampi (1.19 ± 0.32 vs. 0.60 ± 0.22, p < 0.05). When perampanel was given 10 min after the development of first stage 5 behavioral seizures, the surface expression of the GluA2 subunit was similar to that in controls (p > 0.05). Treatment of animals with perampanel alone without the induction of SE did not alter the expression of GluA2 subunits (0.995 ± 0.32, p > 0.05). The absence of the cytoplasmic protein 14-3-3 in surface membrane fraction confirmed the purity of surface proteins (p > 0.05).
Perampanel partially inhibited SE-induced decrease of surface GluA2 subunits. Cell-surface biotinylation of hippocampal slices showed a decrease in GluA2 surface expression after pilocarpine-induced SE compared with control. This effect was inhibited by perampanel. a–e Sample Western blots of the surface protein fraction (a–c) and the total protein fraction (d, e) of the GluA2 (a, d), beta actin (b, e), and 14-3-3 (c) in hippocampal slices obtained from animals in Ctl (n = 4), SE (n = 4), SE + Per. (n = 4), and Per. Ctl (n = 4). The total expression of the GluA2 subunit was normalized with β-actin expression and the ratio of surface/total protein was calculated. Data were expressed as mean ± SD; n = 4. Protein 14-3-3 was absent in surface biotinylation blots, confirming no contamination of biotinylated AMPARs with cytosolic proteins. Mean difference between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad prism 5.0
Discussion
The purpose of this study is to investigate the hypothesis that perampanel and amantadine exert therapeutic effects after status epilepticus. Perampanel is able to terminate ongoing status epilepticus providing a long-lasting inhibition of the seizure whereas amantadine failed to terminate the seizure when given 10 or 60 min after the development of SE. In the experiment to assess the long-term consequences of SE, i.e., the development of cognitive alterations and development of SRSs and hippocampal damage in a pilocarpine rat model of SE, perampanel preserved the memory of rats, retarded the appearance of SRSs, and reduced the SE-induced hippocampal cell death. In contrast with our expectations, the consequences of the treatment with amantadine after SE were largely negative. Rats treated with vehicle or amantadine after SE both developed SRSs. Furthermore, impairment of spatial and recognition memory was observed in both groups compared with controls. The histological examination of the hippocampal formation shows a significant cell loss without any noticeable neuroprotective effect of amantadine treatment.
In our study, early administration of perampanel successfully terminated the ongoing seizures. These results were in agreement with previous studies of AMPA receptor antagonists in animal models of SE [39, 52,53,54]. There was an increase in the latency to terminate the seizure when perampanel was given 60 min post-SE suggestive of tolerance against perampanel’s effect. On the other hand, at a dose of 45 mg/kg, amantadine increased the intensity of behavioral and electrographic seizure. To our knowledge, the mechanism by which amantadine exacerbated the seizures is unknown. This lack of anticonvulsant efficacy of amantadine in the pilocarpine rat model was in agreement with some studies that have demonstrated that NMDA antagonist may lack efficacy during the early stage of SE [55].
Although a wide range of behavioral deficits may follow SE, disturbances in learning and memory are frequently reported [5]. Given the key role of hippocampus in certain form of memory, it is not surprising that hippocampal lesions caused by SE are accompanied by impairments of learning and memory [56, 57]. Using the pilocarpine rat model of SE, we have demonstrated that the saline-treated SE rats exhibited spatial memory deficits early after the induction of SE and this deficit persisted during epileptogenesis. In contrast, the recognition memory appeared not to be significantly affected early after SE. Object recognition memory was impaired only after nearly complete hippocampal lesions (as seen in the CA1, CA3, and hilar regions of the hippocampus of SE rats 5 weeks after the onset of SE). Thus, the recognition memory was relatively spared by smaller lesions that severely impaired spatial memory, 72 h after the induction of SE. These results support the theory that hippocampal region is important for both spatial and recognition memory, and that a sufficient hippocampal damage is required to reveal deficits in the recognition memory. Our results came in agreement with previously published studies that reported deficits in the recognition memory after brain insults including SE [58,59,60] Changes in spatial and recognition memory were prevented by blocking AMPA receptors using perampanel. These finding suggest that the cognitive deficits after SE were mediated in part by AMPA receptor activation.
