Abstract
Neutrophil extracellular traps (NETs), crucial in immune defense mechanisms, are renowned for their propensity to expel decondensed chromatin embedded with inflammatory proteins. Our comprehension of NETs in pathogen clearance, immune regulation and disease pathogenesis, has grown significantly in recent years. NETs are not only pivotal in the context of infections but also exhibit significant involvement in sterile inflammation. Evidence suggests that excessive accumulation of NETs can result in vessel occlusion, tissue damage, and prolonged inflammatory responses, thereby contributing to the progression and exacerbation of various pathological states. Nevertheless, NETs exhibit dual functionalities in certain pathological contexts. While NETs may act as autoantigens, aggregated NET complexes can function as inflammatory mediators by degrading proinflammatory cytokines and chemokines. The delineation of molecules and signaling pathways governing NET formation aids in refining our appreciation of NETs’ role in immune homeostasis, inflammation, autoimmune diseases, metabolic dysregulation, and cancer. In this comprehensive review, we delve into the multifaceted roles of NETs in both homeostasis and disease, whilst discussing their potential as therapeutic targets. Our aim is to enhance the understanding of the intricate functions of NETs across the spectrum from physiology to pathology.
Similar content being viewed by others
Introduction
Neutrophils are the first line of defense within the innate immune system, crucial for protecting the host against pathogens. Alongside traditional defense mechanisms, recent attention has focused on unique fibrous web-like chromatin structures, termed neutrophil extracellular traps (NETs).1,2 NETs aid neutrophils in immobilizing and trapping pathogens, thereby contributing to host defense.3,4,5 This process relies on associated histones, proteolytic enzymes from granules, and enzymatic myeloperoxidase (MPO).1,2 Accumulating evidence strongly supports the direct and indirect regulatory effects of NETs on both adaptive and innate immunity,6,7,8 playing a crucial role in immune homeostasis. Moreover, NETs contribute specific mechanisms to potentiate immunothrombosis,9,10,11,12 potentially playing a protective role in the context of infection.13
NETs are typically formed and exhibit antibacterial activity in a variety of infectious conditions, including bacterial, parasitic, and fungal infections,14,15 where these pathogens can act as stimuli to induce NET formation. Impaired NET function may facilitate pathogen evasion from the immune system and create a niche for chronic infection.16,17,18 Nevertheless, akin to a double-edged sword, sustained inflammation or persistent stimuli can lead to excessive NET formation, thereby exacerbating tissue damage during inappropriate inflammation. Additionally, NET formation is observed in nonpathogenic conditions, including but not limited to sterile inflammation, autoimmune disorders, metabolic dysregulation, vasculitis, thrombosis, and carcinogenesis when dysregulated.19,20,21 Under sterile conditions, NETs can be induced by interleukin-8 (IL-8),22 immune complexes,23 crystals,24 or damage-associated molecular patterns (DAMPs), such as high mobility group Box 1 (HMGB1).25 Evidence thus far suggests that NETs play dual roles in these nonpathogenic conditions. On one hand, NETs may act as autoantigens in autoimmune conditions, contributing to tissue destruction, amplifying the inflammatory cascade, and promoting thrombosis formation.19,20,21 On the other hand, aggregated NETs formed during sterile inflammation, containing a diverse array of enzymes, have the potential to serve as inflammatory mediators by degrading proinflammatory cytokines and chemokines, thereby promoting inflammation resolution and wound healing.10,11 Despite the controversial role of NETs, major studies confirm their more detrimental roles in nonpathogenic inflammation.
Emerging evidence emphasizes the protumorigenic role of NETs in various cancers,26,27,28 primarily due to their contribution to cell damage and regeneration, leading to subsequent excessive inflammation. NETs have been reported to promote tumor cell proliferation,29 metastasis,30,31,32 immunosuppression,33,34 and cancer-associated thrombosis.35 Additionally, NETs can capture circulating tumor cells and facilitate their colonization.36 The antitumor effects of NETs vary depending on tumor type and microenvironment.37 While the debate continues regarding whether NETs inhibit or promote tumor progression, their role in promoting tumor development appears more evident.38 Accumulated NETs provide an immunosuppressive microenvironment favoring the survival of premalignant cells and cancer cells.39 Elevated NET markers correlate with poor clinical outcomes in cancer patients and may serve as prognostic indicators.40,41,42 This review explores the molecular mechanisms underlying NET formation and clearance, along with recent advances in comprehending how NETs contribute to both infection defense and pathologies associated with various diseases, including specific inflammatory, autoimmune, thrombotic, and cancerous conditions. Additionally, we provide an overview of current clinical trials and therapies targeting NETs, offering insights into the development of therapeutic strategies targeting NETs in the clinical practice.
History of research on NETs
NETs have a rich history in research, beginning approximately two decades ago. NETs were first described in the early 2000s as a protective mechanism against pathogenic bacteria,1 which was subsequently expanded to protection against yeast43 and protozoal species. Quickly thereafter, NETs were associated with a variety of human disease processes, first described in the female reproductive tract.44,45,46 As NETs continued to be studied, it was revealed that certain bacteria expressed endonucleases that degraded NETs as a protective mechanism.47,48,49 As these mechanisms for pathogen evasion50,51 became better understood, this led to research developments on harnessing exogenous methods of inhibition or degradation to address human pathology.
In 2007, models of NET activity began to expand into other animal models including fish,52 and zebrafish,53 demonstrating the conserved function of NETs across species. Simultaneously, research shifted toward elucidating the mechanism of NETosis, as well as the structural components that are responsible for their functionality; Pentraxin-3 (PTX3) was identified as a structural protein dotted on NETs54 and the connection with toll-like receptor-mediated activation, which was monumental in the study of NETs in sepsis.
Thus began the era of NETs as prognostic biomarkers in the clinical setting,55,56,57 particularly in the realm of autoimmune disease. Beginning in 2010, the role of NETs in cancer began to emerge,58 first being implicated in non-human animal models. In 2011, exogenous deoxyribonuclease (DNase) came to the forefront as a modality of NET degradation in human disease models59 and has remained a primary agent for NET degradation in current pre-clinical and clinical trials. Causative mechanisms for how their degradation led to these improved outcomes expanded substantially,60,61 leading to studies that focused on inhibiting NET formation62,63 in addition to the degradation that was emphasized previously.
Quickly after the association between human cancers and NETs was made, it became evident that NETs were also responsible for malignancy-related complications such as tumor-associated thrombosis64,65 and metastases.66 Due to the immunogenic environment of cancers, it was natural that at this time the ability of NETs to modulate the innate as well as the adaptive immune microenvironment was also recognized, notably in terms of modulating the T cell compartment.67
The first human observational studies regarding NETs in critical care literature was published in 2014,68 then rapidly expanded to the transplant69 and cardiac70,71 populations. With these observational studies, the in-vivo effects of NETs became better understood72 and the use of NET components in prediction models grew.73,74,75,76 Furthermore, the beginnings of high throughput biomarker detection systems started to be explored.77,78
Beginning in 2016, the concept of iatrogenic NET induction was introduced, with commonly used medical tools such as antibiotics79,80 and ventilators81 implicated in NET formation and subsequent poor outcomes. A key cause of iatrogenic NET induction was found to be chemotherapy, leading to treatment resistance.82 In addition to chemotherapy resistance, significant advances were made in identifying the role of NETs in metastatic disease, with a heavy emphasis in their role in modulating the immune microenvironment,83,84,85 inducing escape mechanisms such as epithelial-mesenchymal transition (EMT),33,86,87 and migration.88,89,90,91 This ultimately led to the expansion of research on NET targeting therapies,92,93,94,95,96 and mitigating the adverse effects of NETs. In the 2020s, agents targeting NET degradation or inhibition have been expanded outside of DNase, exploring thrombomodulin97 or necrostatin-198 as promising agents in the preclinical space. Furthermore, more selective targeting of NET components has become more prominent, demonstrating similar outcome efficacy as degradation.99 Interestingly, the role of exercise in reversing the effects of NETs has become a popular topic of research interest100,101 in recent years.
While the connection of NETs and the immune system, particularly in its modulation of other immune players102 has been well researched in the decades of NET-related research, NETs have also been found to connect to a myriad of homeostatic mechanisms, in particular cellular metabolism.103,104 As the knowledge of NETs multi-functionality and its role in disease has expanded in recent years, research has shifted to elucidating its role as a prognostic and predictive biomarker in acute stages of disease,105,106,107 and strides have been taken to elucidate its role in other disease processes through genomics research108 within the past five years. Research thus far has illustrated the wide breadth and comprehensive scope of NET functionality and continues to make rapid advancements (Fig. 1).
History of research on NETs. The major discoveries related to NETs, from their initial identification and role in pathogen eradication to their involvement in diseases such as cancer. It illustrates the progression of research over time and the increasing recognition of their clinical significance. This figure was created by Adobe Illustrator Artwork 16.0 (Adobe Systems, USA)
Structure of NETs
NETs are web-like extracellular filamentous structures released by activated neutrophils. A distinctive feature of NETs is the exposed DNA fibers with diameters of 15-17 nm formed by decondensed neutrophil nuclear chromatin, which are important components of NETs. Although DNA is extruded from NETs for defense purposes, it has both antimicrobial and pro-inflammatory properties throughout the immune responses.109 High concentrations of DNA can chelate divalent metal cations, which can destroy the membranes of bacteria. As a cue for tissue damage locally or programmed cell death, extracellular DNA can be rapidly degraded by circulating nucleases, as well as engulfed by phagocytes.110,111 Impairment of the process might trigger a strong inflammatory response. Mitochondrial DNA (mtDNA) is another source of NETs and acts as a DAMP capable of triggering a pro-inflammatory response. The rapid activation of important NETs by mtDNA stimulates other neutrophils, which amplify the inflammatory responses by further releasing NETs through a positive feedback mechanism.112,113
Notably, histones, including H1, H2A, H2B, H3, and H4, are also major components of NETs, accounting for ~70% of the proteins of NETs.114 Although unstimulated neutrophils have the same proportion of all core histones, there are higher amounts of H2A and H2B compared with H3 and H4 in NETing neutrophils.115 Posttranslational modifications of histones also have been found in NETs, even during NET formation. As serine proteases shear the histones of NETs during NET formation, histones of NETs are 2–5 kDa smaller than those unstimulated.116 Acetylation is another modification neutralizing the positive charges in histones, allowing them to detach from DNA and chromatin loss.117 The conversion of arginine into citrulline by peptidyl arginine deiminases (PAD) is named histone citrullination, and it is noteworthy that citrullinated histones have been recognized as one of the major sources of autoantibodies in certain autoimmune diseases, such as rheumatoid arthritis (RA).118,119 In addition, histones also have immunophysiological characteristics, such as antimicrobial activity, cytotoxicity, and immunomodulation. Extracellular histones can cause potent pro-inflammatory responses, leading to organ damage and even death.111
Furthermore, cytoplasmic proteins (including S100 calcium-binding proteins A8/A9/A12) and granular proteins (such as MPO), neutrophil elastase (NE), proteinase 3 (PRTN3), cathepsin G, neutrophil defensins) bind in globular patterns to NETs. During the formation and release of NETs, the chromatin swells up, allowing the granule components and cellular components to come into contact.111,120 The toxicity of the various components released by degranulation might cause tissue damage at the site of infection and play an important role in some non-infectious diseases, especially autoimmune diseases and tumors.
Mechanisms of NET formation
Activation of NETs
NETs catch a wide range of bacterial pathogens and prevent their spread. Previous studies have shown that Streptococcus suis (S. suis) can be recognized by toll-like receptors (TLRs), which activate NET formation in an nicotinamide adenine dinucleotide phosphate oxidase (NOX)-dependent manner.121 Although small pathogens exhibit weaker stimulatory effects of NETs, small bacteria have been reported to induce NET formation. This occurs when small microorganisms evade death by phagosomes and tend to aggregate. The size of the external invaders is not a determining factor in activating formation of NETs, but the number of particles in the neutrophil cytoplasm may be a sensitive indicator, as Staphylococcus aureus (S. aureus) aggregates when exposed to plasma in a murine model of sepsis, which triggers NET formation.122,123 Moreover, NET activation has been perceived in response to virus infection caused by respiratory syncytial virus (RSV), human immunodeficiency virus (HIV), hepatitis B virus (HBV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).124,125,126,127 In RSV and HIV-induced infections, NETs seem to be beneficial to the immune systems, whereas NET formation in patients with Coronavirus disease 2019 (COVID-19) has been shown to be deleterious.
In addition to pathogens, different immunological stimuli (including interleukins, interferons, autoantibodies, and immune complexes), tumor-associated stimuli (including granulocyte-colony stimulating factor (G-CSF), C-X-C motif chemokine ligands (CXCLs)), lipopolysaccharides (LPS) and DAMPs can also promote the formation of NETs. The stimuli may activate the cell surface receptors of neutrophils; for example, immune complexes activate the FcgRIIIb receptor, CXCLs recognize CXC chemokine receptors (CXCRs), C3a recognizes C3a receptor (C3aR), as well as HMGB1 recognizes receptor of advanced glycation end products (RAGE) and TLR4.2,128,129 Upon activation of receptors on neutrophils by stimuli, a variety of intracellular signaling mechanisms are further activated, resulting in NET formation. Notably, activated platelets and endothelial cells, the important parts of microenvironment in vivo, have also been reported to exhibit a role in activating NET formation in diseases such as sepsis, stroke and tumors.130,131
Phorbol 12-myristate 13-acetate (PMA) is a well-known activator of NET formation used for scientific studies. Recent studies have demonstrated that certain metabolites and external environmental factors, and also induce NET activation. Metabolites from gut microbiome dysbiosis and free fatty acids are involved in both infectious and non-infectious diseases by promoting NET formation.132 Cigarette smoke and PM2.5 might contribute to pulmonary diseases through activating NETs as well.133,134 Moreover, bleomycin has been shown to induce NET formation and fibrosis in the lungs of mice.135 Diverse particles also have been shown to induce NET formation, such as hydrophobic nanoparticles, acicular microparticles, and other natural and artificial crystals. Nanoparticles with specific surface properties can be used as adjuvants that stimulate NETs.136 Munoz et al. found that lysosomal destabilization and nuclear disassembly occur simultaneously after exposure of neutrophils to nonpolar nanoparticles, followed by the formation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent chromatin externalization, suggesting that, in addition to exogenous factors, lysosomal leakage in neutrophils might also trigger NET formation.137 However, to date, the formation of NETs in response to a variety of stimuli is not fully understood.
NET formation pathways
In various diseases, neutrophils are recruited into the microenvironment by diverse mediators to form NETs. Chemokine concentration gradients influence the direction of neutrophil migration. For instance, local tissue injury can lead to increased production of G-CSF, which stimulates neutrophil recruitment.138 Additionally, CXCLs and C-C motif chemokine ligands (CCLs), such as CXCL1, CXCL5, and CCL2, play key roles in neutrophil recruitment in diseases.139,140
Although the specific process of NET formation differs depending on the stimuli, it can be categorized as two main pathways (Fig. 2). The first is a cell death pathway termed NETosis, which begins with nuclear delobulation, disassembly of nuclear membranes, a constant loss of cellular polarization, decondensation of chromatin, and eventually rupture of plasma membranes. This process of lytic cell death is that taking 2–4 h usually.20,141 An alternative pathway is non-lytic NETosis that can occur without cell death, whereby chromatin expulsion is accompanied by granular proteins release. These components are formed extracellularly, leaving behind active anucleate phagocytes with microbial phagocytosis and chemotaxis capabilities. This pathway occurs relatively quickly, usually within 5–60 min, but depends on the inducer.20,142
NET formation pathways. NET formation can be categorized into two main pathways. The first type is the classic pathway known as NETosis, which initiates with nuclear lobulation, followed by disassembly of nuclear membranes, loss of cellular polarization, chromatin decondensation, and eventual rupture of plasma membranes. An alternative pathway is termed non-lytic NETosis which can occur without cell death, where chromatin expulsion is accompanied by the release of granular proteins. These components are formed extracellularly, leaving behind active anucleate phagocytes with capabilities for microbial phagocytosis and chemotaxis. This figure was created by Adobe Photoshop CS6 (Adobe Systems, USA)
The lytic NETosis
The lytic NETosis pathway is also known as “suicide NETosis”, as well as NOX-dependent NETosis. Antibodies, microorganisms, cholesterol, and PMA can induce the lytic NETosis.143 These stimuli trigger the activation of signaling pathway proteins, leading to increased cytosolic calcium levels and activation of NOX. Further downstream, oxidase converts molecular oxygen to create reactive oxidative species (ROS). NE is located in the granules of phagocytosis in the resting neutrophils, partly bound to MPO and attached to the granule membranes, with another part in the lumen. ROS induces the activation of NE, as well as its release into the cytoplasm from the MPO-containing azurosome complex. NE binds to F-actin and mediates degradation of actin filaments. NE then translocates to the nucleus and partially cleaves histones to promote chromatin decondensation. Hydrogen peroxide releases NE into the cytoplasm selectively, which depends on MPO. However, inhibition of the enzymatic activity of MPO only delays rather than prevents NETosis, most likely because of the role of MPO in activating the hydrolytic activity of NE on bulky protein substrates.144
The role of the MPO-NE pathway is supported by studies of neutrophils from diabetes patients with hereditary MPO deficiency at high risk of infection.145 Bellaaouaj et al. have reported that mice with NE deficiency are more susceptible to sepsis and death,146 and NE inhibition prevents NET formation and rescues mice from ischemia/reperfusion injury, infection, and tumor.147,148,149,150 Lacking the NADPH oxidase in the respiratory burst pathway can decrease the ability to kill microorganisms, leading to recurrent microbial infections. Similarly, neutrophil elastase gene (ELANE) mutation is one of the most common genetic mutations in neutropenic patients. ELANE-induced neutropenia is associated with dysfunction of the theisprotease enzyme rather than due to NE deficiency. Patients with heterozygous mutations in the ELANE gene might develop severe life-threatening congenital neutropenia, or cyclic neutropenia with mild to moderate clinical characteristics.151
Recent studies have shown another nuclear chromatin-binding protein implicated in NETosis is DEK. Both DEK depletion and treatment with DEK-targeted aptamers attenuate inflammation in vivo and greatly impair NET formation, while NETosis can be reversed by addition of exogenous recombinant DEK protein, suggesting that chromatin decondensation mediated by DEK binding is similar to MPO.152,153
Another factor involved in NETosis is PAD4, which decreases the positive charge of histones, as well as their electrostatic interactions with DNA. The formation of a catalytically active conformation of this enzyme requires five calcium ionophores, which are always employed in studies on exploring the role of PAD4 in NETosis.144 ROS also promotes PAD4 activation.154 Citrullination mediated by PAD4 can be triggered by hydrogen peroxide, which can be reduced by inhibiting NADPH oxidase, indicating an association between PAD4 and production of ROS. The results of experiments with PAD4 inhibitor-treated cell lines or with neutrophils from mice with PAD4 deficiency are difficult to interpret because of low NET yields.135 For example, PAD4 inhibition prevents NET formation activated by nicotine rather than cholesterol crystals.24,155 However, studies with a variety of NET markers have shown that inhibition of PAD4 suppresses NET release in murine models of sepsis and cancer. Moreover, recent studies demonstrate that blockade of citrullination inhibits the pro-inflammatory effects of histones and the formation of atherosclerotic plaques in mice, but not NETosis. In contrast, granule proteases in mouse neutrophils may be indispensable for NETosis in response to calcium ionophores. These findings suggest that citrullination mediated by PAD4 and NE-dependent protein hydrolysis of histones share common features but may play a key role in different situations.144,156
Activation of cell cycle and DNA repair signaling is also important in NETosis. The cell cycle protein-dependent kinase (CDK) 4/6 is activated during NETosis. CDK6 is required for NETosis, as a previous study has reported mice with CDK6-deficiency are more susceptible to infection. S-phase events (including DNA synthesis and histone gene transcription) are not found during NETosis, while M-phase events (laminin phosphorylation and centrosome segregation) are important the formation of NETs.157 These results suggest that neutrophils utilize the properties of the cell cycle to break down the nuclear membrane. Upon rupture of the nuclear membrane, the dispersed chromatin mixes with granule proteins in the cytoplasm to form NETs.
The non-lytic NETosis
The non-lytic NETosis, occurs through a NOX-independent pathway as known as ‘vital NETosis’, which can be induced by activated platelets, certain microbes, and calcium ionophore carrier A23187. It does not require ROS generation nor result in cell death and is especially critical for acute invasive infection. In contrast to lytic NETosis, neutrophils do not rupture and die, but rather excrete NETs to the outside of the cell by vesicular transport.128 In this pathway, neutrophils can release mtDNA to form NETs when stimulated by LPS or C5a. Furthermore, it has been illustrated that some pathogens can trigger a rapid non-lytic NETosis by activation of TLR2 and C3, such as S. aureus and Candida albicans (C. albicans).109,123 Moreover, platelets stimulated by LPS can also induce non-lytic NETosis by activating TLR4 in platelets. It is important to note that several studies have described a new formation of NETs containing mainly mitochondrial instead of nuclear DNA. Massive and fast release of mtDNA without loss of viability is detected in neutrophils primed with IL-5/IFNγ or LPS. Unlike the non-lytic NETosis containing nuclear DNA, mitochondrial NET formation depends on ROS, since ROS inhibitor treatment or utilization of neutrophils from patients with granulomatous diseases with ROS deficiency, could not release NETs. However, the detailed molecular mechanisms remain unclear.109,156
More importantly, these pathways of NET formation are not completely independent from each other. For example, acetylation modification of histones in NETs upregulates the immunoreactivity of NETs, and the use of low concentrations of deacetylation inhibitors promotes the formation of NETs, but when the dose is increased to a certain level, the NET formation is inhibited.158
Recently studies have shown that NETs formed by neutrophil subpopulations with varying densities play distinct roles in diverse pathologies. High-density neutrophils (HDNs) are typically found in healthy conditions, whereas low-density neutrophils (LDNs) are predominantly associated with pathological settings. LDNs can be co-segregated with the peripheral blood mononuclear cell fraction after centrifugation.159 LDNs often exhibit immunosuppressive effects and are prone to forming NETs. Elevated levels of LDNs have been observed in the blood of patients with systemic lupus erythematosus (SLE), antiphospholipid syndrome, and lung infections.160,161,162
Molecular mechanisms regulating NET formation
Kinases in NET formation
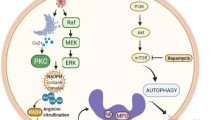
Since 2020, increasing evidence has concentrated on the molecules involved in the regulation of NET formation, particularly kinases and receptors.156,163 The kinases implicated in NETosis include kinases activated by calcium influx, or cell cycle regulators, and cytokines involvement in downstream activation (Fig. 3). The protein kinase C (PKC), which is dependent of phospholipid and activated by ester and calcium, in particular PKCα, PKCβ1, and PKCζ, mediates NET formation induced by different stimuli.164 Dowey et al. have demonstrated that PKC inhibitor, ruboxistaurin, reduces pro-inflammatory and tissue-damaging consequences, as well as NET formation. Downey et al. have completed phase III trials for other indications without safety concerns.165 It is also important to clarify that PKCβ/δ/Cζ are all implicated in the oxidative burst, spreading and activation of NET formation by calcium ionophore A23187, whereas in PMA-activated NET formation, only PKCβ is associated with these functions.164 The regulator of cell cycle G1/S transition CDK6, and the Raf-MEK-ERK pathway are also critical for PMA-induced NETosis.157 In addition, receptor-interacting protein kinase (RIPK), and the mixed lineage kinase domain-like (MLKL) are involved in NET formation induced by antineutrophil cytoplasmic antibody (ANCA) and monosodium urate (MSU) crystals.166,167 Neutrophils from patients with chronic granulomatous diseases are unable to be phosphorylated by PMA-induced MLKL, while RIPK3 genetic depletion in mice blocks NET formation activated by MSU crystals.167
Molecular mechanisms regulating NET formation. The formation of NETs, also known as NETosis, can be initiated by microbial and endogenous stimuli. Various receptors, including those activated by immune complexes, bacteria, fungi, viruses, oxLDL, S100 calcium-binding proteins, and crystals, trigger NETosis via downstream effector proteins. Activated platelets can also induce NETosis through interaction between HMGB1-RAGE and P-electin-PSGL1. Signaling pathways such as MEK/ERK/PKC or JNK induce ROS generation, which is central to triggering NETosis by releasing NE from the azurosome complex. NE degrades the actin cytoskeleton and translocates to the nucleus to drive chromatin decondensation by processing histones. Additionally, chromatin decondensation can be promoted by MPO and DEK binding, as well as the activation of PAD4, which always employs calcium ionophores and mediates histone citrullination. NETosis also relies on CDK4/6 and the segregation of centrosomes. Autophagy and PI3K/AKT/mTOR signaling are also implicated in NET formation. NOD1/NOD2-linked signaling pathways may promote NET formation through both MPO-NE and PAD4 pathways. EVs can act as endogenous danger signals to induce NET formation by activating multiple receptors, including CLECs. Phagocytic receptors like Dectin-1 inhibit NETosis in response to small microorganisms by sequestering NE to phagosomes, while Siglec-5 and Siglec-9 suppress NETosis by limiting neutrophil activation. This figure was created with the assistance of Figdraw (www.figdraw.com)
Oliveira et al. have identified that in response to different NET stimuli, phosphatidylinositol 3-kinase (PI3K) isoforms and related signaling partners can be mobilized, including inflammatory cytokines, growth factors, and chemokines. PI3Kα and PI3Kγ isoforms contribute to NET formation across multiple stimuli, whereas the involvement of other isoforms depends on stimuli. Some PI3K isozymes are found to signal through the typical downstream effector of PI3K, AKT, while others cannot. Downstream of PI3K, all stimuli can regulate NET formation with mammalian target of rapamycin (mTOR) and phospholipase C γ 2 (PLCγ2). Conversely, the participation of other kinases depends on the different stimuli, both tumor necrosis factor alpha (TNFα) and GM-CSF rely on pyruvate dehydrogenase kinase 1 (PDK1) and AKT, and TNFα relies on s6 kinase (S6K).168 In addition, the requirement for PI3K has also suggested the role of autophagy in NET formation, as it also relies on this enzyme. Consistent with this, in a bone marrow-specific murine model of autophagy deficiency, Bhattacharya et al. identified the significance of autophagy in neutrophil degranulation regulation. Neutrophils deficient of autophagy could inhibit degranulation of neutrophils by suppressing ROS production mediated by NADPH oxidase, indicating the correlation of NADPH oxidase with the impacts of autophagy on neutrophil degranulation.169,170,171 Autophagy inhibition (e.g., PI3K inhibitors) can result in a reduction of NET release, while its activation (e.g., rapamycin) enhances the formation of NETs.172 In addition, ROS can rapidly increase the pH value of primary vesicles and then induce autophagy, which is necessary but insufficient to induce NET formation.