Citraro et al. reported that perampanel significantly reduced the development of absence epilepsy in WAG/Rij rat model which suggests that AMPA receptors are involved in the process of epileptiogenesis [61]. In addition, perampanel exhibited anti-epileptogenic in younger rats [62]. In our study, early treatment with perampanel completely blocked epileptogenesis, since no animals developed SRSs. It was significantly better than the untreated SE or the amantadine-treated group. When given 60 min after the onset of SE, 30% of rats developed SRSs. In addition, the frequency of SRSs was less than that seen in the SE control rats. This would possibly reflect a reduction in SE severity.
As previously reported [63], perampanel eliminated SE-induced neuronal cell loss within the CA1, CA3, and the hilar regions when given 10 min after the onset of SE. This effect may be due to its ability to interfere with the initial insult and/or the neuroprotective properties of perampanel. However, perampanel given 60 min after SE is not equally effective in protecting CA1 neurons against pilocarpine-induced seizures. This may suggest that AMPA receptors are not the primary mediator of seizure-induced cell loss later in SE, at least within the CA1 neurons.
Although several studies have demonstrated that NMDA antagonists are effective as a therapeutic intervention for the treatment of status epilepticus [23, 64], however, amantadine was the least effective drug tested in this study. Under our experimental conditions, amantadine lacked any neuroprotective or antiepileptiogenic effects. The failure of amantadine treatment might be due to the ability of glutamate released during the seizures to replace amantadine from its binding sites. Another explanation might be dose inadequacy given the higher rate of elimination in rats compared with humans [32]. However, according to our observation in a pilot study to adjust the dose of amantadine, higher or more frequent doses of amantadine increased the aggressive behavior in treated rats that made it difficult to handle them, making it impractical. Furthermore, it is possible that amantadine decreased the seizure threshold and made the animals brains more susceptible to the SE-induced damage [65].
AMPA receptors containing the GluA2 subunits exhibit low calcium permeability, while GluA2 lacking AMPA receptors are permeable to calcium [66]. In most of the hippocampal neurons, the AMPA receptors contain GluA2 subunits and are impermeable to calcium. The calcium-permeable GluA2 lacking AMPA receptors were reported to be a contributing cause for ischemia-induced neuronal loss [67]. In rodents, SE lead to reduced expression of GluA2 subunits [49]. Recent report indicated that perampanel can block both calcium permeable and calcium in permeable AMPA receptors [68]. Under our experimental conditions, perampanel given 10 min post-SE reduced the SE-induced surface reduction of GluA2 subunits. While the reduction of SE-induced GluA2 internalization after perampanel treatment failed to reach statistical significance, the possibility that it may contribute to blocking the SE-induced GluA2 downregulation cannot be ruled out. More studies are warranted to address its participation in affecting GluA2 trafficking.
The impact of pilocarpine-induced SE on the brain is controversial [69, 70]. However, reports have demonstrated that seizures damage neuronal cells by necrosis or programmed cell death pathways [71,72,73]. Caspase-3 cleavage was also observed in the SE brains. This finding is also supported by studies of caspase-3 activation following experimentally induced SE in different animal models [74,75,76,77]. Our data indicate that programmed cell death is activated in pilocarpine-induced SE and is contributing to SE neuronal death. Furthermore, treatment with perampanel after pilocarpine-induced SE significantly reduced the activation of this mechanism most likely via reducing the seizure severity.
In summary, this study reports improvement in cognitive function along with neuroprotection after administration of perampanel in an experimentally induced SE. In addition, prophylactic administration of perampanel after SE attenuated epileptiogenesis within the 5 weeks study. While it is not possible to draw a clinical conclusion from this animal study, our results support the design of future clinical studies to assess the role of early administration of perampanel in human SE to preserve hippocampal-dependent memory function. In contrast with our expectation, treatment with amantadine was largely negative without significant effect on the development of SRSs, behavioral alteration, or hippocampal damage. This does not mean that amantadine is not a potentially interesting drug; for instance, amantadine was found to be neuroprotective in a TBI animal model [32]. Altogether, the data presented here provide a rational for the further evaluation of perampanel and amantadine for the treatment of SE.
References
Chen JW, Naylor DE, Wasterlain CG (2007) Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl 186:7–15
Walker MC (2016) Pathophysiology of status epilepticus. Neurosci Lett 667:84–91. https://doi.org/10.1016/j.neulet.2016.12.044
Trinka E et al (2015) A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia 56(10):1515–1523. https://doi.org/10.1111/epi.13121
Helmstaedter C et al (2003) Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 54(4):425–432. https://doi.org/10.1002/ana.10692
Helmstaedter C (2007) Cognitive outcome of status epilepticus in adults. Epilepsia 48(Suppl 8):85–90
DeGiorgio CM et al (1992) Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia 33(1):23–27
Provenzale JM et al (2008) Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol 190(4):976–983. https://doi.org/10.2214/AJR.07.2407
Fujisao EK et al (2017) Hippocampal damage and atrophy secondary to status epilepticus in a patient with schizophrenia. Front Neurol 8:24. https://doi.org/10.3389/fneur.2017.00024
Nairismagi J et al (2004) Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia 45(9):1024–1034. https://doi.org/10.1111/j.0013-9580.2004.08904.x
Pohlmann-Eden B et al (2004) Evolution of MRI changes and development of bilateral hippocampal sclerosis during long lasting generalised status epilepticus. J Neurol Neurosurg Psychiatry 75(6):898–900
Gibbons MB et al (2013) Contributions of astrocytes to epileptogenesis following status epilepticus: opportunities for preventive therapy? Neurochem Int 63(7):660–669. https://doi.org/10.1016/j.neuint.2012.12.008
Feng L, Molnar P, Nadler JV (2003) Short-term frequency-dependent plasticity at recurrent mossy fiber synapses of the epileptic brain. J Neurosci 23(12):5381–5390
Treiman DM et al (1998) A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status epilepticus cooperative study group. N Engl J Med 339(12):792–798. https://doi.org/10.1056/NEJM199809173391202
Kapur J, Macdonald RL (1997) Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 17(19):7532–7540
Mazarati AM et al (1998) Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res 814(1–2):179–185
Goodkin HP et al (2008) Subunit-specific trafficking of GABA(a) receptors during status epilepticus. J Neurosci 28(10):2527–2538. https://doi.org/10.1523/JNEUROSCI.3426-07.2008
Naylor DE, Liu H, Wasterlain CG (2005) Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25(34):7724–7733. https://doi.org/10.1523/JNEUROSCI.4944-04.2005
Terunuma M et al (2008) Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 28(2):376–384. https://doi.org/10.1523/JNEUROSCI.4346-07.2008
Niquet J et al (2016) Benzodiazepine-refractory status epilepticus: pathophysiology and principles of treatment. Ann N Y Acad Sci 1378(1):166–173. https://doi.org/10.1111/nyas.13147
Fritsch B et al (2010) Treatment of early and late kainic acid-induced status epilepticus with the noncompetitive AMPA receptor antagonist GYKI 52466. Epilepsia 51(1):108–117. https://doi.org/10.1111/j.1528-1167.2009.02205.x
Naylor DE et al (2013) Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis 54:225–238. https://doi.org/10.1016/j.nbd.2012.12.015
Loss CM, Cordova SD, de Oliveira DL (2012) Ketamine reduces neuronal degeneration and anxiety levels when administered during early life-induced status epilepticus in rats. Brain Res 1474:110–117. https://doi.org/10.1016/j.brainres.2012.07.046
Borris DJ, Bertram EH, Kapur J (2000) Ketamine controls prolonged status epilepticus. Epilepsy Res 42(2–3):117–122
Schauwecker PE (2010) Neuroprotection by glutamate receptor antagonists against seizure-induced excitotoxic cell death in the aging brain. Exp Neurol 224(1):207–218. https://doi.org/10.1016/j.expneurol.2010.03.013
Grooms SY, Opitz T, Bennett MV et al (2000) Status epilepticus decreases glutamate receptor 2 mRNA and protein expression in hippocampal pyramidal cells before neuronal death. Proc Natl Acad Sci U S A 97:3631–3636
Friedman LK (1998) Selective reduction of GluR2 protein in adult hippocampal CA3 neurons following status epilepticus but prior to cell loss. Hippocampus 8:511–525