Recently, c-Jun NH2-terminal kinase, and nonreceptor tyrosine kinase janus kinase (JAK), especially JAK2, have been implicated in NET formation.173,174,175 Jak2V617F has been identified as one of the most common driven factors of myeloproliferative neoplasms. Mice carrying Jak2V617F are more prone to NET and thrombus formation, while ruxolitinib, a clinically available JAK2 inhibitor, can eliminate the formation of NETs in a murine model of deep vein stenosis.175
Receptors in NET formation
Neutrophils recognize PAMPs or DAMPs when they are recruited to infectious sites, thereby activating specific surface receptors (Fig. 3). These receptors activate different intracellular signaling mechanisms to regulate a variety of neutrophil functions, including NET formation.
TLRs play a crucial role in recognizing host cells and responding to microbes. Except TLR3, all other TLRs are expressed on the surface of neutrophils in human. TLR2 and TLR4 are necessary in the induction of NOX-dependent NETosis by the fungus Fonsecaea pedrosoi (F. pedrosoi). In bacteria, Wolbachia endobacteria (W. endobacteria) can be recognized and initiate NETosis by TLR2 or TLR6. Furthermore, HIV-1 is captured and killed by NETs through the mediation of TLR7 and TLR8.123,176 In addition to pathogens, substances such as DAMPs, oxidized low-density lipoprotein (OxLDL), and activated platelets have been reported to promote NET formation via TLRs.131,141,177 Inhibition of TLRs can reduce NET formation, for example, TLR9 antagonist administration significantly abrogates NET formation, as well as cell death mediated by endoplasmic reticulum (ER) stress and induced by NETs.178,179
The cytoplasmic receptors, NOD-like receptors (NLRs), is the second line of defense against pathogens. Alyami et al. found that Fusobacterium nucleatum (F. nucleatum) upregulates the expression of nucleotide-binding oligomerization domain 1 (NOD1) and NOD2 to trig NET formation in a time-dependent manner.180 Another study on diabetic wound healing identified the role of NLRP3/Caspase-1/Gasdermin D (GSDMD) pathway in NET formation and release.181 Uptake or formation of cholesterol crystals in lysosomes can also cause membrane disruption, as well as activation of NLRP3 inflammasomes. Activation of inflammasomes in neutrophils cleaves GSDMD, followed by the formation of membrane pores and release of IL-1β and IL-18, ultimately resulting in pyroptosis or NET formation in hyperlipidemic mouse models.182
Immune cells (including lymphoid and myeloid cells) express a variety of C-type lectin receptors (CLRs) on their surface, for instance, L-selectin, macrophage inducible C type lectin (Mincle), macrophage inhibitory cytokine 1 (MIC1), of which Dectin 1 and Dectin 2 are usually expressed on neutrophils. The CLRs are able to recognize polysaccharides of microbial membranes directly and activate the immune responses by promoting the secretion of inflammatory cytokines and the formation of NETs. Numerous studies have reported that viruses may interact with lectins in immune cells via terminal glycan on their surface.183,184 Among members of the human CLRs, dendritic cell/lymphocyte-specific intercellular adhesion molecule-3-grabbing non-integrin (DC/L-SIGN), LSECtin, as well as spleen tyrosine kinase (Syk)-coupled C-type lectin member 5A (CLEC5A) and CLEC2, have been shown to play roles in virus-associated NET formation and inflammation.185 Stimulation of P-selectin upregulates the expression level of P-selectin glycoprotein ligand-1 (PSGL-1) and increases the phosphorylation of Syk, thus modulating NET formation in neutrophils.186 Sung et al. have illustrated that extracellular vesicles (EVs) from activated platelets can induce NET formation via activation of CLEC5A/TLR2 heterocomplex, while inhibition of CLEC5A and TLR2 by a bi-specific antibody almost completely abolishes NET formation-induced by EVs.187 Interestingly, besides being involved in NET formation, CLRs can inhibit the release of NETs as well. For example, Dectin-1 acts as a size sensor for microbial phagocytosis by neutrophils to prevent NETosis via blocking NE translocation to the nucleus.122,188
Complement receptors (CRs) are also mainly expressed on lymphoid and myeloid cells, and play an important role in the regulation of innate and acquired immune responses. There are specific interactions between complement factors that eliminate circulating antigens and clear apoptotic cells. One of the first evidence showing the importance of a complement system in NET formation is that neutrophils from mice with C3 deficiency have difficulty in NET formation,189 and those from mice with C3aR deficiency cannot form NETs either.190,191 To date, the most common CRs promoting NET formation are CR1, CR3, CR4 and CR5. In addition to CR1 antagonist, blocking of CR3 can inhibit NET formation in response to certain pathogens.192,193 A recent study has indicated that in neutrophils infected with SARS-CoV-2, the process of NETosis might be amplified by C5a/C5aR1 signaling, while treatment of neutrophils with DF2593A, a selective C5aR1 allosteric antagonist, inhibits NET formation, which provides a promising therapeutic strategy for COVID-19.194
RAGE is a multiligand transmembrane pattern recognition receptor, and its ligands include HMGB1, advanced glycation end products (AGEs), and the S100 family, etc. When activated, RAGE activates multiple intracellular signaling pathways and promotes the production of various inflammatory substances. HMGB1, by binding to RAGE, induces neutrophil activation and promotes the formation of NETs, a process that is dependent on the involvement of NADPH oxidase. The disulfide HMGB1 has also been observed in venous thrombosis to promote pro-thrombotic NET formation mediated by RAGE. More importantly, the employment of HMGB1-neutralizing antibodies eliminates NET formation.195 In the lupus-prone mice, NET formation in the glomerulus is remarkably suppressed in RAGE-deficient mice, along with the improvement of renal pathological scores, suggesting that the blockade of RAGE might be a promising therapeutic target for SLE.196 HBV-induced S100A9 accelerates the formation of NETs mediated by TLR4/RAGE-ROS signaling in hepatocellular carcinoma (HCC).126 In addition, S100 family calprotectins are also released upon the formation of NETs, shown as the failure of neutrophils from patients deficient in PMA-induced NETosis to release S100A8 or S100A9 in response to PMA stimulation, indicating that these calprotectins might amplify the activation of NET formation.197
Moreover, other receptors have also been shown to mediate NET formation. Multiple immune cells express Fc receptors (FcRs), thus driving humoral and cellular immune responses by facilitating the uptake of immune complexes. In one report, FcγRIIa directly participates in activation of NETosis, while another report demonstrates that FcγRIIa merely promotes phagocytosis and NET formation can be induced by FcγRIIIb through MEK/ERK signaling pathway.198,199 It remains unclear which receptor plays a major role or whether their interactions are critical for the formation of NETs. FcRs also seem to be involved in NET formation during infection of bacteria, as neutrophil exposure to ammoniated S. aureus suggests that activation of FcRs promotes NET release.200 In addition, neutrophil effector functions (e.g., degranulation and NETosis) are also reported to be mediated by chemokine receptors. Only CXCR1/2/4 have been identified to be implicated in NET formation to date.200 For example, CXCR1 and CXCR2 have been confirmed to be involved in mediating chemokines-promoted NETosis in tumors.201 CXCR2 induces NET formation by cooperating with PSGL-1, which signals the recruitment of neutrophils, thereby further promoting deep vein thrombosis.202 Overlapping subsets of immune cells express sialic acid-binding immunoglobulin-like lectins (Siglecs). Each Siglec binds to specific endogenous glycosylated glycan to initiate signaling programs and participate in cellular responses. Several Siglecs have been reported to play a regulatory role in NET formation, especially Siglec-9. Siglec-9 is considered as a neutrophil checkpoint and can suppress NETosis in inflammation and cancer immune evasion. Delivery of an artificial glycopeptide targeting Siglec-9 to the surface of intact cells could suppress NET formation and induce neutrophil apoptosis. A pair of receptors, Siglec-5 and Siglec-14, are expressed on monocytes and neutrophils, as Siglec-5 promotes bacterial survival through impairing NET formation, while Siglec-14 has opposing effects in the regulation of host immunity.203,204
NETs in health
The bulk of materials associated with NETs are derived from the nucleus, resulting in a significant enrichment of core histones.205 Additionally, these materials contain elevated levels of cytosolic proteins such as S100 proteins, MPO, and granule proteins (NE and proteinase).144 The proteins contained within the reticular structure of NETs serve as the foundation for the physiological functions of NETs.144,206 NETs are integral components in the preservation of homeostasis, as evidenced by their involvement in host defense, immune regulation, immune thrombosis and wound healing, thereby serving beneficial functions to a certain degree (Fig. 4).207,208,209 Comprehending these physiological functions will aid in the formulation of more holistic clinical treatment strategies.210
NETs in health. NETs play a crucial role in maintaining homeostasis. a NET function by capturing and immobilizing pathogens, relying on specific proteins embedded within the NETs to modify the morphological structure of these pathogens, thereby neutralizing and ultimately killing them. b NETs enhance neutrophil defense, promote macrophage polarization, induce pyroptosis, and facilitate pDC differentiation, thereby aiding antiviral functions. They also support CD4+ T cell and B cell activation while potentially impairing NK cell activity. c NETs promote immunothrombosis by activating factor XII, binding VWF, and triggering platelet activation via histones H3 and H4. They also inactivate anticoagulants and facilitate activation of the extrinsic pathway, aiding in pathogen defense. d AggNETs promote inflammation resolution and wound healing by degrading pro-inflammatory cytokines and sequestering NE to protect the extracellular matrix from proteolysis. This figure was created with the assistance of Figdraw (www.figdraw.com)
Host defense
As a foundational element of innate immunity, the primary function of NETs is to defend the host from pathogenic invasion (Fig. 4a).20 NETs effectively combat infections by ensnaring, immobilizing, and neutralizing a diverse array of pathogens, encompassing fungi, Gram-positive and Gram-negative bacteria, parasites and viruses.144,211 Neutrophils possess a distinctive microbe-detection mechanism, which enables them to customize their antimicrobial reactions towards pathogens based on microbial size.212,213 The ineffectiveness of phagocytosis in eliminating the large filamentous form of fungi highlights the necessity of NETs in effectively controlling these pathogens, particularly in individuals with MPO deficiency, leading to recurrent fungal infections.214,215,216
Candida albicans, a significant pathogen in invasive candidiasis, has been demonstrated to be effectively eliminated by calprotectin (S100A8/A9) within NETs in vitro and in vivo.217,218 This antimicrobial protein complex functions as a divalent metal ion chelator, exhibiting strong efficacy against a range of fungal pathogens such as Candida albicans, C. neoformans, and Aspergillus spp.219 Upon interaction, calprotectin demonstrates antifungal properties by sequestering Zn2+ and/or Mn2+, crucial elements for the growth of these pathogens.197,220 Moreover, NETs have the capability to alter the cell wall composition of Candida albicans, resulting in the exposure of β-glucan and increased detection by Dectin-1-positive immune cells.221 Aspergillus spp are widely distributed environmental fungi that emit spores, which are consistently inhaled but effectively eliminated by individuals with intact immune systems.222 As previously stated, calprotectin serves as a crucial antifungal agent in combating Aspergillus spp and has the ability to induce irreversible zinc deprivation at elevated concentrations.214,223 In a clinical investigation of chronic granulomatous disease patients undergoing gene therapy, the restored release of calprotectin is essential for protecting against Aspergillus spp and managing invasive pulmonary aspergillosis.224 NETs have also been observed to influence host immunity to Aspergillus fumigatus by releasing long PTX3, a pattern recognition receptor that triggers complement activation and aids in pathogen detection.225
The antibacterial properties of NETs continue to be a subject of scholarly discussion, with the potential for NETs to exhibit varying degrees of efficacy in the eradication of diverse bacterial strains.20 The morphological effects of NETs in bacterial infections represent a prominent and direct approach. NETs can alter the morphology of bacteria by ensnaring them with the web-like structure.1,211,226 Imaging techniques utilizing flow chamber systems or intravital microscopy effectively demonstrated the capture of E. coli by accumulated NETs in hepatic sinusoids during sepsis.227 In the absence of bactericidal elements, NETs capture pathogens without completely eliminating them, as they may not disrupt the structural integrity of bacterial cell walls or induce further alterations in bacterial morphology.228,229,230,231 Histones, which are rich in positively charged lysine and arginine residues, have been shown to exhibit bactericidal activity at low concentrations.232,233 Likewise, NE eradicates bacteria through the degradation of proteins located on the outer membrane of bacteria, while also focusing on the virulence factors specific to colonic enterobacteria.234 MPO continues to be active on the extruded NETs, producing ROS-like hypochlorous acid to kill bacteria.211,235 Additionally, NETs play a role in disrupting bacterial biofilms, which can also contribute to alterations in bacterial morphology.236,237 Interestingly, the environment in which NETs are formed affects their ability to kill bacteria. NETs formed under dynamic conditions trap more bacteria but kill them less effectively compared to those formed under static conditions.228
The mechanisms by which NETs defend against viral pathogens exhibit a range of diversity.176,238 First of all, the web-like structure can trap and immobilize viral particles, preventing their spread through electrostatic attraction.239 In addition to mechanically trapping, NETs also possess the ability to attract viral envelopes with negative charges, such as those found in influenza A particles, HIV-1, and norovirus, through the presence of positively charged amino acids. This process leads to the aggregation of these viruses, ultimately aiding in the containment and eradication of the pathogens.239,240 Furthermore, antimicrobial proteins such as MPO, cathelicidins, and α-defensin are attached to the chromatin backbone of NETs.241,242 These proteins have demonstrated antiviral activity against both enveloped and non-enveloped viruses.124,239,243 Additionally, the activity of human respiratory syncytial virus is also impeded by NETs, a phenomenon that may be associated with the presence of serine proteases and bactericidal permeability-increasing protein within NETs.244,245
A series of studies have shown that parasite infections can result in significant neutrophil infiltration and the production of NETs, although most parasites are typically captured but not entirely eradicated.246 In vitro formation of NETs has been documented as a mechanism capable of ensnaring E. histolytica; however, NETs do not impede its proliferation, with additional studies indicating that only a minor fraction of trophozoites are eradicated.247,248 Similarly, Strongyloides stercolaris and Brugia malayi can induce neutrophils to release NETs, which may help trap larvae but does not lead to their death in vitro.249,250 NETs cannot kill Trypanosoma cruzi, the cause of Chagas disease, but they can restrict its invasion and replication.251 Overall, the defensive protective role of NETs in parasitic infections remains poorly understood, potentially due to limited availability of experimental models for investigation.20,246,252
In this chapter, we focus on the reported host defense mechanisms related with NETs. Further research and discussion are needed to understand how NETs eliminate microbes. While NETs play a crucial role in combating infections, their tendency to trigger a systemic inflammatory response, referred to as the “waterfall effect,” can negatively impact host survival, particularly in viral infections.253,254,255 In cases of HBV-related acute-on-chronic liver failure, elevated NET levels are associated with poor patient outcomes.256 Similarly, excessive NET release in patients with COVID-19 contributes to complications such as coagulopathy and lung damage.127,257,258 These pathological effects are discussed in detail in subsequent sections. Therefore, precise control over the production and breakdown of NETs is imperative in order to mitigate pathogenic inflammation.
Immune regulation
Recent studies suggest that while NETs are part of the innate immune system, they also play a significant role in modulating the functions of various immune cells (Fig. 4b).42,206,259 In light of the crucial role of immune homeostasis, it is essential to comprehensively investigate the interplay between NETs and both adaptive and innate immune responses.260
Neutrophils exposed to isolated NETs activate various neutrophil functions in a concentration-dependent manner, according to several studies.130,261,262 These functions include the induction of granule exocytosis, generation of ROS and the NADPH oxidase NOX2, formation of NOX2-dependent NETs, increased phagocytosis, and eradication of microbial pathogens. Additionally, it has been observed that the activation of neutrophils by NETs involves pathways that entail the phosphorylation of p38 Akt/ERK1/2. Collectively, NETs stimulate neutrophil effector function and bolster antimicrobial defense. Moreover, NETs possess the capacity to connect the adaptive and innate immune responses through the stimulation of B-cell Activating Factor (BAFF) from neutrophils.262,263,264
The plasticity of macrophages renders them essential in the immune response to pathogens, tissue regeneration, and the preservation of homeostasis.265 Studies have demonstrated that the DNA component of NETs contributes to the activation and polarization of pro-inflammatory macrophages via the TLR9/NF-κB signaling pathway.266,267 In a separate study, it was observed that the levels of iNOS, CD80, and CD86, markers associated with M1 macrophages, were markedly elevated following treatment with NETs. Conversely, the expression of CD206, an M2 marker, was significantly reduced.268 Additionally, NETs aid in the transfer of antimicrobial peptides by macrophages, thereby augmenting their antimicrobial capabilities.259 It is important to acknowledge that NETs have the potential to induce caspase-1-dependent pyroptosis in macrophages via HMGB1.269 This interaction additionally aids in combating extracellular pathogens.270 Upon exposure to Staphylococcus aureus, Streptococcus pneumoniae, and Pseudomonas aeruginosa, it was observed that NET formation enhances antimicrobial efficacy by promoting macrophage phagocytosis and facilitating the transfer of neutrophil-specific antimicrobial peptides to macrophages.270,271,272 These findings underscore the importance of the crosstalk between NETs and macrophages in achieving optimal bactericidal activity through NET formation.
NETs have a dual impact on the function of dendritic cells (DCs).273 They attract DCs and stimulate them through the IgG Fc fragment via the IIa receptor with low affinity (FCγII), resulting in the generation of interferon-alpha (IFN-α) through TLR9.274 Specific granule proteins found in NETs, such as MPO, HMGB1, and secretory leukocyte proteinase inhibitor (SLPI), stimulate plasmacytoid DCs (pDCs) to produce antiviral factor.275 Furthermore, pDCs have the capacity to induce the differentiation of naïve CD4+ T cells into Th17 and Th1 cells subsets.133,276 However, it has been observed that NETs have the potential to impede the differentiation and maturation of DCs in response to LPS stimulation.277 Moreover, the treatment of immature DCs with NE resulted in the generation and secretion of transforming growth factor beta (TGF-β), which in turn facilitates the differentiation of regulatory T cells (Tregs).278
Monocytes possess the capability to undergo differentiation into either DCs (mo-DCs) or macrophages (mo-Macs), with the balance between the mo-DC and mo-Mac fate being subject to adjustable homeostasis.279,280 Furthermore, the incorporation of NETs into monocytes treated with interleukin-4/granulocyte-macrophage colony-stimulating factor (IL-4/GM-CSF) resulted in the downregulation of IL-4 receptor on monocytes, hindering their full differentiation into DCs while promoting their differentiation into M2 macrophages.281 mo-DCs are a significant contributor to the progression of pathogenic processes in chronic inflammation. Consequently, NETs serve a crucial function in regulating immune homeostasis.282,283,284
Natural killer (NK) cells, a significant subset of innate immune cells, are known to have their function predominantly suppressed by NETs.260 The addition of DNase I to degrade NETs in postoperative immunotherapy for HCC has been shown to enhance the infusion of NK cells and reduce the risk of HCC recurrence, indicating a potential alleviation of the inhibitory effects of NETs on NK cell activity.285 RNA-Seq analysis demonstrated that NETs impede NK cell function via the interaction with carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) during the host’s antiviral immune response.286 Furthermore, in a murine model where NET formation was disrupted, a decrease in dNKs was observed.287
The T cell receptor serves as a crucial mechanism for NETs to engage with T cells, leading to a reduction in T cell activation threshold and enhancement of antigen-specific immune responses.288 Research has shown that Toxoplasma gondii-induced NETs enhance the recruitment of CD4+ T cells and the secretion of TNF, IFN-γ, and IL-6, suggesting that the adaptive immune response is partially enhanced by NETs.289 Notably, CD4+ T cells exposed to NETs demonstrate elevated levels of activation markers, including CD69 and CD25. A comparable pattern of activation marker expression is noted in CD8+ T cells subsequent to exposure to NETs.259,290 Furthermore, NET-associated histones have the capacity to induce the differentiation and cytokine production of Th17 cells through a TLR2/MyD88/STAT3/RORγ-dependent pathway.291 It is imperative for bolstering immunity against fungal and bacterial infections, as well as enhancing anticancer immunity.260 While another study concluded that Tregs are modulated by NETs, which enhance mitochondrial oxidative phosphorylation and support the differentiation of Tregs from naïve CD4+ T cells through TLR4 signaling.39 NETs may also enhance antiviral adaptive immunity by lowering the activation threshold of T lymphocytes.242 In summary, NETs have been observed to promote T cell activation, proliferation, and differentiation, thereby modulating adaptive immune responses during periods of necessity.
B cells, another important responder to adaptive immunity, have been identified as associated with NETs, in addition to macrophages, DCs, NK cells, and T cells.229,259,260 Upon encountering antigens, B cells undergo rapid proliferation, with the majority of cells differentiating into plasma cells (effector B cells) and generating antibodies. LL37-DNA complexes originating from NETs have been found to possess the distinctive capability of localizing to endosomal compartments within B cells and inducing polyclonal B cell activation through TLR9, as well as selectively amplifying self-reactive memory B cells that generate anti-LL37 antibodies in response to antigens.292,293 In addition, citrullinated histones are recognized as a classic antigen for B cell activation, and the MAPK-p38 pathway represents an additional mechanism through which NETs induce B cell activation.294,295 B cells play a crucial role in mediating humoral immune responses, as their activation is necessary for antigen presentation, antibody-dependent cell-mediated cytotoxicity against tumors, as well as antibacterial and antiviral activities.296,297,298,299 Hence, it is possible that the beneficial effects of these functions on health conditions could be further augmented following exposure to NETs.
NETs are essential in maintaining immune homeostasis, but they also activate immune cells such as B cells, antigen-presenting cells, and T cells, contributing to autoimmune diseases including RA, ANCA associated vasculitis (AAV), SLE, and antiphospholipid syndrome.109,300 In tumors, NETs create an immunosuppressive environment that weakens the antitumor immune response of macrophages, CD4+ T, and CD8+ T cells, thereby accelerating cancer progression and metastasis.39,301,302 Notably, the impact of NETs on immune cells varies between tumor and non-tumor settings.260 Additional specific details will be provided in subsequent sections.
Immunothrombosis
Researchers introduced the term immunothrombosis, prompting a shift in contemporary research towards investigating its potential protective role in the context of infection.13 To uphold homeostasis and bolster the host defense against infectious pathogens, the innate immune system initiates local coagulation, leading to microvascular thrombosis, a process that is dependent on neutrophils and NETs (Fig. 4c).9 The development of thrombi is initiated by the interaction of activated neutrophils and monocytes infected with pathogens, as well as activated platelets and coagulation factors. This process serves a protective role by restricting, sequestering, and eliminating pathogens, and can manifest in veins, arteries, and microvessels across various anatomical levels.303,304
NETs contribute a cell specific mechanisms to potentiate immunothrombosis.9,10,11,12 NETs can bind to and activate platelets, forming a platform that boosts neutrophil elastase activity and promotes coagulation.304 NE on NETs degrades and inactivates Tissue factor pathway inhibitor (TFPI), with help from activated platelets that aid in NET formation. Neutrophil serine proteases facilitate the activation of coagulation by tissue factor, known as the extrinsic pathway. This process allows platelet-neutrophil conjugates to directly stimulate coagulation by increasing intravascular tissue factor activity. Thrombomodulin may undergo degradation via cleavage by NE and inactivation by neutrophil oxidases in NETs. Factor XIIa can be formed during fibrin formation when extracellular nucleosomes within NETs activate the contact pathway of coagulation. Additionally, histone components in NETs can induce thrombosis by activating platelets through TLR2 and TLR4.13 Platelets directly interact with neutrophils in response to bacterial products, inducing the formation of NETs through a process known as NETosis.12 Additionally, the histone components of NETs, specifically histones H3 and H4, have been found to influence platelets by promoting their recruitment and activation.305,306
Immunothrombosis has been proposed to fulfill a minimum of four distinct physiological roles.13,303,306 Firstly, it aids in the capture and entrapment of circulating pathogens, thereby restricting their spread by confining them within the fibrin network. As a second benefit, microthrombi resulting from immunothrombosis in microvessels inhibit tissue invasion by pathogens. Thirdly, the blood clots create a distinct space that enhances the concentration of antimicrobial strategies and their targets, thereby promoting pathogen eradication. Four, microvascular buildup of fibrinogen or fibrin attracts more immune cells to the infected or damaged tissue, enhancing pathogen recognition and immune response coordination.13 In conclusion, immunothrombosis with NETs helps identify, contain, and eliminate pathogens to protect the host without causing harm.303 Therefore, it has been argued that universal use of anticoagulation in these patients cannot be recommended.307
It is imperative to acknowledge that uncontrolled immunothrombosis can lead to disseminated intravascular coagulation (DIC), especially during sepsis, and increases the risk of thrombosis and cardiovascular issues in individuals with chronic inflammatory or infectious conditions.9,308 The protective phase of immunothrombosis should be rigorously evaluated from a clinical perspective.