Leo A et al (2018) The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia 58(6):1098–1108. https://doi.org/10.1111/epi.14082
Condorelli DF et al (1994) Changes in gene expression of AMPA-selective glutamate receptor subunits induced by status epilepticus in rat brain. Neurochem Int 25:367–376
Rogawski MA (2013) AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol Scand Suppl 197:9–18. https://doi.org/10.1111/ane.12099
Blanpied TA, Clarke RJ, Johnson JW (2005) Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 25(13):3312–3322. https://doi.org/10.1523/JNEUROSCI.4262-04.2005
Rajput A, Rajput AH (2006) Parkinson's disease management strategies. Expert Rev Neurother 6(1):91–99. https://doi.org/10.1586/14737175.6.1.91
Wang T et al (2014) Amantadine improves cognitive outcome and increases neuronal survival after fluid percussion traumatic brain injury in rats. J Neurotrauma 31(4):370–377. https://doi.org/10.1089/neu.2013.2917
Perry MS et al (2012) Amantadine for the treatment of refractory absence seizures in children. Pediatr Neurol 46(4):243–245. https://doi.org/10.1016/j.pediatrneurol.2012.02.004
Shahar EM, Brand N (1992) Effect of add-on amantadine therapy for refractory absence epilepsy. J Pediatr 121(5 Pt 1):819–821
Shields WD, Lake JL, Chugani HT (1985) Amantadine in the treatment of refractory epilepsy in childhood: an open trial in 10 patients. Neurology 35(4):579–581
French JA et al (2012) Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 79(6):589–596. https://doi.org/10.1212/WNL.0b013e3182635735
French JA et al (2015) Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology 85(11):950–957. https://doi.org/10.1212/WNL.0000000000001930
Hanada T et al (2011) Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52(7):1331–1340. https://doi.org/10.1111/j.1528-1167.2011.03109.x
Hanada T, Ido K, Kosasa T (2014) Effect of perampanel, a novel AMPA antagonist, on benzodiazepine-resistant status epilepticus in a lithium-pilocarpine rat model. Pharmacol Res Perspect 2(5):e00063. https://doi.org/10.1002/prp2.63
Eid T et al (2008) Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain 131(Pt 8):2061–2070. https://doi.org/10.1093/brain/awn133
Racine RJ et al (1973) Rates of motor seizure development in rats subjected to electrical brain stimulation: strain and inter-stimulation interval effects. Electroencephalogr Clin Neurophysiol 35(5):553–556
Ghafouri S et al (2016) Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Res 126:37–44. https://doi.org/10.1016/j.eplepsyres.2016.06.010
Brewster AL et al (2013) Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS One 8(3):e57808. https://doi.org/10.1371/journal.pone.0057808
Arida RM et al (1999) The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res 34(2–3):99–107
Hoexter MQ et al (2005) Consequences of prolonged caffeine administration and its withdrawal on pilocarpine- and kainate-induced seizures in rats. Epilepsia 46(9):1401–1406. https://doi.org/10.1111/j.1528-1167.2005.63904.x
Goffin K et al (2007) Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol 205(2):501–505. https://doi.org/10.1016/j.expneurol.2007.03.008
Lopim GM et al (2016) Relationship between seizure frequency and number of neuronal and non-neuronal cells in the hippocampus throughout the life of rats with epilepsy. Brain Res 1634:179–186. https://doi.org/10.1016/j.brainres.2015.12.055
Cavalheiro EA, Naffah-Mazzacoratti MG, Mello LE, Leite JP (2006) The pilocarpine model of seizures. In: Pitkanen A, Schwartzkroin PA, Moshe SL (eds) Models of seizures and epilepsy. Elsevier Academic Press, New York, pp. 433–448
Rajasekaran K, Todorovic M, Kapur J (2012) Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann Neurol 72(1):91–102. https://doi.org/10.1002/ana.23570
Suprynowicz FA et al (2008) HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene 27(8):1071–1078. https://doi.org/10.1038/sj.onc.1210725