Wound healing
Many studies view the role of NETs in wound healing negatively, but there is this is a controversial finding.209 It has been documented that aggregated NETs, which contain a diverse array of enzymes, have the potential to act as inflammatory mediators by degrading pro-inflammatory cytokines and chemokines, thereby promoting inflammation resolution and wound healing.309,310,311 Furthermore, aggregated NETs (aggNETs) have the ability to sequester NE and shield the extracellular matrix (ECM) from NE-mediated proteolysis.309 Bicarbonate-induced aggregated NETs have been observed to encapsulate necrotic regions and wounds. It is evident that aggregated NETs fulfill distinct functions in the context of wound healing compared to other forms of NETs (Fig. 4d).312 Previous research, particularly in diabetic patients, has primarily focused on the association between impaired wound healing and elevated levels of NETs-related proteins. Excessive or persistent NETs have been observed to contribute to delayed healing of diabetic foot ulcers, a topic that will be further detailed subsequently.313,314 In other words, research on the intrinsic mechanisms of different types of NET formation in wound healing is still in its early stage due to the diverse nature of wound formation and healing processes, as well as the various pathways that trigger NET formation.209
In conclusion, NETs are crucial for an antimicrobial defense mechanism within the innate immune system, functioning both as a physical barrier to impede the dissemination of pathogens and inflammatory mediators, and as a means to eliminate microbes through the action of extracellular DNA, citrullinated histones, and enzymes.211,214,226,238 Furthermore, the inflammatory nature of NETs serves to modulate the immune response and activate additional immune cells.205,260,290 NETs exhibit a tendency to aggregate at high neutrophil densities, degrade soluble inflammatory mediators through NET-associated serine proteases, thereby facilitating the resolution of inflammation and tissue regeneration.209,313 It is noteworthy that NETs serve a crucial function in preserving host well-being and physiological equilibrium.
NETs in various diseases
Infectious diseases
As elucidated previously, NETs unequivocally play an essential role in orchestrating the immune response against infectious agents, notably by helping neutrophils immobilize, capture, and kill invading pathogens such as Gram-negative and Gram-positive bacteria,3,4 virus,126,172,257 fungi,214,217,315 and parasites.316,317 Impaired NET function may promote pathogens’ escape from the immune system and provide a niche for chronic infection.16,17,18 Nevertheless, akin to a double-edged sword, the sustained presence of inflammation or persistent stimuli can precipitate excessive NET formation, thereby exacerbating tissue damage in instance of inappropriate inflammation (Fig. 5).
NETs in diseases. NETs are involved in various human diseases. NETs are central to the immune response against infectious agents, yet their role can be linked to a double-edged sword due to their potential to exacerbate tissue damage under conditions of sustained inflammation or persistent stimuli. NETs are implicated in a spectrum of nonpathogenic diseases, including sterile inflammation, autoimmune disorders, metabolic dysregulation, thrombosis, pregnancy-related diseases, and tumors, when dysregulated. Under sterile conditions, various stimuli, such as IL-8, immune complexes, and crystals, can facilitate the formation of NETs, leading to conditions like gouty arthritis. AggNETs facilitate the resolution of sterile inflammation. NETs are also implicated in pancreatitis and I/R injuries such as brain and liver I/R. In autoimmune disorders, beyond their pro-inflammatory function, NETs have emerged as potential autoantigens, contributing to the production of autoantibodies. NETs contribute to the disease process of T1D, while further investigation is required for their involvement in T2D. Circulating NET markers positively correlate with glycated HbA1c levels and the severity of diabetic complications. Additionally, NETs promote the progression of MASLD, from steatosis to MASH-HCC. NETs are also implicated in both venous (DVT and pulmonary embolism) and arterial thrombotic events (atherosclerosis, coronary artery disease, and ischemic stroke). Furthermore, NETs are associated with several pregnancy-related diseases, such as pre-eclampsia, spontaneous abortions, and gestational diabetes, contributing to their pathogenesis. The protumorigenic role of NETs in various cancers has been confirmed, although a bidirectional interplay between cancer cells and NETs is proposed. This figure was created by Adobe Illustrator Artwork 16.0 (Adobe Systems, USA)
While NETs effectively ensnare pathogens, certain pathogens have developed mechanisms to evade this process. Various pathogens, encompassing a spectrum including V. cholerae, Streptococcus, Staphylococcus genera, P. aeruginosa, N. gonorrhoeae, M. tuberculosis, N. brasiliensis, Plasmodium, Mycoplasma, Leishmania, and Leptospira, produce both endogenous and secreted endonucleases. These enzymes degrade the extracellular DNA scaffold of NETs, thereby dismantling and circumventing the entrapment.207,318,319,320 This evasion facilitated by endonuclease promotes subsequent invasion and dissemination from primary sites to distant organs and the circulation,319 which contributes to the exacerbation of inflammatory pathological conditions, including sepsis.
Sepsis represents a condition characterized by lethal dysfunction of multiple organs and is associated with a high rate of morbidity and mortality.130,321 During the early stages of sepsis, neutrophils are recruited from the blood to the infection site and release NETs.208,322 Studies have elucidated that dysregulated NET function during the early stages of infection contributes to the persistent systemic inflammation that initiates the development of sepsis.16,130 In contrast, as sepsis progresses, excess NETs damage tissue, increase vascular permeability and promote organ failure.16,93,322,323 Circulatory NETs in the bloodstream were significantly elevated and NET markers were also increased in patients with sepsis.324,325,326,327 A growing body of evidence reveals that in sepsis and acute injury, NET-bound histones are cytotoxic because of their ability to compromise cell membrane integrity.328,329 Meanwhile, other NET proteins, such as defensins and NE can disrupt cell junctions.20,317 In murine models of sepsis, a study observed marked platelet aggregation, thrombin activation, and fibrin clot formation within NETs in vivo.330 Aggumated accumulated NETs contribute to the sustained hyper-immunothrombosis in sepsis, which leads to lethal DIC complications in patients.131,303,331
NETs are regarded as the main players in antiviral immunity.15 Neutrophils and NETs have been reported to have protective effects in the early stage of viral hepatitis.332,333 A study indicated that NET release was decreased in patients with chronic HBV infection, and correlated negatively with hepatitis B surface Ag, hepatitis B E Ag, and hepatitis B core Ab levels.333 Nevertheless, HBV C protein and HBV E protein might inhibit the release of NETs by decreasing ROS production and autophagy.333 This suggests that impaired NET function may promote viral escape from the immune system and provide a niche for chronic hepatic virus infection. However, in HBV-related acute chronic liver failure (ALF), circulating neutrophils display a significantly heightened propensity to form NETs, which is closely associated with adverse patient outcomes.256,334 Excessive generation of NETs is widely acknowledged as a mediator of further pathophysiological abnormalities following SARS-CoV-2 infection.335,336,337 Elevated NET release has been documented in numerous patients with COVID-19, contributing to detrimental coagulopathy, immunothrombosis, and pulmonary endothelium damage within the alveoli.257,335,338 Inhibition of NETs in patients with COVID-19 has been shown to mitigate thrombotic tissue damage associated with COVID-19-related acute respiratory distress syndrome (ARDS) and mortality.338,339,340 Moreover, NET-derived histones have been identified in bronchoalveolar lavage fluid from patients with ARDS,340 underscoring the pivotal pathogenical role of NETs in lung injury.
In the context of infectious diseases, NETs exhibit dual roles. During the initial phases of infection, their normal function aids in pathogen clearance and prevents the transition of inflammation into a chronic state. However, in conditions such as sepsis and acute injury, NETs assume a detrimental role, compromising cell membrane integrity, exerting cytotoxic effects on epithelial and endothelial cells, and contributing to immunothrombosis formation.303,329,341 NET-mediated damage may exacerbate rather than constrain certain infections during chronic inflammation. Consequently, strategies aimed at optimal NET inhibition at pertinent disease stage represent potential strategies for infection management.
Sterile inflammation
In contrast to pathogen-targeted mechanisms, sterile-associated NETs may entail heightened deleterious effects.20,128 Under sterile conditions, NET formation can be facilitated by various stimuli including but not limited to IL-8,22 immune complexes,23 crystals,24 or DAMPs, such as HMGB1.25 The deleterious impact of NETs on tissues manifests through direct cytotoxicity towards epithelial and endothelial cells, thereby potentiating tissue inflammatory cascades.342,343 Additionally, the influence of NETs extends to the modulation of inflammatory cytokines either through direct or indirect impact on diverse immune cell populations.
In sterile crystal-mediated inflammation, microcrystals including monosodium urate (MSU), calcium pyrophosphate dihydrate, calcium carbonate, calcium phosphate, calcium oxalate, and cholesterol can stimulate neutrophils to release NETs.310,344 Crystals of MSU monihydrate in joints and soft tissues elicit an acute inflammatory condition commonly known as gouty arthritis.345 Within the joint, MSU crystals instigate the release of inflammatory mediators, orchestrating the recruitment of neutrophils and subsequent NET formation.167,346,347 Infiltrated NETs contribute to the acute, profoundly painful, and tissue-damaging inflammation observed within the joints.344 NET formation in MSU crystal-induced arthritis is influenced by diverse factors, including the presence of inflammatory cytokines such as IL-1β.348 Neutrophils demonstrate increased release of NETs in response to synovial fluid from patients with gout, albeit partially abrogated by the IL-1β antagonist.349 Conversely, studies have unveiled that the excessive accumulation of aggNETs facilitates the resolution of gouty inflammation by encapsulating MSU crystals, degrading cytokines and chemokines, and inhibiting neutrophil recruitment and activation.310,350,351 These findings highlight the potential role of aggNETs as a mechanism promoting the spontaneous resolution of gout, thereby presenting novel therapeutic avenues. However, the precise underlying mechanisms are not fully understood.
Within the milieu of atherosclerosis (AS), circulating cholesterol form monohydrate cholesterol crystals, thereby fostering the formation of atherosclerotic lesions.352,353 These cholesterol crystals serve as potent inducers of NET formation, and in concert with cholesterol crystals, NET augment the release of cytokines released from macrophages via the IL-1/IL-17 and NF-κB signaling pathways.24 NETs have been discerned within the luminal regions of murine and human atherosclerotic lesions, as well as arterial thrombi, implying the potential NET formation across all stages of AS progression.354,355,356,357,358 Notably, within an atherosclerosis mouse model deficient in NE and proteinase 3 (PR3), NETs fail to generate, consequently exhibiting diminished plaque size.24,359 Collectively, NETs-derived extracellular components exhibit cytotoxic and pro-inflammatory attributes, culminating in cellular malfunction and tissue injury, thereby suggesting a nexus between lipid metabolism, inflammatory immunity, and atherosclerosis.360 In patients with suspected or established coronary artery disease, heightened levels of dsDNA and MPO-DNA complexes in plasma demonstrate a positive correlation with both the severity and quantity of atherosclerotic vessels.361,362 Consequently, strategies aimed at inhibiting NET release or the dissolution of NETs may present a promising therapeutic avenue in the context of NET-mediated AS and thrombosis.
In pancreatitis, studies substantiated that bicarbonate ions alongside calcium carbonate crystals can elicit the formation of aggNETs within the ductal tree via a PAD4-dependent signaling pathway.344,363 Besides their implication in the inflammatory insult to the pancreas, the presence of aggNETs within pancreatic ducts can precipitate catheter obstruction and foster the onset and progression of severe acute pancreatitis (SAP).363 Histological analyses of tissue specimens and pancreatic juice samples obtained from patients with pancreatitis have revealed the presence of aggNETs.363 A study suggests a fundamental role of NETs in gallstone formation, with inhibition of NET formation demonstrating efficacy in inhibiting gallstone development in vivo.364 Administration of DNase I to mouse models resulted in a marked reduction in neutrophil infiltration and tissue damage within the pancreas.365 Cumulatively, NETs exacerbate biliopancreatic duct obstruction and exacerbate inflammation, culminating in the manifestation of SAP. Furthermore, NETs contribute to multi-organ injury, infected pancreatic necrosis, sepsis, and thrombotic events associated with SAP.365,366
The involvement of NETs in ischemia/reperfusion (I/R) injury has generated recent attention. The reperfusion subsequent to abrupt blood flow restoration frequently triggers cerebral IR injury following an episode of cerebral ischemia.367 Neutrophils are prompted to release NETs in response to various stimuli, including platelet activation and the presence of IL-8, DAMPs, and TNF-α subsequent to ischemic stroke.368 The accumulation of NETs exacerbates inflammatory processes, thrombus formation, and neuron apoptosis.369,370 Constituents of NETs, such as MPO, histones, and other enzymes contribute to the leakage of blood-brain barrier. Furthermore, in individuals afflicted with ischemia-induced Alzheimer’s disease, heightened levels of amyloid-β (Aβ) precipitate platelet activation, leading to release of HMGB1 and subsequent NET formation, exacerbating disease progression.371,372 Notably, inhibition of NETs has been confirmed to facilitate neovascularization,373,374 indicating a potential therapeutic avenue in mitigating ischemic injury. The pro-inflammatory function of NETs has also been substantiated in liver I/R injury, exacerbating the inflammatory response and liver injury subsequent to I/R.100,375,376 DAMPs emanating from stressed hepatocytes, such as HMGB1 and IL-33 released from liver sinusoidal endothelial cells, serve as pivotal instigators for neutrophil infiltration and subsequent NET formation.375,377,378 Moreover, membrane-nonpermeable superoxide generated during I/R implicated TLR-4 signaling pathway activation, which subsequently instigated NOX and subsequent NET formation.379 Remarkably, interventions such as DNase treatment or inhibition of PAD4 have demonstrated considerable efficacy in mitigating liver inflammation in liver I/R.377
The similarity of NETs in infectious diseases and sterile inflammation lies in their dual role of both protecting and causing harm. In infectious diseases, NETs help clear pathogens and prevent chronic inflammation but can also cause cytotoxicity and contribute to immunothrombosis in conditions like sepsis. Similarly, in sterile inflammation, NETs, triggered by stimuli such as IL-8 and DAMPs, can cause direct cytotoxic effects on epithelial and endothelial cells, exacerbating tissue inflammation. In both scenarios, NETs can have beneficial and harmful effects on tissues and overall health.
Autoimmune disorders
Accumulating evidence from in vitro, in vivo and clinical diagnostics suggests significant involvement of NETs in the pathogenesis of various autoimmune disorders, including but not limited to RA, AAV, SLE, and antiphospholipid syndrome (Fig. 5). NETs have emerged as potential disruptors of self-tolerance, serving as reservoirs of autoantigens that contribute to the production of autoantibodies characteristic of autoimmune disorders.380,381 Additionally, components of NETs are implicated in exacerbating the inflammatory milieu by facilitating complement activation and activaion of other specific immune cells, such as B cells and antigen-presenting cells, thus perpetuating the autoimmune responses.292,382,383,384,385
RA represents as a chronic systemic disease characterized by progressive joint inflammation and variable extra-articular manifestations.386 Central to its pathology are the anti-citrullinated protein antibodies (ACPAs), which exhibit high specificity for RA and can instigate the formation of pathogenic immune complexes within the affected joints.387,388 Neutrophils are abundant in the inflamed joints of patients with RA, displaying an augmented propensity for spontaneous NET formation.389,390,391,392 Moreover, this propensity for NET generation escalates upon stimulation with RA synovial fluid and ACPA-positive RA serum.389,392 Elevated levels of MPO-DNA complexes and cell-free nucleosome are observed in the serum of patients with RA,393,394 with their concentrations correlating with clinical parameters and ACPA titers in patient sera.389,393,395,396 Accumulated NETs release novel autoantigens, including citrullinated histones, which may further fuel the autoimmune response in RA.389,397 ACPAs have been reported to recognize autoantigens presented on NETs, especially the citrullinated histones.398,399,400 Additionally, NETs have been implicated in disrupting the cartilage structure and facilitating its citrullination, thereby exacerbating synovial inflammation.401 Overall, NETs play a central inflammatory role in RA and represent a significant source of autoantigens capable of eliciting pro-inflammatory responses within various organs, including the lungs and synovium, in patients with RA.129,402,403 Furthermore, NETs and NET-derived products hold promise as biomarkers for RA disease activity.
AAV represents a group of disorders characterized by inflammation and destruction of small and medium vessels, with autoantibodies against MPO and PRTN3 as key distinguishing markers.404,405 PRTN3 is expressed on the membrane of resting neutrophils, whereas MPO is stored within the granules, both of which are notably enriched within the NET structure.300,406,407 Analogous to RA, neutrophils in patients with AAV exhibit a heightened capacity for NET synthesis.408,409 In turn, NETs may be a key origin of ANCA-autoantigens.408,410 Some studies confirm that release of NETs may be triggered by a response to ANCA stimulation.411,412 Beyond their antigenic role, NETs exert influence on AAV progression by directly inflicting vessel damage through the cytotoxic release of NET-associated histone.413 Importantly, NET structures have been identified within various tissues from patients with AAV, promoting inflammation in multi-organs.414,415 Elevated levels of MPO have been detected in patients with AAV compared to those in remission.416,417 In mouse model with AAV, inhibiting PAD4-mediated NET formation has shown promise in reducing disease severity, indicating a potential therapeutic avenue.417 Thus, NETs may serve as novel biomarkers for disease diagnosis and represent promising targets for future therapeutics of AAV.
SLE is a systemic autoimmune disease characterized by pervasive inflammation across many organs.418 NETs represent a central origin of SLE autoantigens.419,420 Neutrophils sourced from healthy individuals exhibit a heightened propensity for NET formation when exposed to serum or plasma derived from patients with SLE, SLE–SLE-associated immune complexes and autoantibodies reciprocally fostering NET generation.23,421 The compromised clearance of NETs contributes substantively to SLE pathogenesis by extending the exposure duration of autoantigens and elevating levels of SLE-associated autoantibodies.420,422,423 Non-degraded NETs precipitate activation of the complement system, thus perpetuating inflammatory cascades.424 Within the SLE milieu, LDNs demonstrate augmented presence in circulation, with their levels correlating with distinct disease manifestations such as vasculopathy, skin disease, nephritis, and cardiopathy.160,382,425,426 Notably, these specific neutrophils exhibit increased spontaneous NET formation.427 Neutrophils from patients with SLE, particularly LDNs, display enhanced ex vivo NET formation, characterized by elevated levels of modified autoantigens and immunostimulatory molecules within the NET structure compared to those from healthy individuals.23,421 LDNs have been implicated in directly compromising endothelial cell integrity through the NET product MMP-9.428
NETs have also been implicated in other autoimmune disorders including but not limited to antiphospholipid syndrome,429,430,431,432 idiopathic inflammatory myopathies,433,434,435 multiple sclerosis,436,437 psoriasis,438,439 and inflammatory bowel diseases.440,441 Diverse autoantibodies have been shown to directly induce NET formation, with resultant NETs reciprocally promoting the production of autoantibodies. On one hand, NETs exhibit the capacity to directly inflict tissue damage, while on the other hand, they serve to catalyze the initiation and perpetuation of systemic autoimmune disorders, orchestrating intricate inflammatory responses by direct or indirect interactions with other immune cells. Collectively, escalated NET formation coupled with decreased NET degradation contribute to heightened levels of these structures and augmented exposure to modified autoantigens, thereby exacerbating tissue damage in these autoimmune conditions. Clinical interventions ought to ideally focus on selectively modulating dysregulated NET activity while keeping other essential antimicrobial functions.
Metabolic dysregulation
Metabolic diseases such as diabetes mellitus (DM) and its associated complications pose a significant threat to public health, leading to diminished health and quality of life.442,443 The prevalence of DM is steadily increasing in both developing and developed countries, reaching epidemic proportions.444,445,446 Type 1 diabetes (T1D) necessitates insulin and involves the destruction of a significant number of insulin-producing pancreatic β cells, stemming from a chronic and progressive autoimmune dysfunction.446 Type 2 diabetes (T2D) represents a metabolic syndrome marked by reduced insulin sensitivity and impaired insulin production.447 The expression of PAD4 is elevated in neutrophils of patients with both T1D and T2D,448 and these neutrophils exhibit increased susceptibility to NETosis when stimulated in vivo.449 NET formation has been observed in the murine model with T1D,450 and clinical data similarly showed that NETs are elevated in patients with T1D.451,452,453 A recent study demonstrated a significant increase in circulating NE and PR3 levels in patients with T1D, strongly correlated with β cell autoimmunity, indicating a potential role of NETs in the onset and pathogenesis of the disease.451 Increased formation of NETs is associated with gut permeability in individuals with T1D, but not T2D.454 Further, NETs caused by gut leakage can trigger autoimmune response in non-obese diabetic mice.455 Improving gut barrier function via intestinal NETs degradation can prevent T1D in node mice.456 Early inhibition of NE finally resulted in decreased incidence of T1D in murine model.457 NETs can stimulate cytokine production and promote the generation of IFNγ-producing T cells in samples from T1D patients.276 Inhibition NET formation prevents the onset of diabetes in non-obese diabetic mice.458 Furthermore, NET inhibition alleviates vascular dysfunction in T1D mice.459 Based on these results, we posit that akin to autoimmune conditions discussed above, NET might similarly assume an antigenic function in the etiology of T1D, notably triggering the autoimmune disorders in the pancreas. Moreover, NETs may further contribute to systemic inflammation and complications in the progression of T1D.
A diverse array of circulating NET markers, including cell-free DNA, nucleosome DNA, and neutrophil expression of PAD4, have been reported to exhibit elevation in the circulation of individuals with T2D.449,460,461 These circulating NET markers have been observed to positively correlate with the level of glycated hemoglobin A1c.462 Nevertheless, the impact of hyperglycemia on NET formation remains controversial. Neutrophils isolated from diabetic patients have demonstrated spontaneous NET production even in the absence of exogenous stimuli, yet they exhibited impaired NET generation when stimulated with PMA or LPS.463,464 Furthermore, evidence suggests that neutrophils isolated from the blood of patients with diabetic foot ulcers exhibit increased spontaneous NET formation but impaired inducible NET generation.465 In vitro experiments have indicated that oxidative stress in a high-glucose microenvironment promotes NET formation,466 whereas contrasting results have been reported, showing impaired NET production in response to high glucose conditions in vitro.464 In vivo experiments present a conflicting perspective on the role of NETs in the pathogenesis of T2D. NETs are acknowledged to play pivotal roles in fostering diabetic ulcers,181,449,467,468 retinopathy,469,470 and nephropathy.471 Patient data suggest that severe obesity is associated with increased generation of plasmatic NETs, potentially influencing systemic inflammatory status.472 However, in a murine model of obesity, inhibition of PAD4 activity leads to NET reduction and attenuation of adipose tissue inflammation, albeit failing to prevent diabetes.473 Although the precise role of NETs in the initiation of T2D remains unclear, a clear positive correlation between NETs and the development of poorly controlled diabetes has been established.
Metabolic-dysfunction-associated steatotic liver disease (MASLD) is a burgeoning global health challenge,474 ranging from simple steatosis to metabolic-dysfunction-associated steatohepatitis (MASH), liver cirrhosis, and even HCC.475,476 Neutrophil infiltration has long been observed in human MASLD.477 Concurrently, plasma levels of NET markers escalate in patients with MASLD,66 with a gradual increase noted with disease progression.478 Experimental induction of steatosis in murine models correlates with excessive neutrophil infiltration in the liver.479 Free fatty acids (FFAs), such as linoleic acid and palmitic acid are considered to be stimulants for augmented NET formation in MASLD.480,481 Furthermore, cholesterol crystals, prevalent in MASLD livers,482 serve as potent inducer of NETs.24 However, inhibition of NETs through DNAse I or utilization of PAD4 knockout mice dose not impede FFA accumulation, implying that NET formation is a consequence of lipid accumulation rather than a causative factor of steatosis.480 MASH is a progressive form of MASLD that slowly progresses toward cirrhosis and finally leads to the development of HCC.483,484 Our research unveils NET formation in NASH, highlighting elevated serum levels of MPO-DNA in preoperative NASH patients.480 Furthermore, increased intrahepatic platelet accumulation correlates with NET formation in liver biopsies of patients with MASLD.485 Studies underscore the cytotoxic effects of NETs on endothelial cells,66,486,487 fostering a procoagulant and pro-inflammatory phenotype,488,489 thereby accentuating the hypercoagulable state in patients with MASH. Moreover, NETs contribute to the establishment of a protumorigenic inflammatory environment, promoting the progression of HCC in MASH.480 Recent study suggests that NETs play a crucial role in bridging innate and adaptive immunity by promoting Treg differentiation through metabolic reprogramming of naïve CD4+ T cells in MASH,39 thereby fostering an immunosuppressive environment for MASH-HCC initiation. In vivo blockade of NETs using PAD4-/- mice or DNase I treatment attenuates the Treg activity and augments cytotoxic CD4+ and CD8+ T-cell function, thus mitigating MASH-HCC initiation and development. Collectively, NET formation emerges as a pivotal factor driving the transition from steatosis to NASH, perpetuating chronic inflammation, and fostering HCC progression by shaping an immunosuppressive microenvironment conducive to aberrant hepatocyte survival.
Thrombosis
Thrombosis, characterized by the obstruction of normal blood flow due to blood clots in arteries or veins, precipitates various pathologies, including cerebral thrombosis, atherosclerosis, coronary thrombosis, pulmonary embolism, and deep venous thromboembolism (DVT).490,491 Over the past few years, the role of NETs has revolutionized our understanding of thrombosis, with studies elucidating their role in both venous and arterial thrombotic events.308,492 As discussed above, NETs facilitate thrombus formation by acting as a scaffold that triggers platelet activation and coagulation.20 Nevertheless, dysregulation or excessive NET generation precipitates pathological thrombotic processes (Fig. 5).
Recent accumulating evidence from human thrombi underscores the presence of NETs within arterial thrombi across various thrombotic pathologies, including atherosclerosis,24,493,494,495 coronary artery disease,362,496,497,498,499 and ischemic stroke.500,501,502 In atherosclerosis, NETs were observed in both human and murine atherosclerotic lesions,24,354,495,503 with cholesterol crystals identified as potential inducers of NET formation. Consequently, NETs contribute to increased expression of pro-inflammatory cytokines, fostering further immune cell recruitment to atherosclerotic plaques and exacerbating atherosclerosis.24 Inhibiting NET formation has shown promise in reducing atherosclerosis burden in apoliporotein-E deficient mice.504 Although recent histological investigations reveal abundant NETs in coronary thrombi from patients with acute myocardial infarction,71,356,505 the extent to which NET formation contributes to coronary thrombus formation remains unclear. Research suggests that NETs are prevalent in fresh and lytic but not organized coronary thrombi, implicating their role in thrombus propagation and stabilization, with potential degradation occurring in the older thrombi.505 Clinical relevance is underscored by findings linking coronary thrombus NET burden and infarct size, as well as ST-segment resolution, reflecting the potential influence of NETs on myocardial infarction outcomes.71 Evidence further suggests localized NET formation in acute coronary syndrome, supported by elevated NETs in the blood from lesion sites compared to other sites.496 Furthermore, a multicenter European study showed that neutrophils and NETs are recognized features of thrombi retrieved from patients with stent thrombosis post-percutaneous coronary intervention.356 Similarly, in ischemic stroke, abundant NETs are observed in occluding thrombi,506,507 with plasma NET markers correlating with stroke severity and outcomes.500,508,509 However, cerebral thrombi can originate from various sources depending on stroke etiology, with studies indicating the differential abundance of H3Cit, a marker of NETs, in cerebral thrombi of cardioembolic origin compared to other etiologies.506 This indicates the possibility of NETs migrating from thrombi in other locations to the brain, thereby exacerbating inflammation in thrombotic processes.
Venous thromboembolism encompasses DVT, pulmonary embolism, and clot formation in large veins.510,511 Animal models have demonstrated the presence of NETs within venous thrombi.512,513 Studies have indicated elevated levels of circulating extracellular DNA and MPO in patients with DVT compared to DVT-negative individuals.514 Moreover, circulating NET components have been observed to rise alongside venous thrombus development in patients.515,516 The identification of citrullinated histones in the inferior vena cava of DVT mice further support this conclusion.513,517 NET involvement in thrombosis is supported by the finding that treatment with DNase and PAD4 inhibitors blocks DVT in mice.513,518 Venous thrombi may exhibit a lower proportion of NETs compared to arterial thrombi, as evidenced by a study comparing patients with coronary artery thrombi and those with deep vein thrombi.71 NET structures are predominantly localized in the organizing regions of venous thrombi rather than the organized areas,519 suggesting a potential role for NETs in venous thrombus maturation rather than sustained generation. Infections can accelerate neutrophil recruitment, leading to heightened involvement of NETs in venous thrombosis. Staphylococcal infection in mice suffering from inferior vena cava ligation has shown larger thrombi containing increased neutrophils and NETs.513 In thrombotic events triggered by infection, such as those occurring in sepsis, the presence of NETs within lung thrombi can be observed.110 However, clinical data regarding NETs in venous thromboembolism are relatively limited, and the precise contribution of NETs to venous thrombosis remains to be further elucidated.
Pregnancy-related diseases
Elevated white blood cell counts during pregnancy have been documented,520,521 with several studies indicating a mild neutrophilia associated with pregnancy.520,522 Within the context of normal pregnancy, neutrophils exhibit heightened susceptibility to activation with an augmented capacity for phagocytosis in comparison to non-pregnant women.522,523 Nevertheless, the precise mechanism and underlying rationale monitoring the heightened activity of peripheral blood neutrophils during pregnancy remain unknown.
Pre-eclampsia (PE), whereby activation of leukocytes such as neutrophils is enhanced, is a paramount contributor to maternal mortality on a global scale.524,525 Evidence suggests a detrimental role of NETs in the pathogenesis of PE.526,527 Histological analysis of placental tissue from patients with PE reveals the presence of NETs in close proximity with trophoblasts.342,527,528 An elevation of NET levels within the placental inter-villous space of PE pregnancies has also been observed.529,530 Concurrently, elevated levels of maternal cell-free DNA (cfDNA), a hallmark of PE531,532 are observed, correlating with disease severity.533 NETs are observed in PE as they are the main origin ofconnected to the presence of cfDNA in maternal plasma.526,528,534 In vitro experiments demonstrate that placenta fragments stimulate the formation of NETs by neutrophils.534 Meanwhile, the release of particles of syncytiotrophoblast and endothelial cell origin induce NET release.535 Additionally, DNA released from damaged placental cells further augments NET formation, leading to vascular endothelial cell damage through a positive feedback loop, thereby exacerbating pregnancy complications, enhancing blood coagulation, and increasing the risk of thrombotic events.526,535 Furthermore, placental NETs are hypothesized to provoke autoimmune reaction in PE.527,534 However, the precise role of NETs in initiating pathological changes remains unclear, warranting further investigations into whether NETs are triggered by placental deficiency or its consequential outcomes.
Gestational diabetes mellitus (GDM) represents a transient sate of glucose intolerance occurring during pregnancy.536,537 Pregnancies complicated by GDM face an elevated risk of developing PE.538 Notably, circulatory neutrophils in GDM cases demonstrate an exaggerated pro-NETosis phenotype, along with heightened placental infiltration evidenced by the expression of neutrophil elastase (NE).539 Neutrophils in GDM exhibit heightened activation, leading to spontaneous NET generation in vitro.540 The administration of infliximab, a clinically utilized TNF-α antagonist, notably attenuates the pro-NETotic effect of GDM sera.540 Additionally, degranulated neutrophil release NE, which perturbs trophoblast physiology and glucose metabolism via modulation of key signal transduction components.539 A study elucidates hypoadiponectinemia as a trigger for NET formation, which promotes trophoblast apoptosis through ROS-dependent mitochondrial pathway activation mediated by ERK1/2 signaling.541 Furthermore, induction of GDM in NETs-deficient PAD4−/− mice leads to a significant increase in placental weight compared to wild-type mice,542 indicating a potential contribution of altered NET activity to the pathogenesis of PE in GDM.
Moreover, pregnancies frequently encounter complications such as spontaneous abortions, often associated with heightened stress or inflammatory condition.543,544 A study investigated a cohort of 268 women, observing a correlation between spontaneous abortions and elevated fetal cfDNA levels in maternal blood.545 Dysregulated LDNs have been implicated in early spontaneous abortions, exhibiting increased in vitro NET formation.546 Analyses revealed the presence of NETs within placental tissue from miscarried women, accompanied by elevated MPO and pentraxin 3 levels.547 Investigation into NETs associated with spontaneous abortion indicated heightened chorioamniotic NET levels in cases of chorioamnionitis and preterm delivery.548 Interestingly, PAD4-/- mice displayed significantly reduced inflammatory and thrombotic response, leading to a marked decrease in pregnancy losses.549 The inhibition of NETs emerges as a promising therapeutic avenue for disorders associated with impaired placentation.
Tumors
NET components have been directly involved in modifying cancer biology, with emerging evidence emphasizing the protumorigenic role of NETs in various cancers.26,27,28 NETs have even been reported to favor tumor cell proliferation,29 metastasis,30,31,550,551 immunosuppression,33,34 angiogenesis, and cancer-associated thrombosis.35 Moreover, NETs can capture circulating tumor cells (CTCs) and promote their colonization.36 Conversely, NETs can also exhibit anti-inflammatory and anti-tumorigenic functions.552 They have the ability to mitigate inflammation by degrading cytokines and chemokines, as well as coordinate the resolution of sterile cancer-related inflammation.310 Thus, there may exist a bidirectional interplay between cancer cells and NETs (Fig. 5). Conversely, the presence of cancer cells can influence neutrophil activity, maturation, and cell fate (Fig. 6). Tumor cells have the capability to prime neutrophils to form NETs.128 IL-8/CXCL8 produced by cancer cells and several cancer-related stimuli (such as CXCR1/CXCR2 agonists, G-CSF, TGF-β, tumor-derived proteases, and tumor exosomes), can induce the release of NETs from both human and murine neutrophils.201,553,554,555,556 Besides cancer cell-derived factors, cancer-associated fibroblasts have also been identified as drivers of suicidal NETosis.557 Moreover, hypoxia in the TME may also induce NETs, as HIF-1 plays a critical role in NETosis and bacteria-killing activity.558
NETs in modulating cancer biology. NET components play a direct role in shaping the biology of cancer. NETs are implicated in tumor cell immunosuppression, proliferation, metastasis, and cancer-associated thrombosis. In tumor proliferation, NETs directly promote tumor growth, angionenesis, and ECM remodeling. In cancer immune surveillance, NETs may contribute to the suppressive TME by: 1. Directly affecting the killing function of NK cells and cytotoxic T cells. 2. Forming a shield to protect tumor cells from effector cells. 3. Promoting Treg activity to inhibit the function of effector cells targeting abnormal cells. For cancer metastasis, NETs capture CTCs through integrin β1, CEACAM 1, TLRs, and CCDC25. NETs also promote EMT and contribute to endothelial damage and increasing vascular permeability. Moreover, NETs can awaken dormant cancer cells at distant sites. NETs also contribute to cancer-associated thrombosis. These mechanisms are associated with the immunothrombosis function of NETs, wherein they trap platelets, red blood cells, and extracellular vesicles containing tissue factor activity, leading to vessel occlusion and promoting cancer-associated thrombosis. This figure was created by Adobe Illustrator Artwork 16.0 (Adobe Systems, USA)
Tumor immune surveillance
Evidence indicates that NETs contribute to the creation of a suppressive inflammatory microenvironment at primary or secondary sites, thereby promoting the seeding, survival, proliferation and metastasis of primary tumor cells.33,559,560 CD8+ T cells, key effectors in the anti-cancer immune response,561,562 interact with NETs in the TME, as confirmed by the negative correlation between NET density in the serum of patients with cancer and CD8+ T cells in the TME.560 Furthermore, neutrophils isolated from patients undergoing resection of colorectal liver metastases were found to be predisposed to forming NETs, resulting in exhaustion and dysfunction of human CD4+ and CD8+ T cells,33 accompanied by increased expression of exhaustion markers PD-1, Tim-3, and LAG-3, along with diminished production of effector cytokines IL-2, IFN-y, and TNF-a.33 Mechanistic studies revealed that PD-L1 is embedded within the NET structure, suggesting that targeting PD-L1-containing NETs may prevent tumor growth, offering a novel strategy to enhance immune surveillance in the TME. NK cells, key cells in immune responses,563 are affected by NETs, as demonstrated by an in vitro study showing that NETs can inhibit NK cell migration and motility.201 In a TME abundant in NETs, the therapeutic efficacy of NK cells is impaired,285 possibly due to MMP9 in NETs contributing to NK cell dysfunction and tumor invasion.564 Inhibition of NETs in a murine model of HCC enhanced anti-tumor immunity mediated by NK cells.
Evidence has also shown that CXCR1 and CXCR2 agonists produced by tumor cells promote NET formation, which act as a protective shield against cytotoxicity mediated by NK cells and T cells.201,565 Additionally, studies have validated that NETs protect tumor cells by creating a physical barrier at the tumor/stroma interface,566,567 thus preventing the infiltration of CD8+ T cells into tumor cell areas. Moreover, NETs contribute to an immune suppressive microenvironment for tumor survival by interacting with Tregs. Our recent finding indicates that accumulated NETs can cause extensive hepatocyte damage and establish an immunosuppressive microenvironment for premalignant hepatocytes and cancer cell survival by promoting Treg activity,39 thereby facilitating the initiation and development of HCC.480 Inhibiting NETs may reduce the number and suppressive function of Treg and enhance the cytotoxicity of effector CD4+ and CD8+ T cells, thus preventing tumor progression. Moreover, inhibiting NET formation may sensitize cancer cells to immune checkpoint blockade.568 In summary, NETs may contribute to the suppressive TME through: 1) Directly affecting the killing function of NK cells and cyctoxic T cells. 2) Forming a shield to protect tumor cells from effector cells. 3) Promoting Treg activity to inhibit the function of effector cells killing abnormal cells. Targeting NET function may reprogram the impaired immune surveillance in the TME, thereby hindering tumor initiation and progression.
Tumor proliferation
Elevated levels of plasma biomarkers of NETs such as cfDNA, NE and citH3, have been observed in various cancers, including but not limited to pancreatic cancer,512,568,569,570 gastric cancer,89,571,572 and breast cancer.42,83 In most reports, NETs have been linked to a protumorigenic role in both experimental murine models and patients with cancers. NETs have been shown to induce endothelial-to-mesenchymal transition (EMT) in several type of cancers.86,573,574 In an experimental melanoma model, NETs accumulated in the TME and promoted cancer growth,575 a phenomenon also observed in HCC development.126,480 In a murine model of orthotropic pancreatic adenocarcinoma, NETs activated pancreatic stellate cells, promoting tumor proliferation, while inhibiting NETs reduced stromal activation and tumor growth.576 In vitro experiments have further confirmed that NETs promote tumor cell proliferation. Another mechanism through which NETs promote tumor growth is their pro-antigenic effects,577 possibly mediated by NETs-induced activation of endothelial cells via TLR-4/NF-kb signaling or upregulation of proangiogenic factors such as vascular endothelial growth factor.577,578,579 Whereas most evidence supports the tumor-promoting role of NETs, several studies have also demonstrated their protective role in tumors.552 Co-culture of melanoma cells with NETs resulted in decreased melanoma cell migration and viability.580 Additionally, experimental evidence suggests that NETs inhibit the proliferation of colon carcinoma cells.581 These controversial findings may reflect the dual role of NETs in the TME, which may vary depending on the disease stage.
Tumor metastasis
Several studies involving patients with various cancer types offer additional evidence supporting the involvement of NETs in promoting metastasis. Recent investigations have shown a correlation between NET levels and metastasis in HCC and breast cancer.36,94 The highest levels of NETs were found in metastatic lesions from patients with triple-negative breast cancer, a subtype characterized by aggressive tumor progression and high risk of metastatic spread.582 In a mice model with lung and colon cancer, tumour-induced NETs contribute to cancer cell adhesion to liver sinusoids.583 IL-8/CXCL8 mediates a positive loop connecting NET formation and colorectal cancer liver metastasis.584 NETs have also been identified as promoting factors in the metastasis of other cancer types, including but not limited to ovarian cancer,551 pancreatic dual adenocarcinoma (PDAC),90 cholangiocarcinoma,585 esophagogastric cancer,89,583 and also non-solid cancers such as diffuse large B cell lymphoma.586 Enhanced metastasis has been suppressed by treatments that inhibit NETs, such as PAD4 knockout or DNase I or NE inhibitor therapy.
Mechanistically, NETs have been implicated in promoting metastasis through several mechanisms: 1) Capturing CTCs. NETs with their web-like structure and adhesive properties, can ensnare CTCs, facilitating their spread in circulation and favoring the metastatic process.66,587,588 Integrin β1589 and CEACAM590 have been identified as crucial for this interaction. Additionally, the DNA component of NETs in the liver exhibits chemotactic properties for CTCs, interacting with the coiled-coil domain containing protein 25, a transmembrane protein expressed on CTCs.36 2) Promoting EMT. NETs induce EMT, as evidenced in both murine model and patients.86,97,573,574 This ability to induce EMT in both normal and neoplastic epithelial cells suggests that NETs may contribute early in the process of neoplastic transformation.31 3) Causing endothelial damage and increasing vascular permeability. Circulating NETs rapidly disrupt endothelial cells contacts, leading to endothelial damage and vascular leakage.591 NET-associated proteases, including NE, MPO, and MMPs, compromise junction integrity and promote vascular permeability.592,593 4) Creating a premetastatic niche for cancer cells. NETs create an immune-suppressive niche for CTCs, particularly in the development of liver metastasis.84,584 NETs can also contribute to the premetastatic niche in lungs in mice with breast cancer.594 5) Enhancing cancer cells’ metastatic abilities via NETs. The primary tumor can induce NET formation, with metastatic cancer cells showing an enhanced capacity to induce NETs compared to poorly metastatic tumor cells.566 Tumor-induced NETs increase breast cancer cell motility and promoted lung metastasis.566 Tumor-derived cathepsin C promotes metastasis through NET-dependent mechanisms.83
It is worth noting that NETs have been implicated in postoperative infection-related metastasis and occurrence. In 2016, we first proposed an enhanced metastatic role of NETs induced by surgical stress using a mouse model of hepatic I/R injury,595 which prevented metastasis by NET inhibition with DNase I or PAD4 inhibitors. Recently, we further demonstrated that I/R injury in the liver and the subsequent NET formation promote the formation of colon cancer metastasis in the lung.84 In this study, NETs were shown to have a higher propensity to bind CTCs aggregated with platelets. Additional evidence provided by a study confirmed that cecal ligation and puncture in mice contributed to NET formation, enhanced trapping of CTCs, and increased formation of liver metastasis.66 These findings suggest that infection-induced NETs enhance the trapping of tumor cells. LPS-induced NET formation was also shown to promote tumor metastases in a mouse model of CRC.596 Although surgical removal of the tumor may be curative clinically, inhibiting NETs as a preventive measure for postoperative infection and subsequent recurrence may provide clinical insights.
Tumor-associated thrombosis
The prothrombotic nature of NETs has been implicated in cancer-associated thrombosis,109 as evidenced by clinical data76,597 and mouse studies.64,512,554 NET complexes or components have been detected in coronary, cerebral and pulmonary thrombi in patients with various cancer types.40,598 Elevated circulating NET markers predict a higher risk of VTE in patient with cancer.76 Moreover, circulating NET markers are elevated in HCC-associated portal vein thrombosis599 and cancer-related stroke.509 These mechanisms are related to the immunothrombosis function of NETs, which trap platelets, red blood cells, and extracellular vesicles with tissue factor activity, occluding vessels and promoting cancer-associated thrombosis. Specifically, 1) cancer-induced platelet activation and NET release contribute to the hypercoagulable state in cancer;600,601 2) tumor-derived pro-coagulant micro particles promote DVT by carrying tissue factor and adhering to thrombus-associated NETs;602 3) NETs released from cancer patients increase levels of thrombin-ant thrombin complexes and enhance the ability of control plasma to generate fibrin.41 Administration of DNase I reduced thrombus size in mice bearing human tumors.512,603
Tumor prognosis
While the clinical significance of circulating NET molecules as cancer biomarkers remains a debate, recent evidence suggests a direct correlation between the high levels of NET markers and poor clinical outcomes in patients with cancer.40,41,42 Elevated level of H3Cit has been identified as an independent prognostic factor for short-term survival in cancer patients.604 In patients with colorectal cancer, elevated pre-operative circulating levels of cfDNA have been linked to persistent disease one year after resection.605 Additionally, in patients with metastatic colorectal cancer undergoing curative liver resection, high levels of circulating NET markers are associated with a high risk of recurrence and worse prognosis.595,596 Similarly, in patients with breast cancer, cfDNA correlated with tumor size, nodal involvement, and clinical stage.606 Serum NET levels can predict the occurrence of liver metastasis in patients with early-stage breast cancer.36 High NET density is correlated with lower recurrence-free survival in patients with cervical cancer,607 suggesting that combining NET density with the TNM staging system could improve prognostic accuracy. NETs are also reported as a novel biomarker to predict recurrence and overall survival,608 and they correlate with the degree of liver dysfunction in patients with HCC. In human large B cell lymphomas, intratumoral and circulating NETs correlate with worse overall survival and progression-free survival.586 Plasma NET markers have been documented to correlate with poor prognosis in head and neck cancer,609,610 gastric cancer,611,612 rectal cancer,613 renal cancer,614 and pancreatic cancer.570 Moreover, cancer cells from a primary tumor can enter a dormant state and remain clinically undetectable for extended periods (Fig. 6). NETs have been shown to awaken dormant cancer cells at distant sites,615 suggesting that therapies targeting the prevention of dormant cell awakening by NETs could potentially extend the survival of cancer patients.
NET-targeting therapies
Targeting NET formation
Multiple pathways have been identified in the formation of NETs, and have been exploited in attempts to inhibit formation in order to abrogate negative downstream effects. A majority of the work in inhibition of NET formation has been done in the pre-clinical setting, with peptidyl arginine deaminase (PAD) being the most common target of interest (Table 1). The PAD family of enzymes catalyze the citrullination of histone proteins, a key component of NET formation.616 Multiple prior studies have demonstrated the correlation between NET reduction and PAD inhibition, and genetic knock outs of PAD have demonstrated similar phenotypic endpoints as prohibiting NET formation. Cl-amidine has been a recently explored PAD inhibitor, used in a variety of inflammatory disease models, including lupus, diabetes, and endometritis.458,617,618 Shen et al. demonstrated the utility of inhibiting PAD4-mediated NET formation with Cl-amidine as a means of preventing diabetes development.458 In their study, Cl-amidine was administered orally at a dose of 5μg/g, resulting in a delay in onset, decreased disease incidence, and decreased type 1 diabetes-associated antibodies, which was simultaneously associated with a reduction in serum NET markers. Furthermore, these findings translated phenotypically, with inhibited pancreatic inflammation and increased regulatory T cell presence within pancreatic lymph nodes. Separately, Knight et al. demonstrated Cl-amidine could confer protective effects against specific lupus phenotypes.617 In their model, MRL/lpr mice, which are more prone to accelerated lupus phenotypes, were treated with subcutaneous injections of either 10 mg/kg/day of Cl-amidine, 1 mg/kg/day of BB-Cl-amidine, a more bioavailable form of Cl-amidine. PAD inhibition with these agents resulted in reduced proteinuria and immune complex deposition, as well as downregulation of type I interferon production in a murine model otherwise prone to developing severe disease.
NET formation can also be targeted through blocking histone citrullination directly. Agents such as thrombomodulin have been studied in this role and applied to a broad range of disease, including sepsis-mediated injury, coagulopathy, and cancer.97,619 Helms et al. explored the use of recombinant human thrombomodulin in rat models of shock-induced coagulopathy, and found that administration of rhThrombomodulin not only decreased histone-induced NETosis, but attenuated the coagulopathy control rats experienced.620 In a model of endotoxin-mediated renal injury, Harada et al. established that intraperitoneal administration of 6 mg/kg of rTM following LPS-induced septic injury decreased citrullinated histone H3 levels in the serum and renal medulla,619 suggesting rTM could suppress NET production. Although this study did not connect these immunohistologic and serologic findings with a phenotypic benefit, other groups have demonstrated the phenotypic benefits of rTM. Kajioka et al. studied this in the context of pancreatic cancer,97 finding that thrombomodulin degraded HMGB1 with consequential inhibition of NET induction, leading to prevention of surgically-induced pancreatic metastases to liver.
In addition to these novel agents, there has been a wave of repurposing commercially available drugs to target NET formation. Hydroxyethyl starch, which no longer has utility as a colloid agent, was administered at a dose of 20 mg/kg by tail vein injection in a group of mice undergoing cecal ligation and puncture as a sepsis model by Rossaint et al. This model was found to reduce NET formation and reduce platelet-neutrophil aggregates and transmigration of neutrophils under inflammatory conditions.621 Zinc chelators have additionally been found to modulate NET formation through multiple studies from Kuzmicka et al.622,623 These in vivo and in vitro studies have demonstrated that low levels of zinc either through decreased dietary ingestion or through direct chelation led to increased NET release and enhanced neutrophil degradation, and that supplementation of zinc can inhibit histone citrullination and subsequent NET release.
While research with these agents is still in its infancy, certain drugs have already been associated with clinically relevant outcomes. Disulfiram, for example, has been found to reduce NET expression through gasdermin D inhibition, and alleviated severe inflammatory injury in acute pancreatitis.624 Ling et al. demonstrated in a murine model of severe acute pancreatitis induced by caerulein and LPS that treatment with either 50 mg/kg or 100 mg/kg of disulfiram led to inhibition of gasdermin D and resultant decrease in in-vivo NET formation, in turn alleviating inflammatory injury.624
Targeting NET structure
Aside from prohibiting NET formation altogether, multiple preclinical studies have examined how to degrade or diminish the functionality of already formed NETs (Table 1). DNase has been the longest-studied agent, targeting the extracellular DNA component of NETs. Exogenous DNase administration has been utilized in a variety of disease states, and has consistently demonstrated reductions in measurable biomarkers, as well as associated with outcome improvements, including reversal of coagulopathies and thrombotic burdens, decreased cancer growth and metastasis, and suppression of pro-inflammatory cytokine production.
While extracellular DNA is often the target for NET degradation, there is an increasing amount of research focusing on targeting NET-associated proteins, which contribute to its functional properties. A 2020 study from Rayes et al. explored CEACAM1, a NET-associated molecule, as a therapeutic target to prevent the metastatic progression of colon adenocarcinoma. Using a murine model, they were able to identify that blocking CEACAM1 or knocking it out led to a decrease in cancer cell adhesion, migration, and metastasis.590 In 2023, Zhang et al. examined the effects of epigallocatechin-3-gallate (EGCG), a naturally occurring neutrophil elastase inhibitor. Through co-culturing neutrophils from peripheral blood samples from human subjects and co-culturing them with SW480 colon cancer cells and inducing NETs, treatment with varying concentrations of EGCG led to suppressed NET formation, decreased expression of STAT3 and CXCL8 in colon cancer cell-derived neutrophils, and impaired cancer cell migration and invasion.88
Other groups have attempted to induce endogenous endonuclease function as opposed to delivering an exogenous agent. Ondracek et al. found that endurance training led to an increase in endogenous DNase activity and a decrease in cfDNA levels, theorizing this could result in improved cardiovascular outcomes.625 Furthermore, some groups have opted to use agents that target downstream functions, as opposed to direct structural targeting. For example, Chen et al. examined exenatide, a glycemic control agent that had been demonstrated to downregulate ROS in prior studies, and found that as a byproduct, NET reduction was observed.626 After subcutaneous inoculation of MC38 colon cancer cells, 24 nmol/kg/day of exenatide, twice weekly 250 μg doses of anti PD-1 or a combination of therapy was administered. Exenatide treatment led to decreased infiltration of NETs in tumor, and decreased peripheral MPO-DNA. In vitro studies demonstrated exenatide alone decreased NET formation and release. However, combining exenatide with anti-PD-1 therapy was superior at restricting tumor growth to either agent alone, and confirmed this was related to NET interaction by demonstrating that NET degradation with 5 mg/kg DNase weakened the efficacy of the combination therapy. Generally, these preclinical studies show consensus that NET degradation or functional NET inhibition is achievable through multiple mechanisms, and results in favorable outcomes.