Dinic J, Ashrafzadeh P, Parmryd I (2013) Actin filaments attachment at the plasma membrane in live cells cause the formation of ordered lipid domains. Biochim Biophys Acta 1828(3):1102–1111. https://doi.org/10.1016/j.bbamem.2012.12.004
Pitkanen A (2002) Drug-mediated neuroprotection and antiepileptogenesis: animal data. Neurology 59(9 Suppl 5):S27–S33
Pitkanen A, Halonen T (1998) Prevention of epilepsy. Trends Pharmacol Sci 19(7):253–255
Loscher W (2002) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50(1–2):105–123
Mazarati AM, Wasterlain CG (1999) N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett 265(3):187–190
Chauviere L et al (2009) Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 29(17):5402–5410. https://doi.org/10.1523/JNEUROSCI.4699-08.2009
Winson J (1978) Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201(4351):160–163
Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20(23):8853–8860
Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101(40):14515–14520. https://doi.org/10.1073/pnas.0406344101
Gould TJ et al (2002) Effects of hippocampal lesions on patterned motor learning in the rat. Brain Res Bull 58(6):581–586
Citraro R et al (2017) Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive-like behavior. Epilepsia 58(2):231–238
Dubuis N et al (2017) Anti-ictogenic and anti-epileptogenic properties of perampanel in mature and immature rats. Epilepsia 58(11):1985–1192. https://doi.org/10.1111/epi.13894
Wu T et al (2017) The neuroprotective effect of perampanel in lithium-pilocarpine rat seizure model. Epilepsy Res 137:152158–152158. https://doi.org/10.1016/j.eplepsyres.2017.06.002
Fang Y, Wang X (2015) Ketamine for the treatment of refractory status epilepticus. Seizure 30:14–20. https://doi.org/10.1016/j.seizure.2015.05.010
Hosenbocus S, Chahal R (2013) Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry 22(1):55–60
Plant K et al (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9(5):602–604. https://doi.org/10.1038/nn1678
Kwak S, Weiss JH (2006) Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol 16(3):281–287. https://doi.org/10.1016/j.conb.2006.05.004
Barygin OI (2016) Inhibition of calcium-permeable and calcium-impermeable AMPA receptors by perampanel in rat brain neurons. Neurosci Lett 633:146–151. https://doi.org/10.1016/j.neulet.2016.09.028
Fujikawa DG, Shinmei SS, Cai B (2000) Seizure-induced neuronal necrosis: implications for programmed cell death mechanisms. Epilepsia 41(Suppl 6):S9–S13
Salmenpera T et al (1998) Hippocampal damage caused by seizures in temporal lobe epilepsy. Lancet 351(9095):35. https://doi.org/10.1016/S0140-6736(05)78092-2
Fujikawa DG et al (2002) Caspase-3 is not activated in seizure-induced neuronal necrosis with internucleosomal DNA cleavage. J Neurochem 83(1):229–240
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460(2):525–542. https://doi.org/10.1007/s00424-010-0809-1
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15(11):1382–1402. https://doi.org/10.1007/s10495-010-0481-0
Niquet J et al (2007) Status epilepticus triggers caspase-3 activation and necrosis in the immature rat brain. Epilepsia 48(6):1203–1206. https://doi.org/10.1111/j.1528-1167.2007.01102.x
Faherty CJ, Xanthoudakis S, Smeyne RJ (1999) Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res 70(1):159–163
Narkilahti S et al (2003) Expression and activation of caspase 3 following status epilepticus in the rat. Eur J Neurosci 18(6):1486–1496
Tzeng TT et al (2013) Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid. J Biomed Sci 20:90. https://doi.org/10.1186/1423-0127-20-90
Funding
This work was supported by Saskatchewan Health Research Foundation (SHRF) Establishment Grant (SHRF No. 3543).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mohammad, H., Sekar, S., Wei, Z. et al. Perampanel but Not Amantadine Prevents Behavioral Alterations and Epileptogenesis in Pilocarpine Rat Model of Status Epilepticus. Mol Neurobiol 56, 2508–2523 (2019). https://doi.org/10.1007/s12035-018-1230-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1230-6