Clinical trials
In the realm of human clinical trials, substantial work has been done with observational methodology, specifically post-hoc analysis of other randomized trials (Table 2). The 2022 study from Schaid et al. utilized post-hoc analysis of the COMBAT randomized control trial to evaluate proteomic markers of NETs in injured trauma patients. They found that more severely injured patients had elevated markers of Serpin B1 (a NETosis marker), and that elevation of serpinB correlated to higher levels of nonsurvival, fewer ICU-free days, and fewer ventilator-free days, supporting NETosis as a potential mediator of post-injury organ dysfunction. Additionally, Qiao et al. performed a post-hoc analysis of plasma biomarkers in patients from the CITRIS-ALI trial, examining the effects of high-dose IV vitamin C on surrogates of NET formation, cfDNA and syndecan1 in patients with sepsis-induced ARDS. The treatment arm displayed greater cfDNA reduction, and increased syndecan1 levels, suggesting amelioration of NETosis. Furthermore, an exploratory open-label randomized phase-2 sub-study of the PANAMO trial in 2022 examined the role of vilobelimab treatment and its effects on biomarkers of inflammation and coagulation. The PANAMO study evaluated whether vilibelimab, an anti-C5a antibody, improved survival in critically ill COVID patients. NET markers were measured over multiple time points, and it was found that the treatment arm had decreased rates of NET biomarkers, and suppressed IL8 secretion.
Observational work is not limited to post-hoc analysis of existing studies. Multiple studies have utilized serum NET biomarkers to form prognostication and prediction models for outcomes across a variety of pathologic states. Boettcher et al utilized cfDNA and CitH3 levels as predictive markers for appendicitis in adult populations, which demonstrated superior performance compared to standard-of-care white blood cell count and c-reactive protein levels.627 Li et al. examined serum NET markers after cardiac arrest, identifying that cfDNA and CitH3 were independent predictors of 28-day all-cause mortality.628 Yang et al. found that higher serum NET-specific markers, particularly CitH3, were predictive for wound healing impairment in diabetic foot ulcers and future amputation.629
Currently, available interventional trials are limited, and the majority use DNase analogs as the intervention of interest. Dornase alpha, an agent known to directly degrade the extracellular DNA in NETs, was tested in a 2021 nonrandomized trial of patients with ARDS secondary to COVID-19. Inhaled administration led to reduced bronchoalveolar lavage fluid MPO-DNA complexes, improved PF ratio, and improved static lung compliance, suggesting that degradation of NETs can be beneficial in this population. However, results were not sustained at 14 days, suggesting the benefit may be short-lived.340
Additionally, existing agents have been studied after modifications with attempts to improve drug delivery and subsequent outcomes. In 2020, a recombinant DNase1 coated with a polymer nanoparticulate was administered in COVID-19 patients to explore whether this would improve delivery and mediate neutrophil-mediated activity. Findings suggested that this nanoparticulate coating led to reduced cfDNA levels and neutrophil activation, and may be used as a therapeutic modification.630
Interventional trials have also taken advantage of other existing and commercially available agents, repurposing them to target NETs. A 2018 single-arm phase 2a proof of concept study examined the effect of the combinatorial rituximab and belimumab, an antibody that leads to sustained inhibition of B cell activation, to address whether autoantibodies were related to excessive NET formation. The combination therapy administered resulted in reduced NETs in patients with systemic lupus erythematosus. It had been previously demonstrated that SLE impairs NET degradation, and those NETs propagate the inflammatory response through immune complex deposition.631
Another agent explored in interventional trials is intravenous lidocaine, particularly in the setting of improving disease-specific outcomes after oncologic surgery. In 2020, intraoperative IV lidocaine use was explored in breast cancer surgery and associated with decreased expression of NET markers post-operatively. While this study did not directly evaluate outcomes, the study authors set a future goal of evaluating if utilizing IV lidocaine in curative intent surgery may reduce recurrence.632 Shortly thereafter, a multicenter randomized controlled trial in 2022 evaluated intravenous intraoperative lidocaine during pancreatectomy for malignancy. Lidocaine in this setting transiently lowered circulating NETs, however there was no difference in intra-tumoral NETs, and did not improve overall or disease-free survival.633
Conclusion and outstanding questions
In recent years, the growing understanding of NETs as pivotal players in both physiological defense mechanisms and pathological processes underscores their significance in human health and disease. NETs act as a double-edged sword, offering fundamental antimicrobial defense while also contributing to tissue damage and inflammation in various diseases. The intricate interplay between NETs and the immune system, coagulation pathways, and tissue remodeling processes emphasizes their multifaceted functions. However, it is worth noting that their immune-regulatory characteristics remain largely unknown, which could be beneficial in immune defense. Several factors, including the microenvironment of the disease sites and various stimuli, determine whether NETs are beneficial or detrimental in certain conditions.
The investigation into the molecular, cellular, and biophysical mechanisms governing NET formation in physiological or pathological processes is at an early stage. Various extracellular and intracellular microbes stimulate neutrophils to initiate NETs through suicidal and vital NETosis. Current research predominantly focuses on determining the factors that induce NET formation, yet show limited elucidation of their underlying cellular mechanisms. It remains uncertain whether NET formation varies between physiological and pathological conditions, such as during immunomodulatory or antimicrobial progress, autoimmune disorders, or cancer. Additionally, there is insufficient understanding of potential variations in NET components across different contexts. The functional role of NETs depends on variations in their composition and structure. Given that NETosis follows a defined sequence of events, understanding molecules inhibiting NET formation will enhance our comprehension of the fundamental mechanisms underlying NET formation and identify new targets for modulating NETs in diseases.
The spectrum of diseases associated with NETs is gradually broadening, encompassing inflammatory disorders, thrombosis, and cancer. In autoimmune diseases, NETs, serving as potential sources of autoantigens and immune-cell activators, could significantly contribute to autoimmunity development and the break of immune tolerance. Further investigations to identify auto antigenic components in NETs structure are crucial for designing new therapies for autoimmune disease therapies. The immunomodulatory properties of NETs might be necessary for enabling an appropriate inflammatory response or for limiting inflammation and maintaining homeostasis, which necessitates further investigations. Moreover, understanding their impact on other immune cells involved in both adaptive and innate immune responses will be pivotal for future research.
Despite numerous studies identifying NETs as having tumor-promoting effects, some studies have demonstrated tumor-inhibiting effects, especially in early-stage cancer or metastasis. Generally, elevated NET levels are associated with poor outcomes in various cancers, suggesting their potential clinical utility as biomarkers. A deeper comprehension of the interplay among NETs, cancer cells, and immune responses in the TME can enhance our understanding of cancer immunotherapy resistance. Moreover, the role of NETs in immune surveillance has not been sufficiently evaluated. It is likely that NETs in blood vessels versus tissues have different consequences, indicating diverse roles for NETs depending on their location.
Existing clinical and basic research highlights the importance of developing novel therapeutics targeting both the process of NET formation and the NET structures. Future research should focus on designing interventions tailored to the specific characteristics and stages of different diseases. For instance, in the early stages of infectious diseases, it is crucial to enhance the function of NETs to eradicate pathogens. Conversely, for sterile inflammation and most advanced-stage cancers, inhibiting the formation of NETs is more advantageous. When considering NET inhibition, it is more promising to focus on regulating NET formation rather than eliminating already formed NETs. This objective can be achieved by identifying and targeting the factors implicated in the pathways initiating NET formation. Given the presence of NETs in multiple organs of the human body, they hold potential as significant modulators of both health and disease states. The dynamic regulation of NET levels in the body to sustain homeostasis presents an exciting research avenue. Although researchers have already integrated NETs into various clinical trials, the primary remaining objective in the field is to translate NET-targeted therapies into clinical practice.
References
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Sørensen, O. E. & Borregaard, N. Neutrophil extracellular traps—the dark side of neutrophils. J. Clin. Investig. 126, 1612–1620 (2016).
Driouich, A. et al. Root extracellular traps versus neutrophil extracellular traps in host defence, a case of functional convergence? Biol. Rev. Camb. Philos. Soc. 94, 1685–1700 (2019).
Rawat, S., Vrati, S. & Banerjee, A. Neutrophils at the crossroads of acute viral infections and severity. Mol. Asp. Med. 81, 100996 (2021).
Singhal, A. & Kumar, S. Neutrophil and remnant clearance in immunity and inflammation. Immunology 165, 22–43 (2022).
Wang, X. et al. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front. Immunol. 9, 2456 (2018).
Yang, F. et al. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation 40, 311–323 (2017).
Scapini, P. & Cassatella, M. A. Social networking of human neutrophils within the immune system. Blood 124, 710–719, (2014).
Grover, S. P. & Mackman, N. Neutrophils, NETs, and immunothrombosis. Blood 132, 1360–1361 (2018).
Knight, J. S. & Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 44, 347–362 (2022).
Leberzammer, J. & von Hundelshausen, P. Chemokines, molecular drivers of thromboinflammation and immunothrombosis. Front. Immunol. 14, 1276353 (2023).
Zhu, S. et al. Neutrophil extracellular traps contribute to immunothrombosis formation via the STING pathway in sepsis-associated lung injury. Cell Death Discov. 9, 315 (2023).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Zhong, H., Lu, R.-Y. & Wang, Y. Neutrophil extracellular traps in fungal infections: a seesaw battle in hosts. Front Immunol. 13, 977493 (2022).
Schultz, B. M., Acevedo, O. A., Kalergis, A. M. & Bueno, S. M. Role of extracellular trap release during bacterial and viral infection. Front. Microbiol. 13, 798853 (2022).
Bukong, T. N. et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 69, 1145–1154 (2018).
Mulet, M. et al. Dysregulated neutrophil extracellular traps formation in sepsis. Immunology 170, 374–387 (2023).
Balazs, I. & Stadlbauer, V. Circulating neutrophil anti-pathogen dysfunction in cirrhosis. JHEP Rep. 5, 100871 (2023).
Lee, K. H. et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun. Rev. 16, 1160–1173 (2017).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23, 279–287 (2017).
Teijeira, A. et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin. Cancer Res. 27, 2383–2393 (2021).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320, (2015).
Denning, N. L., Aziz, M., Gurien, S. D. & Wang, P. DAMPs and NETs in sepsis. Front. Immunol. 10, 2536 (2019).
De Meo, M. L. & Spicer, J. D. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin. Immunol. 57, 101595 (2021).
Demkow, U. Neutrophil Extracellular Traps (NETs) in cancer invasion, evasion and metastasis. Cancers 13, 4495 (2021).
Zhao, J. & Jin, J. Neutrophil extracellular traps: New players in cancer research. Front. Immunol. 13, 937565 (2022).
Yazdani, H. O. et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. 79, 5626–5639 (2019).
Kaltenmeier, C., Simmons, R. L., Tohme, S. & Yazdani, H. O. Neutrophil extracellular traps (NETs) in cancer metastasis. Cancers 13, 6131 (2021).
Yang, D. & Liu, J. Neutrophil extracellular traps: a new player in cancer metastasis and therapeutic target. J. Exp. Clin. Cancer Res. 40, 233 (2021).
Yang, L.-Y. et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 13, 3 (2020).
Kaltenmeier, C. et al. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front. Immunol. 12, 785222 (2021).
Wang, H. et al. The regulatory mechanism of neutrophil extracellular traps in cancer biological behavior. Cell Biosci. 11, 193 (2021).
Martinod, K. & Wagner, D. D. Thrombosis: tangled up in NETs. Blood 123, 2768–2776, (2014).
Yang, L. et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020).
Liu, Y. & Liu, L. The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps. Biosci. Trends 13, 469–475 (2020).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446, (2016).
Wang, H. et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 75, 1271–1283 (2021).
Thalin, C. et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 139, 56–64 (2016).
Yang, C. et al. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int. J. Clin. Exp. Pathol. 8, 14075–14086 (2015).
Taifour, T. et al. The tumor-derived cytokine Chi3l1 induces neutrophil extracellular traps that promote T cell exclusion in triple-negative breast cancer. Immunity 56, 2755–2772.e2758 (2023).
Urban, C. F., Reichard, U., Brinkmann, V. & Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 8, 668–676, (2006).
Gupta, A. K. et al. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 66, 1146–1154 (2005).
Alghamdi, A. S. & Foster, D. N. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol. Reprod. 73, 1174–1181, (2005).
Gupta, A. et al. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann. N.Y. Acad. Sci. 1075, 118–122 (2006).
Buchanan, J. T. et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16, 396–400 (2006).
Beiter, K. et al. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16, 401–407 (2006).
Berends, E. T. et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2, 576–586 (2010).
Wartha, F. et al. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 9, 1162–1171 (2007).
Walker, M. J. et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13, 981–985 (2007).
Palic, D., Ostojic, J., Andreasen, C. B. & Roth, J. A. Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev. Comp. Immunol. 31, 805–816, (2007).
Palic, D. et al. Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J. Immunol. Methods 319, 87–97 (2007).
Jaillon, S. et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 204, 793–804 (2007).
Margraf, S. et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 30, 352–358 (2008).
Urbonaviciute, V. & Voll, R. E. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J. Intern. Med. 270, 309–318, (2011).
Hamaguchi, S. et al. Identification of neutrophil extracellular traps in the blood of patients with systemic inflammatory response syndrome. J. Int. Med. Res. 41, 162–168 (2013).
Wardini, A. B. et al. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 91, 259–264 (2010).
Papayannopoulos, V., Staab, D. & Zychlinsky, A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One 6, e28526 (2011).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Hakkim, A. et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7, 75–77 (2011).
Kirchner, T. et al. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators Inflamm. 2013, 710239 (2013).
Gray, R. D. et al. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 10, 12 (2013).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA 109, 13076–13081 (2012).
Demers, M. & Wagner, D. D. Neutrophil extracellular traps: a new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology 2, e22946 (2013).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 123, 3446–3458 (2013).
Tillack, K., Breiden, P., Martin, R. & Sospedra, M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J. Immunol. 188, 3150–3159, (2012).
Hirose, T. et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS One 9, e111755 (2014).
Sayah, D. M. et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 191, 455–463 (2015).
Ge, L. et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Heart Circ. Physiol. 308, H500–H509 (2015).
Mangold, A. et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 116, 1182–1192 (2015).
Nomura, K. et al. Citrullinated histone H3: early biomarker of neutrophil extracellular traps in septic liver damage. J. Surg. Res. 234, 132–138 (2019).
Jin, W. et al. Tumor-Infiltrating NETs predict postsurgical survival in patients with pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 26, 635–643 (2019).
Singel, K. L. et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br. J. Cancer 120, 207–217 (2019).
Caldarone, L. et al. Neutrophil extracellular traps in ex vivo lung perfusion perfusate predict the clinical outcome of lung transplant recipients. Eur. Respir. J. 53, 1801736 (2019).
Mauracher, L. M. et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 16, 508–518 (2018).
Kraaij, T. et al. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun. Rev. 15, 577–584 (2016).
Sil, P. et al. High throughput measurement of extracellular DNA release and quantitative NET formation in human neutrophils in vitro. J. Vis. Exp. 18, 52779 (2016).
Bystrzycka, W. et al. The effect of clindamycin and amoxicillin on neutrophil extracellular trap (NET) release. Cent. Eur. J. Immunol. 41, 1–5 (2016).
Manda-Handzlik, A. et al. Antibiotics modulate the ability of neutrophils to release neutrophil extracellular traps. Adv. Exp. Med. Biol. 944, 47–52 (2017).
Yildiz, C. et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology 122, 864–875 (2015).
Mousset, A. et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-beta activation. Cancer Cell 41, 757–775 e710 (2023).
Xiao, Y. et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 39, 423–437.e427 (2021).
Ren, J. et al. Platelet TLR4-ERK5 axis facilitates NET-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. 81, 2373–2385 (2021).
Takesue, S. et al. Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer‑associated fibroblasts. Int J. Oncol. 56, 596–605 (2020).
Zhu, T. et al. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial‑mesenchymal transition. Int. J. Mol. Med. 48, 127 (2021).
Martins-Cardoso, K. et al. Neutrophil extracellular traps (NETs) promote pro-metastatic phenotype in human breast cancer cells through epithelial-mesenchymal transition. Cancers 12, 1542 (2020).
Zhang, Z., Zhu, Q., Wang, S. & Shi, C. Epigallocatechin-3-gallate inhibits the formation of neutrophil extracellular traps and suppresses the migration and invasion of colon cancer cells by regulating STAT3/CXCL8 pathway. Mol. Cell Biochem. 478, 887–898 (2023).
Xia, X. et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat. Commun. 13, 1017 (2022).
Deng, J. et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight 6, e146133 (2021).
Shi, L. et al. Endogenous PAD4 in breast cancer cells mediates cancer extracellular chromatin network formation and promotes lung metastasis. Mol. Cancer Res. 18, 735–747 (2020).
Xia, Y. et al. AAV-mediated gene transfer of DNase I in the liver of mice with colorectal cancer reduces liver metastasis and restores local innate and adaptive immune response. Mol. Oncol. 14, 2920–2935 (2020).
Boufenzer, A. et al. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cell Mol. Immunol. 18, 452–460 (2021).
Guan, X. et al. The crosstalk between cancer cells and neutrophils enhances hepatocellular carcinoma metastasis via neutrophil extracellular traps-associated cathepsin G component: a potential therapeutic target. J. Hepatocell. Carcinoma 8, 451–465 (2021).
Wu, Y. et al. Neutrophil mediated postoperative photoimmunotherapy against melanoma skin cancer. Nanoscale 13, 14825–14836 (2021).
Wang, C. Y. et al. Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy. EBioMedicine 90, 104499 (2023).
Kajioka, H. et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett. 497, 1–13 (2021).
Han, X. A. et al. Necrostatin-1 ameliorates neutrophilic inflammation in asthma by suppressing MLKL phosphorylation to inhibiting NETs release. Front Immunol. 11, 666 (2020).
Cui, C. et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 184, 3163–3177.e3121 (2021).
Yazdani, H. O. et al. Exercise training decreases hepatic injury and metastases through changes in immune response to liver ischemia/reperfusion in mice. Hepatology 73, 2494–2509 (2021).
Shi, Y. et al. Aerobic exercise attenuates acute lung injury through NET inhibition. Front. Immunol. 11, 409 (2020).
Donis-Maturano, L. et al. Prolonged exposure to neutrophil extracellular traps can induce mitochondrial damage in macrophages and dendritic cells. Springerplus 4, 161 (2015).
Rodriguez-Espinosa, O. et al. Metabolic requirements for neutrophil extracellular traps formation. Immunology 145, 213–224 (2015).
Azevedo, E. P. et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. J. Biol. Chem. 290, 22174–22183 (2015).
Wu, Z. R., Zhou, T. Q. & Ai, S. C. Neutrophil extracellular traps correlate with severity and prognosis in patients with ischemic stroke: a systematic review and meta-analysis. Acta Neurol. Belg. 124, 513–522 (2024).
Ichimura, S. et al. Neutrophil extracellular traps in myocardial tissue drive cardiac dysfunction and adverse outcomes in patients with heart failure with dilated cardiomyopathy. Circ. Heart Fail. 17, e011057 (2024).
Xu, S. S. et al. Neutrophil extracellular traps and macrophage extracellular traps predict postoperative recurrence in resectable nonfunctional pancreatic neuroendocrine tumors. Front. Immunol. 12, 577517 (2021).
Borrego-Yaniz, G. et al. Risk loci involved in giant cell arteritis susceptibility: a genome-wide association study. Lancet Rheumatol. 6, e374–e383 (2024).
Herre, M., Cedervall, J., Mackman, N. & Olsson, A. K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol. Rev. 103, 277–312 (2023).
Jimenez-Alcazar, M. et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358, 1202–1206 (2017).
Morales-Primo, A. U., Becker, I. & Zamora-Chimal, J. Neutrophil extracellular trap-associated molecules: a review on their immunophysiological and inflammatory roles. Int. Rev. Immunol. 41, 253–274 (2022).
McIlroy, D. J. et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J. Crit. Care 29, 1133.e1131–e1135 (2014).
Takishita, Y. et al. Formation of neutrophil extracellular traps in mitochondrial DNA-deficient cells. J. Clin. Biochem. Nutr. 66, 15–23 (2020).
Thiam, H. R., Wong, S. L., Wagner, D. D. & Waterman, C. M. Cellular mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 36, 191–218 (2020).
Richards, C. M., McRae, S. A., Ranger, A. L. & Klegeris, A. Extracellular histones as damage-associated molecular patterns in neuroinflammatory responses. Rev. Neurosci. 34, 533–558 (2023).
Papayannopoulos, V., Metzler, K. D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691, (2010).
Hamam, H. J., Khan, M. A. & Palaniyar, N. Histone acetylation promotes neutrophil extracellular trap formation. Biomolecules 9, 32 (2019).
Spengler, J. et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 67, 3135–3145 (2015).
Sanchez-Tirado, E. et al. Serum autoantibody biomarkers for management of rheumatoid arthritis disease. Biosensors 13, 381 (2023).
Doring, Y., Libby, P. & Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ. Res. 126, 1228–1241 (2020).
de Buhr, N. et al. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell Microbiol. 19, e12649 (2017).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Tabrizi, Z. A. et al. Multi-facets of neutrophil extracellular trap in infectious diseases: moving beyond immunity. Micro. Pathog. 158, 105066 (2021).
Saitoh, T. et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12, 109–116 (2012).
Muraro, S. P. et al. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 8, 14166 (2018).
Zhan, X. et al. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun. 43, 225–245 (2023).
Veras, F. P. et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 217, e20201129 (2020).
Cristinziano, L. et al. Neutrophil extracellular traps in cancer. Semin Cancer Biol. 79, 91–104 (2022).
Wigerblad, G. & Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 23, 274–288 (2023).
Zhang, H. et al. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin. Transl. Med. 13, e1170 (2023).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017).
Tian, Z. et al. Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe 30, 1450–1463.e1458 (2022).
Qiu, S. L. et al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax 72, 1084–1093 (2017).
He, X. et al. PM2.5 aggravates NQO1-induced mucus hyper-secretion through release of neutrophil extracellular traps in an asthma model. Ecotoxicol. Environ. Saf. 218, 112272 (2021).
Suzuki, M. et al. PAD4 deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am. J. Respir. Cell Mol. Biol. 63, 806–818 (2020).
Vaseruk, A., Bila, G. & Bilyy, R. Nanoparticles for stimulation of neutrophil extracellular trap-mediated immunity. Eur. J. Immunol. 54, e2350582 (2024).
Munoz, L. E. et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl Acad. Sci. USA 113, E5856–E5865 (2016).
Metzemaekers, M., Gouwy, M. & Proost, P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol. Immunol. 17, 433–450 (2020).
Bai, W. et al. TRAF1 suppresses antifungal immunity through CXCL1-mediated neutrophil recruitment during Candida albicans intradermal infection. Cell Commun. Signal. 18, 30 (2020).
Ahmad, S., Ramadori, G. & Moriconi, F. Modulation of chemokine- and adhesion-molecule gene expression and recruitment of neutrophil granulocytes in rat and mouse liver after a single gadolinium chloride or zymosan treatment. Int. J. Mol. Sci. 19, 3891 (2018).
Aube, F. A., Bidias, A. & Pepin, G. Who and how, DNA sensors in NETs-driven inflammation. Front Immunol. 14, 1190177 (2023).
Byrd, A. S. et al. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 190, 4136–4148 (2013).
Kenny, E. F. et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 6, e24437 (2017).
Hidalgo, A. et al. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc Res. 118, 2737–2753 (2022).
Dinauer, M. C. Neutrophil defects and diagnosis disorders of neutrophil function: an overview. Methods Mol. Biol. 2087, 11–29 (2020).
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4, 615–618 (1998).
Okeke, E. B. et al. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 238, 119836 (2020).
Crocetti, L., Quinn, M. T., Schepetkin, I. A. & Giovannoni, M. P. A patenting perspective on human neutrophil elastase (HNE) inhibitors (2014-2018) and their therapeutic applications. Expert Opin. Ther. Pat. 29, 555–578 (2019).
Uchida, Y. et al. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 89, 1050–1056 (2010).
Chu, X. et al. Human antibody domains and fragments targeting neutrophil elastase as candidate therapeutics for cancer and inflammation-related diseases. Int J. Mol. Sci. 22, 11136 (2021).
Rydzynska, Z. et al. Neutrophil elastase defects in congenital neutropenia. Front. Immunol. 12, 653932 (2021).
Mor-Vaknin, N. et al. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat. Commun. 8, 14252 (2017).
Cao, J. et al. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol. Pharm. 18, 305–316 (2021).
Guo, W. et al. GPR109A controls neutrophil extracellular traps formation and improve early sepsis by regulating ROS/PAD4/Cit-H3 signal axis. Exp. Hematol. Oncol. 12, 15 (2023).
Hosseinzadeh, A., Thompson, P. R., Segal, B. H. & Urban, C. F. Nicotine induces neutrophil extracellular traps. J. Leukoc. Biol. 100, 1105–1112 (2016).
Vorobjeva, N. V. & Chernyak, B. V. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry 85, 1178–1190 (2020).
Amulic, B. et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev. Cell 43, 449–462.e445 (2017).
Hamam, H. J. & Palaniyar, N. Histone deacetylase inhibitors dose-dependently switch neutrophil death from NETosis to apoptosis. Biomolecules 9, 184 (2019).
Tay, S. H., Celhar, T. & Fairhurst, A. M. Low-density neutrophils in systemic lupus erythematosus. Arthritis Rheumatol. 72, 1587–1595 (2020).
Mehdipour, P. et al. Epigenetic therapy induces transcription of inverted SINEs and ADAR1 dependency. Nature 588, 169–173 (2020).
Mauracher, L. M. et al. Neutrophil subpopulations and their activation potential in patients with antiphospholipid syndrome and healthy individuals. Rheumatology 60, 1687–1699 (2021).
Rankin, A. N., Hendrix, S. V., Naik, S. K. & Stallings, C. L. Exploring the role of low-density neutrophils during mycobacterium tuberculosis infection. Front. Cell Infect. Microbiol. 12, 901590 (2022).
Huang, J., Hong, W., Wan, M. & Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm 3, e162 (2022).
Vorobjeva, N. et al. Protein kinase C isoforms mediate the formation of neutrophil extracellular traps. Int. Immunopharmacol. 114, 109448 (2023).
Dowey, R. et al. Enhanced neutrophil extracellular trap formation in COVID-19 is inhibited by the protein kinase C inhibitor ruboxistaurin. ERJ Open Res. 8, 00596–02021 (2022).
Schreiber, A. et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA 114, E9618–E9625 (2017).
Desai, J. et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur. J. Immunol. 46, 223–229 (2016).
de Carvalho Oliveira, V., Tatsiy, O. & McDonald, P. P. Phosphoinositol 3-kinase-driven NET formation involves different isoforms and signaling partners depending on the stimulus. Front. Immunol. 14, 1042686 (2023).
Huang, Z. et al. Autophagy-driven neutrophil extracellular traps: the dawn of sepsis. Pathol. Res. Pract. 234, 153896 (2022).
Bhattacharya, A. et al. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 12, 1731–1739 (2015).
Guo, Y. et al. Spontaneous formation of neutrophil extracellular traps is associated with autophagy. Sci. Rep. 11, 24005 (2021).
Zhang, R. et al. Neutrophil autophagy and NETosis in COVID-19: perspectives. Autophagy 19, 758–767 (2023).
Cheng, Z. et al. SHIP1 is required for the formation of neutrophil extracellular traps in rheumatoid arthritis. Int. Immunopharmacol. 115, 109625 (2023).
Lv, X. et al. Tetrachlorobenzoquinone exhibits immunotoxicity by inducing neutrophil extracellular traps through a mechanism involving ROS-JNK-NOX2 positive feedback loop. Environ. Pollut. 268, 115921 (2021).
Wolach, O. et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 10, eaan8292 (2018).
de Jesus Gonzalez-Contreras, F. & Zarate, X. Neutrophil extracellular traps: modulation mechanisms by pathogens. Cell Immunol. 382, 104640 (2022).
Obama, T. & Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci. 21, 8312 (2020).
Mi, L. et al. Neutrophil extracellular traps aggravate neuronal endoplasmic reticulum stress and apoptosis via TLR9 after traumatic brain injury. Cell Death Dis. 14, 374 (2023).
Sun, S. et al. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 12, 606 (2021).
Alyami, H. M. et al. Role of NOD1/NOD2 receptors in Fusobacterium nucleatum mediated NETosis. Micro. Pathog. 131, 53–64 (2019).
Yang, S. et al. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Transl. Res 254, 115–127 (2023).
Tall, A. R. & Westerterp, M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J. Lipid Res. 60, 721–727 (2019).
Hardison, S. E. & Brown, G. D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13, 817–822, (2012).
Torigoe, S., Schutt, C. R. & Yamasaki, S. Immune discrimination of the environmental spectrum through C-type lectin receptors. Int. Immunol. 33, 847–851 (2021).
Sung, P. S. & Hsieh, S. L. C-type lectins and extracellular vesicles in virus-induced NETosis. J. Biomed. Sci. 28, 46 (2021).
Xu, Q. et al. High expression of P-selectin induces neutrophil extracellular traps via the PSGL-1/Syk/Ca(2+)/PAD4 pathway to exacerbate acute pancreatitis. Front. Immunol. 14, 1265344 (2023).
Sung, P. S., Huang, T. F. & Hsieh, S. L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 10, 2402 (2019).
Bachiega, T. F. et al. Participation of dectin-1 receptor on NETs release against Paracoccidioides brasiliensis: role on extracellular killing. Immunobiology 221, 228–235 (2016).
Yipp, B. G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012).
de Bont, C. M., Boelens, W. C. & Pruijn, G. J. M. NETosis, complement, and coagulation: a triangular relationship. Cell Mol. Immunol. 16, 19–27 (2019).
Guglietta, S. et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 7, 11037 (2016).
Palmer, L. J., Damgaard, C., Holmstrup, P. & Nielsen, C. H. Influence of complement on neutrophil extracellular trap release induced by bacteria. J. Periodontal Res. 51, 70–76, (2016).
Behnen, M. et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J. Immunol. 193, 1954–1965 (2014).
Silva, B. M. et al. C5aR1 signaling triggers lung immunopathology in COVID-19 through neutrophil extracellular traps. J. Clin. Investig. 133, e163105 (2023).
Kim, S. W. & Lee, J. K. Role of HMGB1 in the interplay between NETosis and thrombosis in ischemic stroke: a review. Cells 9, 1794 (2020).
Watanabe, H. et al. Amelioration of nephritis in receptor for advanced glycation end-products (RAGE)-deficient lupus-prone mice through neutrophil extracellular traps. Clin. Immunol. 250, 109317 (2023).
Sprenkeler, E. G. G. et al. S100A8/A9 is a marker for the release of neutrophil extracellular traps and induces neutrophil activation. Cells 11, 236 (2022).
Aleman, O. R. et al. Differential use of human neutrophil fcgamma receptors for inducing neutrophil extracellular trap formation. J. Immunol. Res. 2016, 2908034 (2016).
Aleman, O. R. et al. Transforming growth factor-beta-activated kinase 1 is required for human fcgammariiib-induced neutrophil extracellular trap formation. Front. Immunol. 7, 277 (2016).
Chen, T. et al. Receptor-mediated NETosis on neutrophils. Front. Immunol. 12, 775267 (2021).
Teijeira, A. et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 52, 856–871.e858 (2020).
Yago, T., Liu, Z., Ahamed, J. & McEver, R. P. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 132, 1426–1437 (2018).
Delaveris, C. S. et al. Synthetic Siglec-9 agonists inhibit neutrophil activation associated with COVID-19. ACS Cent. Sci. 7, 650–657 (2021).
Liu, Y. C., Yu, M. M., Chai, Y. F. & Shou, S. T. Sialic acids in the immune response during sepsis. Front. Immunol. 8, 1601 (2017).
Sadeghi, M. et al. Neutrophil extracellular trap: a key player in the pathogenesis of autoimmune diseases. Int. Immunopharmacol. 116, 109843 (2023).
Mulay, S. R. & Anders, H. J. Neutrophils and neutrophil extracellular traps regulate immune responses in health and disease. Cells 9, 2130 (2020).
Islam, M. M. & Takeyama, N. Role of neutrophil extracellular traps in health and disease pathophysiology: recent insights and advances. Int. J. Mol. Sci. 24, 15805 (2023).
Chen, Z. et al. Review: the emerging role of neutrophil extracellular traps in sepsis and sepsis-associated thrombosis. Front. Cell Infect. Microbiol 11, 653228 (2021).
Zhu, S. et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 12, 984 (2021).
Németh, T., Sperandio, M. & Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 19, 253–275 (2020).
Burgener, S. S. & Schroder, K. Neutrophil extracellular traps in host defense. Cold Spring Harb. Perspect. Biol. 12, a037028 (2020).
Siwicki, M. & Kubes, P. Neutrophils in host defense, healing, and hypersensitivity: dynamic cells within a dynamic host. J. Allergy Clin. Immunol. 151, 634–655 (2023).
Liang, C., Lian, N. & Li, M. The emerging role of neutrophil extracellular traps in fungal infection. Front. Cell Infect. Microbiol. 12, 900895 (2022).
Urban, C. F. & Nett, J. E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 89, 47–57 (2019).
Zerbe, C. S. & Holland, S. M. Functional neutrophil disorders: chronic granulomatous disease and beyond. Immunol. Rev. 322, 71–80 (2024).
Milligan, K. L. et al. Complete myeloperoxidase deficiency: beware the “false-positive” dihydrorhodamine oxidation. J. Pediatr. 176, 204–206 (2016).
He, Y. et al. Neutrophil extracellular traps in Candida albicans infection. Front. Immunol. 13, 913028 (2022).
Shankar, M. et al. Immune resolution dilemma: host antimicrobial factor S100A8/A9 modulates inflammatory collateral tissue damage during disseminated fungal peritonitis. Front. Immunol. 12, 553911 (2021).
Fang, X. et al. A positive feedback cycle between the alarmin S100A8/A9 and NLRP3 inflammasome-GSDMD signalling reinforces the innate immune response in Candida albicans keratitis. Inflamm. Res. 72, 1485–1500 (2023).
Jukic, A. et al. Calprotectin: from biomarker to biological function. Gut 70, 1978–1988 (2021).
Hopke, A. et al. Neutrophil attack triggers extracellular trap-dependent candida cell wall remodeling and altered immune recognition. PLoS Pathog. 12, e1005644 (2016).
Casadevall, A. Immunity to invasive fungal diseases. Annu. Rev. Immunol. 40, 121–141 (2022).
Earle, K. et al. Pathogenicity and virulence of Aspergillus fumigatus. Virulence 14, 2172264 (2023).
Bianchi, M. et al. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127, 1243–1252.e1247 (2011).
Porte, R. et al. The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front. Immunol. 10, 794 (2019).
Baz, A. A. et al. Neutrophil extracellular traps in bacterial infections and evasion strategies. Front. Immunol. 15, 1357967 (2024).
McDonald, B. et al. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 (2012).
Azzouz, L. et al. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria. Mol. Immunol. 97, 71–81 (2018).
Li, X. et al. Role and therapeutic targeting strategies of neutrophil extracellular traps in inflammation. Int. J. Nanomed. 18, 5265–5287 (2023).
Halverson, T. W. et al. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 11, e1004593 (2015).
Sollberger, G., Tilley, D. O. & Zychlinsky, A. Neutrophil extracellular traps: the biology of chromatin externalization. Dev. Cell 44, 542–553 (2018).
Hoeksema, M., van Eijk, M., Haagsman, H. P. & Hartshorn, K. L. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 11, 441–453, (2016).
Wang, X., Yu, D. & Chen, L. Antimicrobial resistance and mechanisms of epigenetic regulation. Front. Cell Infect. Microbiol. 13, 1199646 (2023).
Domon, H. & Terao, Y. The role of neutrophils and neutrophil elastase in pneumococcal pneumonia. Front. Cell Infect. Microbiol. 11, 615959 (2021).
Amunugama, K., Kolar, G. R. & Ford, D. A. Neutrophil myeloperoxidase derived chlorolipid production during bacteria exposure. Front. Immunol. 12, 701227 (2021).
Thanabalasuriar, A. et al. Neutrophil extracellular traps confine Pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe 25, 526–536.e524 (2019).
Chen, W. A. & Boskovic, D. S. Neutrophil extracellular DNA traps in response to infection or inflammation, and the roles of platelet interactions. Int. J. Mol. Sci. 25, 3025 (2024).
Schönrich, G. & Raftery, M. J. Neutrophil extracellular traps go viral. Front Immunol. 7, 366 (2016).
Barr, F. D., Ochsenbauer, C., Wira, C. R. & Rodriguez-Garcia, M. Neutrophil extracellular traps prevent HIV infection in the female genital tract. Mucosal Immunol. 11, 1420–1428 (2018).
Kozlowski, H. N. et al. Extracellular histones identified in crocodile blood inhibit in-vitro HIV-1 infection. AIDS 30, 2043–2052 (2016).
Radic, M. & Muller, S. LL-37, a multi-faceted amphipathic peptide involved in NETosis. Cells 11, 2463 (2022).
Nagaoka, I., Tamura, H. & Reich, J. Therapeutic potential of cathelicidin peptide LL-37, an antimicrobial agent, in a murine sepsis model. Int. J. Mol. Sci. 21, 5973 (2020).
Mojoli, A. et al. Neutrophil extracellular traps from healthy donors and HIV-1-infected individuals restrict HIV-1 production in macrophages. Sci. Rep. 10, 19603 (2020).
Lopes, B. R. P. et al. Serine proteases in neutrophil extracellular traps exhibit anti-respiratory syncytial virus activity. Int. Immunopharmacol. 106, 108573 (2022).
Souza, P. S. S. et al. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: possible interaction with the viral F protein. Virus Res. 251, 68–77 (2018).
Díaz-Godínez, C. & Carrero, J. C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci. Rep. 39, BSR20180916 (2019).
Díaz-Godínez, C. et al. Entamoeba histolytica trophozoites induce a rapid non-classical NETosis mechanism independent of NOX2-derived reactive oxygen species and PAD4 activity. Front. Cell Infect. Microbiol. 8, 184 (2018).
Fonseca, Z. et al. Pathogenic Entamoeba histolytica, but not Entamoeba dispar, induce neutrophil extracellular trap (NET) formation. J. Leukoc. Biol. 105, 1167–1181 (2019).
Bonne-Année, S. et al. Extracellular traps are associated with human and mouse neutrophil and macrophage-mediated killing of larval Strongyloides stercoralis. Microbes Infect. 16, 502–511 (2014).
McCoy, C. J. et al. Human leukocytes Kill Brugia malayi microfilariae independently of DNA-based extracellular trap release. PLoS Negl. Trop. Dis. 11, e0005279 (2017).
Sousa-Rocha, D. et al. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating toll-like receptors. PLoS One 10, e0139569 (2015).
Aitken, E. H., Alemu, A. & Rogerson, S. J. Neutrophils and malaria. Front Immunol. 9, 3005 (2018).
Cortjens, B. et al. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 238, 401–411 (2016).
Yi, T. et al. Neutrophil extracellular traps mediate severe lung injury induced by influenza A virus H1N1 in mice coinfected with Staphylococcus aureus. Micro. Pathog. 166, 105558 (2022).
Al-Kuraishy, H. M. et al. Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int. Immunopharmacol. 104, 108516 (2022).
Wu, W. et al. Circulating neutrophil dysfunction in HBV-related acute-on-chronic liver failure. Front. Immunol. 12, 620365 (2021).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Ackermann, M. et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 28, 3125–3139 (2021).
Shrestha, S. & Hong, C. W. Extracellular mechanisms of neutrophils in immune cell crosstalk. Immune Netw. 23, e38 (2023).
Yan, M., Gu, Y., Sun, H. & Ge, Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front. Immunol. 14, 1135086 (2023).
Dömer, D. et al. Neutrophil extracellular traps activate proinflammatory functions of human neutrophils. Front. Immunol. 12, 636954 (2021).
Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 108, 377–396 (2020).
Möckel, T., Basta, F., Weinmann-Menke, J. & Schwarting, A. B cell activating factor (BAFF): Structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun. Rev. 20, 102736 (2021).
Giordano, D. et al. B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease. Front. Immunol. 14, 1050528 (2023).
Louiselle, A. E., Niemiec, S. M., Zgheib, C. & Liechty, K. W. Macrophage polarization and diabetic wound healing. Transl. Res. 236, 109–116 (2021).
Wei, X. et al. EDIL3 deficiency ameliorates adverse cardiac remodelling by neutrophil extracellular traps (NET)-mediated macrophage polarization. Cardiovasc. Res. 118, 2179–2195 (2022).
An, Z. et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 18, 2928–2938 (2019).
Song, C. et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp. Cell Res. 382, 111486 (2019).
Chen, L. et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 9, 597 (2018).
Monteith, A. J. et al. Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens. Sci. Adv. 7, eabj2101 (2021).
Pollitt, E. J. G., Szkuta, P. T., Burns, N. & Foster, S. J. Staphylococcus aureus infection dynamics. PLoS Pathog. 14, e1007112 (2018).
Sutton, J. A. F. et al. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 17, e1009468 (2021).
Su, B. et al. TIM-3 regulates the NETs-mediated dendritic cell activation in myeloperoxidase-ANCA-associated vasculitis. Clin. Exp. Rheumatol. 39, 13–20 (2021).
Matta, B. & Barnes, B. J. Coordination between innate immune cells, type I IFNs and IRF5 drives SLE pathogenesis. Cytokine 132, 154731 (2020).
Kotov, D. I. et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis. Cell 186, 5536–5553.e5522 (2023).
Parackova, Z. et al. Neutrophil extracellular trap induced dendritic cell activation leads to Th1 polarization in type 1 diabetes. Front. Immunol. 11, 661 (2020).
Barrientos, L. et al. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J. Immunol. 193, 5689–5698 (2014).
Tateosian, N. L. et al. Neutrophil elastase treated dendritic cells promote the generation of CD4(+)FOXP3(+) regulatory T cells in vitro. Cell Immunol. 269, 128–134 (2011).
Villar, J. et al. ETV3 and ETV6 enable monocyte differentiation into dendritic cells by repressing macrophage fate commitment. Nat. Immunol. 24, 84–95 (2023).
Devalaraja, S. et al. Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell 180, 1098–1114.e1016 (2020).
Guimarães-Costa, A. B. et al. Neutrophil extracellular traps reprogram IL-4/GM-CSF-induced monocyte differentiation to anti-inflammatory macrophages. Front. Immunol. 8, 523 (2017).
Wang, X. et al. Characteristic gene expression in the liver monocyte-macrophage-DC system is associated with the progression of fibrosis in NASH. Front. Immunol. 14, 1098056 (2023).
Goudot, C. et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity 47, 582–596.e586 (2017).
Rigamonti, A., Villar, J. & Segura, E. Monocyte differentiation within tissues: a renewed outlook. Trends Immunol. 44, 999–1013 (2023).
Cheng, Y. et al. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma. Biomaterials 284, 121506 (2022).
Wang, J. et al. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Front. Immunol. 11, 2063 (2020).
Jiang, M. et al. The enrichment of neutrophil extracellular traps impair the placentas of systemic lupus erythematosus through accumulating decidual NK cells. Sci. Rep. 11, 6870 (2021).
Bert, S., Nadkarni, S. & Perretti, M. Neutrophil-T cell crosstalk and the control of the host inflammatory response. Immunol. Rev. 314, 36–49 (2023).
Miranda, F. J. B. et al. Toxoplasma gondii-induced neutrophil extracellular traps amplify the innate and adaptive response. mBio 12, e0130721 (2021).
Tate, M. D., Brooks, A. G., Reading, P. C. & Mintern, J. D. Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol. Cell Biol. 90, 197–205 (2012).
Wilson, A. S. et al. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 13, 528 (2022).
Gestermann, N. et al. Netting neutrophils activate autoreactive B cells in lupus. J. Immunol. 200, 3364–3371 (2018).
Lande, R. et al. Complementary effects of carbamylated and citrullinated ll37 in autoimmunity and inflammation in systemic lupus erythematosus. Int. J. Mol. Sci. 22, 1650 (2021).
Corsiero, E. et al. NETosis as source of autoantigens in rheumatoid arthritis. Front. Immunol. 7, 485 (2016).
Fang, H. et al. Neutrophil extracellular traps contribute to immune dysregulation in bullous pemphigoid via inducing B-cell differentiation and antibody production. FASEB J. 35, e21746 (2021).
Downs-Canner, S. M., Meier, J., Vincent, B. G. & Serody, J. S. B cell function in the tumor microenvironment. Annu. Rev. Immunol. 40, 169–193 (2022).
Akkaya, M., Kwak, K. & Pierce, S. K. B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 20, 229–238 (2020).
Moir, S. & Fauci, A. S. B-cell responses to HIV infection. Immunol. Rev. 275, 33–48 (2017).
Engelhard, V. et al. B cells and cancer. Cancer Cell 39, 1293–1296 (2021).
Fousert, E., Toes, R. & Desai, J. Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells 9, 915 (2020).
Fang, Q. et al. No NETs no TIME: crosstalk between neutrophil extracellular traps and the tumor immune microenvironment. Front. Immunol. 13, 1075260 (2022).
Khan, U. et al. Neutrophil extracellular traps in colorectal cancer progression and metastasis. Int. J. Mol. Sci. 22, 7260 (2021).
Iba, T., Levi, M. & Levy, J. H. Intracellular communication and immunothrombosis in sepsis. J. Thromb. Haemost. 20, 2475–2484 (2022).
Campos, J. et al. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 5, 2319–2324 (2021).
Perdomo, J. et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 10, 1322 (2019).
Patel, P., Michael, J. V., Naik, U. P. & McKenzie, S. E. Platelet FcγRIIA in immunity and thrombosis: adaptive immunothrombosis. J. Thromb. Haemost. 19, 1149–1160 (2021).
van der Poll, T. & Opal, S. M. Should all septic patients be given systemic anticoagulation? No. Intensive Care Med. 43, 455–457 (2017).
Thakur, M. et al. NETs-induced thrombosis impacts on cardiovascular and chronic kidney disease. Circ. Res 132, 933–949 (2023).
Knopf, J. et al. Aggregated NETs sequester and detoxify extracellular histones. Front. Immunol. 10, 2176 (2019).
Schauer, C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014).
Schoen, J. et al. Neutrophils’ extracellular trap mechanisms: from physiology to pathology. Int. J. Mol. Sci. 23, 12855 (2022).
Podolska, M. J. et al. Treatment with DNases rescues hidden neutrophil elastase from aggregated NETs. J. Leukoc. Biol. 106, 1359–1366 (2019).
Yang, S. et al. Neutrophil extracellular traps delay diabetic wound healing by inducing endothelial-to-mesenchymal transition via the Hippo pathway. Int. J. Biol. Sci. 19, 347–361 (2023).
Chu, Z. et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. Bioact. Mater. 27, 257–270 (2023).
Karkowska-Kuleta, J. et al. Proteinous components of neutrophil extracellular traps are arrested by the cell wall proteins of candida albicans during fungal infection, and can be used in the host invasion. Cells 10, 2736 (2021).
Knackstedt, S. L. et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci. Immunol. 4, eaaw0336 (2019).
Guimaraes-Costa, A. B. et al. 3’-nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect. Immun. 82, 1732–1740 (2014).
Meurer, M. et al. Role of bacterial and host dnases on host-pathogen interaction during streptococcus suis meningitis. Int. J. Mol. Sci. 21, 5289 (2020).
Liao, C., Mao, F., Qian, M. & Wang, X. Pathogen-derived nucleases: an effective weapon for escaping extracellular traps. Front. Immunol. 13, 899890 (2022).
Chalmers, C. et al. Streptococcus pyogenes nuclease A (SpnA) mediated virulence does not exclusively depend on nuclease activity. J. Microbiol. Immunol. Infect. 53, 42–48 (2020).
Zhang, Y. Y. & Ning, B. T. Signaling pathways and intervention therapies in sepsis. Signal Transduct. Target Ther. 6, 407 (2021).
Colon, D. F. et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 23, 113 (2019).
Silva, C. M. S. et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood 138, 2702–2713 (2021).
Su, M. et al. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat. Cardiovasc. Res 1, 732–747 (2022).
Maruchi, Y. et al. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit. Care 22, 176 (2018).
Stiel, L. et al. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb. Res. 183, 153–158 (2019).
Lenz, M. et al. cfDNA and DNases: new biomarkers of sepsis in preterm neonates-a pilot study. Cells 11, 192 (2022).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PloS One 7, e32366 (2012).
Abrams, S. T. et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 187, 160–169 (2013).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357-1367. Blood 139, 952 (2022).
Abrams, S. T. et al. Damage-associated cellular markers in the clinical and pathogenic profile of vaccine-induced immune thrombotic thrombocytopenia. J. Thromb. Haemost. 22, 1145–1153 (2024).
Kubes, P. & Jenne, C. Immune responses in the liver. Annu. Rev. Immunol. 36, 247–277 (2018).
Hu, S. et al. Hepatitis B virus inhibits neutrophil extracellular trap release by modulating reactive oxygen species production and autophagy. J. Immunol. 202, 805–815 (2019).
von Meijenfeldt, F. A. et al. Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome. Hepatology 75, 623–633 (2022).
Kuo, Y. M. et al. Temporal changes in biomarkers of neutrophil extracellular traps and NET-promoting autoantibodies following adenovirus-vectored, mRNA, and recombinant protein COVID-19 vaccination. J. Med. Virol. 96, e29556 (2024).
Feys, S. et al. Lower respiratory tract single-cell RNA sequencing and neutrophil extracellular trap profiling of COVID-19-associated pulmonary aspergillosis: a single centre, retrospective, observational study. Lancet Microbe 5, e247–e260 (2024).
Giaglis, S. Poised to cast wide NETs in long COVID. J. Thromb. Haemost. 21, 2362–2364 (2023).
Barnes, B. J. et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 217, e20200652 (2020).
Cesta, M. C. et al. Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis. Eur. J. Immunol. 53, e2250010 (2023).
Holliday, Z. M. et al. Non-randomized trial of dornase alfa for acute respiratory distress syndrome secondary to Covid-19. Front. Immunol. 12, 714833 (2021).
Poon, I. et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife 3, e01808 (2014).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Block, H., Rossaint, J. & Zarbock, A. The fatal circle of NETs and NET-associated DAMPs contributing to organ dysfunction. Cells 11, 1919 (2022).
Li, Y. et al. Neutrophil extracellular traps formation and aggregation orchestrate induction and resolution of sterile crystal-mediated inflammation. Front. Immunol. 9, 1559 (2018).
Dehlin, M., Jacobsson, L. & Roddy, E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 16, 380–390 (2020).
So, A. K. & Martinon, F. Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 13, 639–647 (2017).
Hahn, J. et al. Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin. Exp. Rheumatol. 34, 6–8 (2016).
Sil, P., Wicklum, H., Surell, C. & Rada, B. Macrophage-derived IL-1beta enhances monosodium urate crystal-triggered NET formation. Inflamm. Res. 66, 227–237 (2017).
Mitroulis, I. et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PloS One 6, e29318 (2011).
Liu, L. et al. Neutrophil extracellular trap-borne elastase prevents inflammatory relapse in intercritical gout. Arthritis Rheumatol. 75, 1039–1047 (2023).
Cao, X. et al. Transient receptor potential melastatin 2 regulates neutrophil extracellular traps formation and delays resolution of neutrophil-driven sterile inflammation. J. Inflamm. 20, 7 (2023).
Sorci-Thomas, M. G. & Thomas, M. J. Microdomains, inflammation, and atherosclerosis. Circ. Res. 118, 679–691, (2016).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target Ther. 7, 131 (2022).
Megens, R. T. et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 107, 597–598 (2012).
da Silva, R. F. et al. Anti-apolipoprotein A-1 IgG influences neutrophil extracellular trap content at distinct regions of human carotid plaques. Int. J. Mol. Sci. 21, 7721 (2020).
Riegger, J. et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European Effort Consortium. Eur. Heart J. 37, 1538–1549 (2016).
Stark, K. et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128, 2435–2449 (2016).
Gu, C. et al. Neutrophil extracellular traps contributing to atherosclerosis: from pathophysiology to clinical implications. Exp. Biol. Med. 248, 1302–1312 (2023).
An, Z. et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-kappaB signaling in macrophages. Cell Cycle 18, 2928–2938 (2019).
Molinaro, R. et al. Targeted delivery of protein arginine deiminase-4 inhibitors to limit arterial intimal NETosis and preserve endothelial integrity. Cardiovasc. Res. 117, 2652–2663 (2021).
Borissoff, J. I. et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 33, 2032–2040 (2013).
Langseth, M. S. et al. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur. J. Prev. Cardiol. 25, 762–769 (2018).
Leppkes, M. et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun. 7, 10973 (2016).
Munoz, L. E. et al. Neutrophil extracellular traps initiate gallstone formation. Immunity 51, 443–450.e444 (2019).
Merza, M. et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology 149, 1920–1931.e1928 (2015).
Kang, H. et al. Role of neutrophil extracellular traps in inflammatory evolution in severe acute pancreatitis. Chin. Med. J. 135, 2773–2784 (2022).
Dong, X. et al. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano 13, 1272–1283 (2019).
Luo, H. et al. Neutrophil extracellular traps in cerebral ischemia/reperfusion injury: friend and foe. Curr. Neuropharmacol. 21, 2079–2096 (2023).
Kim, S. W. et al. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol. Commun. 7, 94 (2019).
Gou, X. et al. The roles of high mobility group box 1 in cerebral ischemic injury. Front. Cell Neurosci. 14, 600280 (2020).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Volkman, R. et al. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer’s disease. Front. Neurosci. 13, 990 (2019).
Lim, S. et al. Senolytic therapy for cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 22, 11967 (2021).
Otxoa-de-Amezaga, A. et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke 50, 1548–1557 (2019).
Huang, H. et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 62, 600–614 (2015).
Zhang, F. et al. The role of extracellular traps in ischemia reperfusion injury. Front. Immunol. 13, 1022380 (2022).
Yazdani, H. O. et al. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J. Hepatol. S0168-8278, 32291–32292 (2017).
Tohme, S. et al. Computational analysis supports IL-17A as a central driver of neutrophil extracellular trap-mediated injury in liver ischemia reperfusion. J. Immunol. 202, 268–277 (2019).
Al-Khafaji, A. B. et al. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol. Med. 22, 621–631 (2016).
Liu, Y. et al. Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight 3, e124729 (2018).
Tsourouktsoglou, T. D. et al. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 31, 107602 (2020).
Rahman, S. et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann. Rheum. Dis. 78, 957–966 (2019).
Carmona-Rivera, C. et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358 (2017).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66, 2532–2544 (2014).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Ding, Q. et al. Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct. Target Ther. 8, 68 (2023).
Sokolove, J. et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 66, 813–821 (2014).
Krishnamurthy, A. et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 75, 721–729 (2016).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra140 (2013).
Catrina, A., Krishnamurthy, A. & Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 7, e001228 (2021).
Demoruelle, M. K. et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheumatol. 70, 516–527 (2018).
Sur Chowdhury, C. et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122 (2014).
Wang, W., Peng, W. & Ning, X. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. Int. J. Rheum. Dis. 21, 415–421 (2018).
Perez-Sanchez, C. et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J. Autoimmun. 82, 31–40 (2017).
Wright, H. L., Makki, F. A., Moots, R. J. & Edwards, S. W. Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J. Leukoc. Biol. 101, 599–611 (2017).
Ramanathan, K. et al. Neutrophil activation signature in juvenile idiopathic arthritis indicates the presence of low-density granulocytes. Rheumatology 57, 488–498 (2018).
Pratesi, F. et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 73, 1414–1422 (2014).
Ribon, M. et al. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 98, 122–131 (2019).
Corsiero, E. et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 75, 1866–1875 (2016).
Lloyd, K. A. et al. Differential ACPA binding to nuclear antigens reveals a PAD-independent pathway and a distinct subset of acetylation cross-reactive autoantibodies in rheumatoid arthritis. Front. Immunol. 9, 3033 (2018).
Carmona-Rivera, C. et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 5, e139388 (2020).
Song, W. et al. Neutrophil extracellular traps tied to rheumatoid arthritis: points to ponder. Front. Immunol. 11, 578129 (2020).
Apel, F., Zychlinsky, A. & Kenny, E. F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 14, 467–475 (2018).
Kronbichler, A. et al. Diagnosis and management of ANCA-associated vasculitis. Lancet 403, 683–698 (2024).
Trivioli, G. et al. Genetics of ANCA-associated vasculitis: role in pathogenesis, classification and management. Nat. Rev. Rheumatol. 18, 559–574 (2022).
Cornec, D., Cornec-Le Gall, E., Fervenza, F. C. & Specks, U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 12, 570–579, (2016).
Grayson, P. C. et al. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 67, 1922–1932 (2015).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Michailidou, D. et al. Neutrophil extracellular trap formation in anti-neutrophil cytoplasmic antibody-associated and large-vessel vasculitis. Clin. Immunol. 249, 109274 (2023).
Panda, R. et al. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front. Immunol. 8, 439 (2017).
Heeringa, P., Rutgers, A. & Kallenberg, C. G. M. The net effect of ANCA on neutrophil extracellular trap formation. Kidney Int. 94, 14–16 (2018).
Kusunoki, Y. et al. Peptidylarginine deiminase inhibitor suppresses neutrophil extracellular trap formation and MPO-ANCA production. Front. Immunol. 7, 227 (2016).
Nakazawa, D., Masuda, S., Tomaru, U. & Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 15, 91–101 (2019).
Negreros, M. & Flores-Suarez, L. F. A proposed role of neutrophil extracellular traps and their interplay with fibroblasts in ANCA-associated vasculitis lung fibrosis. Autoimmun. Rev. 20, 102781 (2021).
Aendekerk, J. P. et al. Assessment of longitudinal serum neutrophil extracellular trap-inducing activity in anti-neutrophil cytoplasmic antibody-associated vasculitis and glomerulonephritis in a prospective cohort using a novel bio-impedance technique. Kidney Int. 104, 151–162 (2023).
Soderberg, D. et al. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology 54, 2085–2094 (2015).
Yoshida, M. et al. Myeloperoxidase anti-neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis. Nephrology 21, 624–629 (2016).
Tian, J. et al. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 82, 351–356 (2023).
Dieker, J. et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 68, 462–472 (2016).
Wang, H. et al. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
van der Linden, M. et al. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology 57, 1228–1234 (2018).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Lyons, P. A. et al. Novel expression signatures identified by transcriptional analysis of separated leucocyte subsets in systemic lupus erythematosus and vasculitis. Ann. Rheum. Dis. 69, 1208–1213 (2010).
Leffler, J. et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188, 3522–3531 (2012).
Carlucci, P. M. et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 3, e99276 (2018).
Lopez, P. et al. Low-density granulocytes and monocytes as biomarkers of cardiovascular risk in systemic lupus erythematosus. Rheumatology 59, 1752–1764 (2020).
Blanco, L. P. et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 73, 2282–2292 (2021).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M. J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424, (2015).
Leffler, J. et al. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 32, 66–70 (2014).
Pisetsky, D. S. Antibodies to neutrophil extracellular traps: novel markers for the antiphospholipid syndrome. Arthritis Rheumatol. 75, 1331–1333 (2023).
Zuo, Y. et al. Anti-neutrophil extracellular trap antibodies in antiphospholipid antibody-positive patients: results from the antiphospholipid syndrome alliance for clinical trials and international networking clinical database and repository. Arthritis Rheumatol. 75, 1407–1414 (2023).
Ali, R. A. et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 10, 1916 (2019).
Seto, N. et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 5, e134189 (2020).
Ma, W. et al. The role of neutrophil extracellular traps and proinflammatory damage-associated molecular patterns in idiopathic inflammatory myopathies. Clin. Exp. Immunol. 213, 202–208 (2023).
Moon, S. J. et al. Molecular signature of neutrophil extracellular trap mediating disease module in idiopathic inflammatory myopathy. J. Autoimmun. 138, 103063 (2023).
Tillack, K. et al. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J. Neuroimmunol. 261, 108–119 (2013).
Paryzhak, S. et al. Neutrophil-released enzymes can influence composition of circulating immune complexes in multiple sclerosis. Autoimmunity 51, 297–303 (2018).
Ding, Y. et al. Tyrosine phosphatase SHP2 exacerbates psoriasis-like skin inflammation in mice via ERK5-dependent NETosis. MedComm 3, e120 (2022).
Lambert, S. et al. Neutrophil extracellular traps induce human Th17 cells: effect of psoriasis-associated TRAF3IP2 genotype. J. Investig. Dermatol. 139, 1245–1253 (2019).
Xu, C. et al. Cyclosporine A alleviates colitis by inhibiting the formation of neutrophil extracellular traps via the regulating pentose phosphate pathway. Mol. Med. 29, 169 (2023).
Cao, D. et al. Thymopentin ameliorates experimental colitis via inhibiting neutrophil extracellular traps. Int. Immunopharmacol. 124, 110898 (2023).
Liu, P. et al. Ferroptosis: mechanisms and role in diabetes mellitus and its complications. Ageing Res. Rev. 94, 102201 (2024).
Li, Y. et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct. Target Ther. 8, 152 (2023).
Abel, E. D. et al. Type 2 diabetes—controlling the epidemic, episode 1: understanding and preventing type 2 diabetes. N. Engl. J. Med. 389, e18 (2023).
Lin, C. H. et al. Evaluation of disease complications among adults with type 1 diabetes and a family history of type 2 diabetes in Taiwan. JAMA Netw. Open 4, e2138775 (2021).
Liu, C. et al. Incidence of type 1 diabetes may be underestimated in the Chinese population: evidence from 21.7 million people between 2007 and 2017. Diabetes Care 44, 2503–2509 (2021).
Ahmad, E. et al. Type 2 diabetes. Lancet 400, 1803–1820 (2022).
Giovenzana, A. et al. Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes. Diabetes Metab. Res. Rev. 38, e3483 (2022).
Wong, S. L. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 21, 815–819 (2015).
Bissenova, S. et al. NET proteome in established type 1 diabetes is enriched in metabolic proteins. Cells 12, 1319 (2023).
Wang, Y. et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with beta-cell autoimmunity in patients with type 1 diabetes. Diabetes 63, 4239–4248 (2014).
Klocperk, A. et al. Elevated biomarkers of NETosis in the serum of pediatric patients with type 1 diabetes and their first-degree relatives. Front. Immunol. 12, 699386 (2021).
Skoglund, C. et al. Increase of neutrophil extracellular traps, mitochondrial DNA and nuclear DNA in newly diagnosed type 1 diabetes children but not in high-risk children. Front. Immunol. 12, 628564 (2021).
You, Q. et al. Increased formation of neutrophil extracellular traps is associated with gut leakage in patients with type 1 but not type 2 diabetes. J. Diabetes 11, 665–673 (2019).
You, Q. et al. Neutrophil extracellular traps caused by gut leakage trigger the autoimmune response in nonobese diabetic mice. Front. Immunol. 12, 711423 (2021).
Liang, Y. et al. Ameliorating gut microenvironment through staphylococcal nuclease-mediated intestinal NETs degradation for prevention of type 1 diabetes in NOD mice. Life Sci. 221, 301–310 (2019).
Shu, L. et al. Neutrophil elastase triggers the development of autoimmune diabetes by exacerbating innate immune responses in pancreatic islets of non-obese diabetic mice. Clin. Sci. 134, 1679–1696 (2020).
Shen, Y., You, Q., Wu, Y. & Wu, J. Inhibition of PAD4-mediated NET formation by cl-amidine prevents diabetes development in nonobese diabetic mice. Eur. J. Pharm. 916, 174623 (2022).
Liu, C. et al. Inhibition of neutrophil extracellular trap formation alleviates vascular dysfunction in type 1 diabetic mice. Sci. Adv. 9, eadj1019 (2023).
Menegazzo, L. et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 52, 497–503 (2015).
Miyoshi, A. et al. Circulating neutrophil extracellular trap levels in well-controlled type 2 diabetes and pathway involved in their formation induced by high-dose glucose. Pathobiology 83, 243–251 (2016).
Carestia, A. et al. NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PloS One 11, e0168647 (2016).
Wang, L. et al. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front. Immunol. 9, 3076 (2018).
Joshi, M. B. et al. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 587, 2241–2246 (2013).
Fadini, G. P. et al. NETosis delays diabetic wound healing in mice and humans. Diabetes 65, 1061–1071 (2016).
Tong, Y. et al. Excessive neutrophil extracellular trap formation induced by Porphyromonas gingivalis lipopolysaccharide exacerbates inflammatory responses in high glucose microenvironment. Front. Cell Infect. Microbiol. 13, 1108228 (2023).
Roth Flach, R. J. & Czech, M. P. NETs and traps delay wound healing in diabetes. Trends Endocrinol. Metab. 26, 451–452, (2015).
Liu, D. et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin. Sci. 133, 565–582 (2019).
Binet, F. et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 369, eaay5356 (2020).
Magana-Guerrero, F. S. et al. Spontaneous neutrophil extracellular traps release are inflammatory markers associated with hyperglycemia and renal failure on diabetic retinopathy. Biomedicines 11, 1791 (2023).
Zheng, F. et al. Neutrophil extracellular traps induce glomerular endothelial cell dysfunction and pyroptosis in diabetic kidney disease. Diabetes 71, 2739–2750 (2022).
D’Abbondanza, M. et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 9, 14678 (2019).
Braster, Q. et al. Inhibition of NET release fails to reduce adipose tissue inflammation in mice. PloS One 11, e0163922 (2016).
Xu, X. et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target Ther. 7, 287 (2022).
Younossi, Z. M. & Henry, L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 3, 100305 (2021).
Negro, F. Natural history of NASH and HCC. Liver Int. 40, 72–76 (2020).
Liu, K., Wang, F. S. & Xu, R. Neutrophils in liver diseases: pathogenesis and therapeutic targets. Cell Mol. Immunol. 18, 38–44 (2021).
Maretti-Mira, A. C. et al. Cholesterol-Induced M4-Like macrophages recruit neutrophils and induce NETosis. Front. Immunol. 12, 671073 (2021).
Gonzalez-Teran, B. et al. p38gamma and p38delta reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 35, 536–552 (2016).
van der Windt, D. J. et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 68, 1347–1360 (2018).
Wu, J. et al. Polyunsaturated fatty acids drive neutrophil extracellular trap formation in nonalcoholic steatohepatitis. Eur. J. Pharm. 945, 175618 (2023).
Ioannou, G. N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. 27, 84–95 (2016).
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18, 223–238 (2021).
Foerster, F., Gairing, S. J., Muller, L. & Galle, P. R. NAFLD-driven HCC: safety and efficacy of current and emerging treatment options. J. Hepatol. 76, 446–457 (2022).
Miele, L. et al. Nonalcoholic fatty liver disease (NAFLD) severity is associated to a nonhemostatic contribution and proinflammatory phenotype of platelets. Transl. Res 231, 24–38 (2021).
Islam, R. et al. Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance. Cancer Metastasis Rev. 41, 771–787 (2022).
Minciuna, I., Taru, M. G., Procopet, B. & Stefanescu, H. The interplay between liver sinusoidal endothelial cells, platelets, and neutrophil extracellular traps in the development and progression of metabolic dysfunction-associated steatotic liver disease. J. Clin. Med. 13, 1406 (2024).
Tripodi, A. et al. Hypercoagulability in patients with non-alcoholic fatty liver disease (NAFLD): causes and consequences. Biomedicines 10, 249 (2022).
Du, J. et al. Neutrophil extracellular traps induced by pro-inflammatory cytokines enhance procoagulant activity in NASH patients. Clin. Res. Hepatol. Gastroenterol. 46, 101697 (2022).
Violi, F., Pastori, D., Pignatelli, P. & Carnevale, R. Nutrition, thrombosis, and cardiovascular disease. Circ. Res. 126, 1415–1442 (2020).
Wendelboe, A. M. & Raskob, G. E. Global burden of thrombosis: epidemiologic aspects. Circ. Res. 118, 1340–1347, (2016).
Laridan, E., Martinod, K. & De Meyer, S. F. Neutrophil extracellular traps in arterial and venous thrombosis. Semin. Thromb. Hemost. 45, 86–93 (2019).
Doring, Y., Soehnlein, O. & Weber, C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 120, 736–743 (2017).
Knight, J. S. et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114, 947–956 (2014).
Franck, G. et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ. Res. 123, 33–42 (2018).
Stakos, D. A. et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 36, 1405–1414 (2015).
Farkas, A. Z. et al. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb. Res. 175, 46–52 (2019).
Gorog, D. A. & Massberg, S. Neutrophil extracellular traps in the infarct-related coronary artery—a marker or mediator of adverse outcome? Thromb. Haemost. 122, 1251–1254 (2022).
Kluge, K. E. et al. Complement activation in association with markers of neutrophil extracellular traps and acute myocardial infarction in stable coronary artery disease. Mediat. Inflamm. 2020, 5080743 (2020).
Valles, J. et al. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb. Haemost. 117, 1919–1929 (2017).
Wang, R. et al. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood 138, 91–103 (2021).
Dhanesha, N. et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood 139, 1234–1245 (2022).
Wang, Y. et al. Neutrophil extracellular trap burden correlates with the stenosis of coronary atherosclerosis. PeerJ 11, e15471 (2023).
Liu, Y. et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front. Immunol. 9, 1680 (2018).
de Boer, O. J. et al. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 109, 290–297 (2013).
Laridan, E. et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 82, 223–232 (2017).
Ducroux, C. et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 49, 754–757 (2018).
Lim, H. H. et al. Evaluation of neutrophil extracellular traps as the circulating marker for patients with acute coronary syndrome and acute ischemic stroke. J. Clin. Lab. Anal. 34, e23190 (2020).
Bang, O. Y. et al. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: the OASIS-cancer study. Stroke 50, 2944–2947 (2019).
Henke, P. K. et al. Call to action to prevent venous thromboembolism in hospitalized patients: a policy statement from the American Heart Association. Circulation 141, e914–e931 (2020).
Wolberg, A. S. et al. Venous thrombosis. Nat. Rev. Dis. Prim. 1, 15006 (2015).
Hisada, Y. et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica 105, 218–225 (2020).
Sharma, S. et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood 137, 1104–1116 (2021).
Diaz, J. A. et al. Plasma DNA is elevated in patients with deep vein thrombosis. J. Vasc. Surg. Venous Lymphat Disord. 1, 341–348.e341 (2013).
van Montfoort, M. L. et al. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 33, 147–151 (2013).
Medeiros, S. K. et al. Does cell-free DNA promote coagulation and inhibit fibrinolysis in patients with unprovoked venous thromboembolism? Thromb. Res. 186, 13–19 (2020).
Brill, A. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 10, 136–144 (2012).
Martinod, K. et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl Acad. Sci. USA 110, 8674–8679 (2013).
Savchenko, A. S. et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 12, 860–870 (2014).
Silvestre-Roig, C., Fridlender, Z. G., Glogauer, M. & Scapini, P. Neutrophil diversity in health and disease. Trends Immunol. 40, 565–583 (2019).
Ye, Y. X. et al. Blood cell parameters from early to middle pregnancy and risk of gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 108, e1702–e1711 (2023).
Gimeno-Molina, B., Muller, I., Kropf, P. & Sykes, L. The role of neutrophils in pregnancy, term and preterm labour. Life 12, 1512 (2022).
Lampe, R. et al. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 107, 26–30 (2015).
Dimitriadis, E. et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 9, 8 (2023).
Deer, E. et al. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 19, 257–270 (2023).
Hu, Y. et al. Increased neutrophil activation and plasma DNA levels in patients with pre-eclampsia. Thromb. Haemost. 118, 2064–2073 (2018).
Marder, W. et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci. Med. 3, e000134 (2016).
Niedzwiedzka-Rystwej, P., Repka, W., Tokarz-Deptula, B. & Deptula, W. “In sickness and in health”—how neutrophil extracellular trap (NET) works in infections, selected diseases and pregnancy. J. Inflamm. 16, 15 (2019).
Moodley, M., Moodley, J. & Naicker, T. Neutrophil extracellular traps: the synergy source in the placentae of HIV infected women with pre-eclampsia. Preg. Hypertens. 20, 69–74 (2020).
Moodley, M., Moodley, J. & Naicker, T. The role of neutrophils and their extracellular traps in the synergy of pre-eclampsia and HIV infection. Curr. Hypertens. Rep. 22, 41 (2020).
De Borre, M. et al. Cell-free DNA methylome analysis for early preeclampsia prediction. Nat. Med. 29, 2206–2215 (2023).
Moufarrej, M. N. et al. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 602, 689–694 (2022).
Rolnik, D. L. et al. Maternal plasma cell-free DNA in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 45, 106–111 (2015).
Hahn, S., Giaglis, S., Hoesli, I. & Hasler, P. Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss. Front. Immunol. 3, 362 (2012).
Gupta, A. K. et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584, 3193–3197 (2010).
Zhang, C. & Catalano, P. Screening for gestational diabetes. JAMA 326, 487–489 (2021).
Ye, W. et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 377, e067946 (2022).
Simmons, D. et al. Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N. Engl. J. Med. 388, 2132–2144 (2023).
Vokalova, L. et al. Excessive neutrophil activity in gestational diabetes mellitus: could it contribute to the development of preeclampsia? Front. Endocrinol. (Lausanne) 9, 542 (2018).
Stoikou, M. et al. Gestational diabetes mellitus is associated with altered neutrophil activity. Front. Immunol. 8, 702 (2017).
Shen, D. et al. Mechanism of neutrophil extracellular traps generation and their role in trophoblasts apoptosis in gestational diabetes mellitus. Cell Signal 88, 110168 (2021).
Lin, X. et al. The choline metabolite TMAO inhibits NETosis and promotes placental development in GDM of humans and mice. Diabetes 70, 2250–2263 (2021).
Li, Y. et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol. Immunol. 16, 908–920 (2019).
Pershad, J. et al. Prevalence and determinants of self-reported anxiety and stress among women with abortion-related complications admitted to health facilities in Eastern and Southern Africa: a cross-sectional survey. Int. J. Gynaecol. Obstet. 156, 53–62 (2022).
Lim, J. H. et al. Cell-free fetal DNA and cell-free total DNA levels in spontaneous abortion with fetal chromosomal aneuploidy. PloS One 8, e56787 (2013).
Ye, H. et al. Dysregulated low-density granulocyte contributes to early spontaneous abortion. Front. Immunol. 14, 1119756 (2023).
Omeljaniuk, W. J. et al. Biomarkers of neutrophil extracellular traps (NETs) and nitric oxide-(NO)-dependent oxidative stress in women who miscarried. Sci. Rep. 10, 13088 (2020).
Gomez-Lopez, N. et al. Neutrophil extracellular traps in acute chorioamnionitis: a mechanism of host defense. Am. J. Reprod. Immunol. 77, https://doi.org/10.1111/aji.12617 (2017).
Erpenbeck, L. et al. PAD4 deficiency decreases inflammation and susceptibility to pregnancy loss in a mouse model. Biol. Reprod. 95, 132 (2016).
Hu, W. et al. Neutrophil extracellular traps facilitate cancer metastasis: cellular mechanisms and therapeutic strategies. J. Cancer Res. Clin. Oncol. 149, 2191–2210 (2023).
Lee, W. et al. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 216, 176–194 (2019).
Adrover, J. M. et al. NETworking with cancer: the bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 41, 505–526 (2023).
Alfaro, C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res 22, 3924–3936 (2016).
Cedervall, J. et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 75, 2653–2662 (2015).
Cedervall, J., Zhang, Y. & Olsson, A. K. Tumor-induced NETosis as a risk factor for metastasis and organ failure. Cancer Res. 76, 4311–4315, (2016).
Kwak, S. B. et al. Tumor regionalization after surgery: roles of the tumor microenvironment and neutrophil extracellular traps. Exp. Mol. Med. 54, 720–729 (2022).
Munir, H. et al. Stromal-driven and Amyloid beta-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat. Commun. 12, 683 (2021).
McInturff, A. M. et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 120, 3118–3125 (2012).
Ronchetti, L. et al. Neutrophil extracellular traps in cancer: not only catching microbes. J. Exp. Clin. Cancer Res. 40, 231 (2021).
de Andrea, C. E. et al. Heterogenous presence of neutrophil extracellular traps in human solid tumours is partially dependent on IL-8. J. Pathol. 255, 190–201 (2021).
Sun, L. et al. T cells in health and disease. Signal Transduct. Target Ther. 8, 235 (2023).
Yang, C. et al. Circulating tumor cells shielded with extracellular vesicle-derived CD45 evade T cell attack to enable metastasis. Signal Transduct. Target Ther. 9, 84 (2024).
Cozar, B. et al. Tumor-infiltrating natural killer cells. Cancer Discov. 11, 34–44 (2021).
Peng, Y. P. et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer 14, 738 (2014).
Ireland, A. S. & Oliver, T. G. Neutrophils create an ImpeNETrable shield between tumor and cytotoxic immune cells. Immunity 52, 729–731 (2020).
Park, J. et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 8, 361ra138 (2016).
Shinde-Jadhav, S. et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat. Commun. 12, 2776 (2021).
Zhang, Y. et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 217, e20190354 (2020).
Yang, J. et al. KDM6A loss recruits tumor-associated neutrophils and promotes neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 82, 4247–4260 (2022).
Schoeps, B. et al. TIMP1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer Res 81, 3568–3579 (2021).
Zhang, F. et al. TGF-beta-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer. Cell Death Dis. 15, 218 (2024).
Li, J. et al. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell Commun. Signal 21, 86 (2023).
Pieterse, E. et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 37, 1371–1379 (2017).
Stehr, A. M. et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J. Pathol. 256, 455–467 (2022).
Demers, M. et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 5, e1134073 (2016).
Miller-Ocuin, J. L. et al. DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology 8, e1605822 (2019).
Aldabbous, L. et al. Neutrophil extracellular traps promote angiogenesis: evidence from vascular pathology in pulmonary hypertension. Arterioscler Thromb. Vasc. Biol. 36, 2078–2087 (2016).
Yuan, K. et al. Neutrophil extracellular traps promote corneal neovascularization-induced by alkali burn. Int. Immunopharmacol. 88, 106902 (2020).
Lasch, M. et al. RNase A treatment interferes with leukocyte recruitment, neutrophil extracellular trap formation, and angiogenesis in ischemic muscle tissue. Front Physiol. 11, 576736 (2020).
Schedel, F. et al. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 33, 63–73 (2020).
Arelaki, S. et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. Plos One 11, e0154484 (2016).
Bianchini, G. et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016).
Rayes, R. F. et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 5, e128008 (2019).
Yang, L. et al. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J. Cancer 11, 4384–4396 (2020).
Yoshimoto, M. et al. Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. 567, 216260 (2023).
Nie, M. et al. Neutrophil extracellular traps induced by IL8 promote diffuse large b-cell lymphoma progression via the TLR9 signaling. Clin. Cancer Res. 25, 1867–1879 (2019).
Spicer, J. D. et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 72, 3919–3927 (2012).
Chen, J. et al. Localized degradation of neutrophil extracellular traps by photoregulated enzyme delivery for cancer immunotherapy and metastasis suppression. ACS Nano 16, 2585–2597 (2022).
Najmeh, S. et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int. J. Cancer 140, 2321–2330 (2017).
Rayes, R. F. et al. Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J. Immunol. 204, 2285–2294 (2020).
Snoderly, H. T., Boone, B. A. & Bennewitz, M. F. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 21, 145 (2019).
Manchanda, K. et al. MPO (myeloperoxidase) reduces endothelial glycocalyx thickness dependent on its cationic charge. Arterioscler. Thromb. Vasc. Biol. 38, 1859–1867 (2018).
Liu, X. et al. Neutrophils activated by membrane attack complexes increase the permeability of melanoma blood vessels. Proc. Natl Acad. Sci. USA 119, e2122716119 (2022).
Zheng, Z. et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat. Commun. 12, 6202 (2021).
Tohme, S. et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 76, 1367–1380 (2016).
Wang, W. W. et al. Lipopolysaccharides increase the risk of colorectal cancer recurrence and metastasis due to the induction of neutrophil extracellular traps after curative resection. J. Cancer Res. Clin. Oncol. 147, 2609–2619 (2021).
Grilz, E. et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br. J. Haematol. 186, 311–320 (2019).
Rosell, A., Martinod, K., Mackman, N. & Thalin, C. Neutrophil extracellular traps and cancer-associated thrombosis. Thromb. Res. 213, S35–S41 (2022).
Seo, J. D. et al. Contact system activation and neutrophil extracellular trap markers: risk factors for portal vein thrombosis in patients with hepatocellular carcinoma. Clin. Appl. Thromb. Hemost. 25, 1076029618825310 (2019).
Suzuki-Inoue, K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood 134, 1912–1918 (2019).
Lazar, S. & Goldfinger, L. E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 137, 3192–3200 (2021).
Thomas, G. M. et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J. Thromb. Haemost. 13, 1310–1319 (2015).
Varady, C. B. S., Oliveira, A. C., Monteiro, R. Q. & Gomes, T. Recombinant human DNase I for the treatment of cancer-associated thrombosis: A pre-clinical study. Thromb. Res. 203, 131–137 (2021).
Thalin, C. et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PloS One 13, e0191231 (2018).
Czeiger, D. et al. Measurement of circulating cell-free DNA levels by a new simple fluorescent test in patients with primary colorectal cancer. Am. J. Clin. Pathol. 135, 264–270 (2011).
Agassi, R. et al. Measurement of circulating cell-free DNA levels by a simple fluorescent test in patients with breast cancer. Am. J. Clin. Pathol. 143, 18–24 (2015).
Yan, B., Dai, X., Ma, Q. & Wu, X. Stromal neutrophil extracellular trap density is an independent prognostic factor for cervical cancer recurrence. Front. Oncol. 11, 659445 (2021).
Kaltenmeier, C. T. et al. Neutrophil extracellular traps as a novel biomarker to predict recurrence-free and overall survival in patients with primary hepatic malignancies. HPB 23, 309–320 (2021).
Decker, A. S. et al. Prognostic role of blood NETosis in the progression of head and neck cancer. Cells 8, 946 (2019).
Chen, N., He, D. & Cui, J. A neutrophil extracellular traps signature predicts the clinical outcomes and immunotherapy response in head and neck squamous cell carcinoma. Front. Mol. Biosci. 9, 833771 (2022).
Zhang, Y. et al. Diagnostic, therapeutic predictive, and prognostic value of neutrophil extracellular traps in patients with gastric adenocarcinoma. Front. Oncol. 10, 1036 (2020).
Qu, Z. et al. A novel neutrophil extracellular traps signature for overall survival prediction and tumor microenvironment identification in gastric cancer. J. Inflamm. Res. 16, 3419–3436 (2023).
Zhong, W. et al. Neutrophil extracellular trap is surrogate biomarker for prognosis and response to neoadjuvant therapy in locally advanced rectal cancer. J. Inflamm. Res. 16, 6443–6455 (2023).
Li, R., Jiang, X., Wang, P. & Liu, X. Prognostic value of neutrophil extracellular trap signature in clear cell renal cell carcinoma. Front. Oncol. 13, 1205713 (2023).
Sanz-Moreno, V. & Balkwill, F. R. Mets and NETs: the awakening force. Immunity 49, 798–800 (2018).
Bicker, K. L. & Thompson, P. R. The protein arginine deiminases: structure, function, inhibition, and disease. Biopolymers 99, 155–163, (2013).
Knight, J. S. et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206 (2015).
Shen, W. et al. Inhibition of neutrophil extracellular traps formation by Cl-amidine alleviates lipopolysaccharide-induced endometritis and uterine tissue damage. Animals 12, 1151 (2022).
Harada, T. et al. Effects of recombinant human soluble thrombomodulin on neutrophil extracellular traps in the kidney of a mouse model of endotoxin shock. Fujita Med. J. 9, 225–230 (2023).
Helms, J. et al. Thrombomodulin favors leukocyte microvesicle fibrinolytic activity, reduces NETosis and prevents septic shock-induced coagulopathy in rats. Ann. Intensive Care 7, 118 (2017).
Rossaint, J. et al. Hydroxyethyl starch 130/0.4 decreases inflammation, neutrophil recruitment, and neutrophil extracellular trap formation. Br. J. Anaesth. 114, 509–519 (2015).
Kuzmicka, W. et al. Zinc supplementation modulates NETs release and neutrophils’ degranulation. Nutrients 13, 51 (2020).
Kuzmicka, W. et al. Influence of iron- and zinc-chelating agents on neutrophil extracellular trap formation. Cent. Eur. J. Immunol. 46, 135–139 (2021).
Ling, X. et al. Disulfiram relieves severe acute pancreatitis by inhibiting GSDMD-dependent NETs formation. J. Dig. Dis. 24, 359–368 (2023).
Ondracek, A. S. et al. Physical exercise promotes DNase activity enhancing the capacity to degrade neutrophil extracellular traps. Biomedicines 10, 2849 (2022).
Chen, D. et al. Exenatide enhanced the antitumor efficacy on PD-1 blockade by the attenuation of neutrophil extracellular traps. Biochem. Biophys. Res. Commun. 619, 97–103 (2022).
Boettcher, M. et al. Markers of neutrophil activation and extracellular trap formation predict appendicitis. Surgery 171, 312–319 (2022).
Li, P. et al. Predictive value of neutrophil extracellular trap components for 28-day all-cause mortality in patients with cardiac arrest: a pilot observational study. Shock 60, 664–670 (2023).
Yang, S. et al. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv. Wound Care 9, 16–27 (2020).
Lee, Y. Y. et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 267, 120389 (2021).
Kraaij, T. et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J. Autoimmun. 91, 45–54 (2018).
Galos, E. V. et al. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br. J. Anaesth. 125, 712–721 (2020).
Zhang, H. et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br. J. Anaesth. 129, 244–253 (2022).
Zabieglo, K. et al. The inhibitory effect of secretory leukocyte protease inhibitor (SLPI) on formation of neutrophil extracellular traps. J. Leukoc. Biol. 98, 99–106 (2015).
Haute, G. V. et al. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol. Vitr. 30, 309–317 (2015).
Shishikura, K. et al. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br. J. Pharm. 173, 319–331 (2016).
Healy, L. D. et al. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J. Biol. Chem. 292, 8616–8629 (2017).
Bystrzycka, W. et al. Azithromycin and chloramphenicol diminish neutrophil extracellular traps (NETs) release. Int. J. Mol. Sci. 18, 2666 (2017).
Fetz, A. E. et al. Localized delivery of Cl-amidine from electrospun polydioxanone templates to regulate acute neutrophil NETosis: a preliminary evaluation of the PAD4 inhibitor for tissue engineering. Front. Pharm. 9, 289 (2018).
Shrestha, B. et al. Recombinant thrombomodulin suppresses histone-induced neutrophil extracellular trap formation. Front. Immunol. 10, 2535 (2019).
Fortner, K. A. et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 7, e000387 (2020).
Li, M. et al. A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol. Cancer Ther. 19, 1530–1538 (2020).
Zeng, J. et al. Kaempferol blocks neutrophil extracellular traps formation and reduces tumour metastasis by inhibiting ROS-PAD4 pathway. J. Cell Mol. Med. 24, 7590–7599 (2020).
Ivey, A. D. et al. Chloroquine reduces neutrophil extracellular trap (NET) formation through inhibition of peptidyl arginine deiminase 4 (PAD4). Clin. Exp. Immunol. 211, 239–247 (2023).
Chen, C. et al. Low-dose vitamin D protects hyperoxia-induced bronchopulmonary dysplasia by inhibiting neutrophil extracellular traps. Front. Pediatr. 8, 335 (2020).
Sudo, M. et al. Blockade of tumor necrosis factor by etanercept prevents postoperative adhesion formation in mice. Cell Physiol. Biochem. 54, 1041–1053 (2020).
Du, M. et al. Inhibition of peptidyl arginine deiminase-4 prevents renal ischemia-reperfusion-induced remote lung injury. Mediat. Inflamm. 2020, 1724206 (2020).
Tanaka, K. I. et al. Thioredoxin-albumin fusion protein prevents urban aerosol-induced lung injury via suppressing oxidative stress-related neutrophil extracellular trap formation. Environ. Pollut. 268, 115787 (2021).
Wadehn, H. et al. Time- and dose-dependent inhibition of neutrophil extracellular trap formation by blocking of the interleukin-1 receptor. Cent. Eur. J. Immunol. 46, 419–426 (2021).
Schulz, A. et al. The inhibitory effect of curosurf((R)) and alveofact((R)) on the formation of neutrophil extracellular traps. Front. Immunol. 11, 582895 (2020).
Sudo, M. et al. Antithrombin together with NETs inhibitor protected against postoperative adhesion formation in mice. Cell Physiol. Biochem. 55, 400–412 (2021).
Ou, Q. et al. TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4. Nat. Commun. 12, 3481 (2021).
Strich, J. R. et al. Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: a potential therapeutic. J. Infect. Dis. 223, 981–984 (2021).
Liu, J. et al. Modulation of HMGB1 release in APAP-induced liver injury: a possible strategy of chikusetsusaponin V targeting NETs formation. Front. Pharm. 12, 723881 (2021).
Liu, Y. et al. Tetramethylpyrazine inhibits neutrophil extracellular traps formation and alleviates hepatic ischemia/reperfusion injury in rat liver transplantation. Exp. Cell Res. 406, 112719 (2021).
Totani, L. et al. Type-4 phosphodiesterase (PDE4) blockade reduces NETosis in cystic fibrosis. Front. Pharm. 12, 702677 (2021).
Mutua, V., Cavallo, F. & Gershwin, L. J. Neutrophil extracellular traps (NETs) in a randomized controlled trial of a combination of antiviral and nonsteroidal anti-inflammatory treatment in a bovine model of respiratory syncytial virus infection. Vet. Immunol. Immunopathol. 241, 110323 (2021).
Zha, Y. F. et al. Senkyunolide I protect against lung injury via inhibiting formation of neutrophil extracellular trap in a murine model of cecal ligation and puncture. Int. Immunopharmacol. 99, 107922 (2021).
Alsabani, M. et al. Reduction of NETosis by targeting CXCR1/2 reduces thrombosis, lung injury, and mortality in experimental human and murine sepsis. Br. J. Anaesth. 128, 283–293 (2022).
Monteith, A. J. et al. Increased dietary manganese impairs neutrophil extracellular trap formation rendering neutrophils ineffective at combating Staphylococcus aureus. Infect. Immun. 90, e0068521 (2022).
Burczyk, G., Cichon, I. & Kolaczkowska, E. Itaconate suppresses formation of neutrophil extracellular traps (NETs): involvement of hypoxia-inducible factor 1alpha (Hif-1alpha) and heme oxygenase (HO-1). Front. Immunol. 13, 864638 (2022).
Liu, Q. et al. Salvianolic acid A protects against lipopolysaccharide-induced acute lung injury by inhibiting neutrophil NETosis. Oxid. Med. Cell Longev. 2022, 7411824 (2022).
Zhu, D. et al. Self-assembling, pH-responsive nanoflowers for inhibiting PAD4 and neutrophil extracellular trap formation and improving the tumor immune microenvironment. Acta Pharm. Sin. B 12, 2592–2608 (2022).
Li, M. et al. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 13, 927215 (2022).
Zhang, H., Xu, X., Xu, R. & Ye, T. Drug repurposing of ivermectin abrogates neutrophil extracellular traps and prevents melanoma metastasis. Front. Oncol. 12, 989167 (2022).
Chen, D. et al. Liraglutide enhances the effect of checkpoint blockade through the inhibition of neutrophil extracellular traps in murine lung and liver cancers. FEBS Open Bio. https://doi.org/10.1002/2211-5463.13499 (2022).
Chen, H. et al. (+)-Borneol inhibits the generation of reactive oxygen species and neutrophil extracellular traps induced by phorbol-12-myristate-13-acetate. Front. Pharm. 13, 1023450 (2022).
Li, B. et al. Interleukin-37 alleviates myocardial injury induced by coxsackievirus B3 via inhibiting neutrophil extracellular traps formation. Int. Immunopharmacol. 113, 109343 (2022).
Zhao, H. et al. Dihydrotanshinone I inhibits the lung metastasis of breast cancer by suppressing neutrophil extracellular traps formation. Int. J. Mol. Sci. 23, 15180 (2022).
Gajendran, C. et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci. Rep. 13, 3189 (2023).
Rysenga, C. E. et al. Taxifolin inhibits NETosis through activation of Nrf2 and provides protective effects in models of lupus and antiphospholipid syndrome. Rheumatology 63, 2006–2015 (2023).
Han, F. et al. Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice. Redox Biol. 64, 102787 (2023).
Gao, T., Li, J., Shi, L. & Hu, B. Rosavin inhibits neutrophil extracellular traps formation to ameliorate sepsis-induced lung injury by regulating the MAPK pathway. Allergol. Immunopathol. 51, 46–54 (2023).
Yu, W. et al. The activation of SIRT1 by resveratrol reduces breast cancer metastasis to lung through inhibiting neutrophil extracellular traps. J. Drug Target 31, 962–975 (2023).
Heuer, A. et al. Therapeutic targeting of neutrophil extracellular traps improves primary and secondary intention wound healing in mice. Front. Immunol. 12, 614347 (2021).
Hao, H. et al. DNaseI protects lipopolysaccharide-induced endometritis in mice by inhibiting neutrophil extracellular traps formation. Microb. Pathog. 150, 104686 (2021).
Jarrahi, A. et al. Recombinant human DNase-I improves acute respiratory distress syndrome via neutrophil extracellular trap degradation. J. Thromb. Haemost. 21, 2473–2484 (2023).
Boettcher, M. et al. Therapeutic targeting of extracellular DNA improves the outcome of intestinal ischemic reperfusion injury in neonatal rats. Sci. Rep. 7, 15377 (2017).
Tan, Q. et al. Targeting neutrophil extracellular traps enhanced tPA fibrinolysis for experimental intracerebral hemorrhage. Transl. Res. 211, 139–146 (2019).
Zhang, J. et al. DNase I improves corneal epithelial and nerve regeneration in diabetic mice. J. Cell Mol. Med. 24, 4547–4556 (2020).
Pavan, C. et al. DNase treatment prevents cerebrospinal fluid block in early experimental pneumococcal meningitis. Ann. Neurol. 90, 653–669 (2021).
Pena-Martinez, C. et al. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke 50, 3228–3237 (2019).
Wu, X., Guo, Y., Zeng, H. & Chen, G. DNase-1 treatment exerts protective effects in neurogenic pulmonary edema via regulating the neutrophil extracellular traps after subarachnoid hemorrhage in mice. J. Clin. Med. 11, 4349 (2022).
Goswami, J. et al. Dnase-mediated dissolution of neutrophil extracellular traps accelerates in vitro thrombin generation kinetics in trauma patients. Shock 58, 217–223 (2022).
Veras, F. P. et al. Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model. Respir. Res. 24, 66 (2023).
Chen, X. Q. et al. DNase I targeted degradation of neutrophil extracellular traps to reduce the damage on IgAV rat. PLoS One 18, e0291592 (2023).
Zhang, H. et al. Neutrophils extracellular traps inhibition improves PD-1 blockade immunotherapy in colorectal cancer. Cancers 13, 5333 (2021).
Wang, C. L. et al. DNase I and sivelestat ameliorate experimental hindlimb ischemia-reperfusion injury by eliminating neutrophil extracellular traps. J. Inflamm. Res. 16, 707–721 (2023).
Kessinger, C. W. et al. Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS One 10, e0116621 (2015).
Wong, S. L., Goverman, J., Staudinger, C. & Wagner, D. D. Recombinant human ADAMTS13 treatment and anti-NET strategies enhance skin allograft survival in mice. Am. J. Transpl. 20, 1162–1169 (2020).
Inaba, I. et al. Inhibiting neutrophil extracellular traps protects against ultraviolet B-induced skin damage: effects of Hochu-ekki-to and DNase I. Int. J. Mol. Sci. 25, 1723 (2024).
Smith, P. et al. Markers of neutrophil activation and neutrophil extracellular traps in diagnosing patients with acute venous thromboembolism: a feasibility study based on two VTE cohorts. PLoS One 17, e0270865 (2022).
Zabczyk, M. et al. Prothrombotic fibrin clot properties associated with NETs formation characterize acute pulmonary embolism patients with higher mortality risk. Sci. Rep. 10, 11433 (2020).
Ferre-Vallverdu, M. et al. Neutrophil extracellular traps (NETs) in patients with STEMI. Association with percutaneous coronary intervention and antithrombotic treatments. Thromb. Res. 213, 78–83 (2022).
Wan, W. et al. The association between circulating neutrophil extracellular trap related biomarkers and retinal vein occlusion incidence: a case-control pilot study. Exp. Eye Res. 210, 108702 (2021).
Hally, K. E. et al. Linking neutrophil extracellular traps and platelet activation: a composite biomarker score for predicting outcomes after acute myocardial infarction. Thromb. Haemost. 121, 1637–1649 (2021).
Abd El Hafez, A., Mohamed, A. S., Shehta, A. & Sheta, H. Neutrophil extracellular traps-associated protein peptidyl arginine deaminase 4 immunohistochemical expression in ulcerative colitis and its association with the prognostic predictors. Pathol. Res Pract. 216, 153102 (2020).
Mazetto, B. M. et al. Association between neutrophil extracellular traps (NETs) and thrombosis in antiphospholipid syndrome. Thromb. Res. 214, 132–137 (2022).
Ibrahim, I. et al. Neutrophil extracellular traps (NETs) are associated with type 2 diabetes and diabetic foot ulcer related amputation: a prospective cohort study. Diabetes Ther. 15, 1333–1348 (2024).
Whittall-Garcia, L. P. et al. Circulating neutrophil extracellular trap remnants as a biomarker to predict outcomes in lupus nephritis. Lupus Sci. Med. 11, e001038 (2024).
Ng, H. et al. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb. Vasc. Biol. 41, 988–994 (2021).
Kuo, Y. M. et al. Biomarker of neutrophil extracellular traps is associated with deep-seated infections and predicts mortality and cardiovascular morbidity in commensal streptococcal bacteremia. J. Microbiol Immunol. Infect. 55, 860–869 (2022).
Carmona-Rivera, C. et al. Multicenter analysis of neutrophil extracellular trap dysregulation in adult and pediatric COVID-19. JCI Insight 7, e160332 (2022).
Twaddell, S. H., Gibson, P. G., Grainge, C. & Baines, K. J. Parapneumonic effusions are characterized by elevated levels of neutrophil extracellular traps. Chest 160, 1645–1655 (2021).
Duan, Z. et al. Neutrophil extracellular trap formation index predicts occurrences of deep surgical site infection after laparotomy. Ann. Transl. Med. 9, 1373 (2021).
Feng, C. et al. A neutrophil extracellular traps-related classification predicts prognosis and response to immunotherapy in colon cancer. Sci. Rep. 13, 19297 (2023).
Jiang, T. et al. Neutrophil extracellular traps (NETs)-related lncRNAs signature for predicting prognosis and the immune microenvironment in breast cancer. Front. Cell Dev. Biol. 11, 1117637 (2023).
Tomas-Perez, S. et al. Increased levels of NETosis biomarkers in high-grade serous ovarian cancer patients’ biofluids: Potential role in disease diagnosis and management. Front. Immunol. 14, 1111344 (2023).
Li, M. et al. Neutrophil extracellular traps-related signature predicts the prognosis and immune infiltration in gastric cancer. Front. Med. 10, 1174764 (2023).
Sun, G. & Liu, W. The neutrophil extracellular traps-related gene signature predicts the prognosis of glioblastoma multiforme. Folia Neuropathol. 62, 59–75 (2024).
Fang, C. et al. A innovative prognostic symbol based on neutrophil extracellular traps (NETs)-related lncRNA signature in non-small-cell lung cancer. Aging 13, 17864–17879 (2021).
Rivera-Franco, M. M. et al. Neutrophil extracellular traps associate with clinical stages in breast cancer. Pathol. Oncol. Res. 26, 1781–1785 (2020).
Li, Q. et al. A novel neutrophil extracellular trap signature to predict prognosis and immunotherapy response in head and neck squamous cell carcinoma. Front. Immunol. 13, 1019967 (2022).
Ivey, A. D. et al. Pancreatectomy induces cancer-promoting neutrophil extracellular traps. Ann. Surg. Oncol. 31, 3707–3717 (2024).
Wang, M. et al. Biomarkers of peripheral blood neutrophil extracellular traps in the diagnosis and progression of malignant tumors. Cancer Med. 13, e6935 (2024).
Teng, Z. H. et al. Neutrophil extracellular traps-associated modification patterns depict the tumor microenvironment, precision immunotherapy, and prognosis of clear cell renal cell carcinoma. Front. Oncol. 12, 1094248 (2022).
Quan, J. & Huang, B. Identification and validation of the molecular subtype and prognostic signature for clear cell renal cell carcinoma based on neutrophil extracellular traps. Front. Cell Dev. Biol. 10, 1021690 (2022).
Zhao, J. & Xie, X. Prediction of prognosis and immunotherapy response in breast cancer based on neutrophil extracellular traps-related classification. Front. Mol. Biosci. 10, 1165776 (2023).
Novotny, J. et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 94, e2346–e2360 (2020).
Zhang, H. et al. Preoperative leukocytosis is associated with increased tumor-infiltrating neutrophil extracellular traps and worse outcomes in esophageal cancer. Ann. Transl. Med. 8, 441 (2020).
Ebrahimi, F. et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur. Respir. J. 51, 1701389 (2018).
Chen, X. et al. Intratumoral neutrophil extracellular traps are associated with unfavorable clinical outcomes and immunogenic context in pancreatic ductal adenocarcinoma. Front. Immunol. 13, 1027459 (2022).
Huang, M. Y. et al. Neutrophil extracellular trap formation during surgical procedures: a pilot study. Sci. Rep. 13, 15217 (2023).
Sabbatini, M. et al. Aging hampers neutrophil extracellular traps (NETs) efficacy. Aging Clin. Exp. Res. 34, 2345–2353 (2022).
Vidal-Seguel, N. et al. High-intensity interval training reduces the induction of neutrophil extracellular traps in older men using live-neutrophil imaging as biosensor. Exp. Gerontol. 181, 112280 (2023).
Fisher, J. et al. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Mol. Cell Proteom. 20, 100113 (2021).
Menegazzo, L. et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 55, 593–601 (2018).
Vaidya, K. et al. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J. Am. Heart Assoc. 10, e018993 (2021).
Ren, B. et al. Perioperative lidocaine and dexmedetomidine intravenous infusion reduce the serum levels of NETs and biomarkers of tumor metastasis in lung cancer patients: a prospective, single-center, double-blinded, randomized clinical trial. Front. Oncol. 13, 1101449 (2023).
Funding
This work was supported by the National Institute of Health grants R01-CA214865 to A.T. State funding within the UVA Comprehensive Cancer Center “IDEA-Cancer pilot award”, “Cancer Therapeutics (CRX) pilot award” to H.Z. National Natural Science Foundation of China Grant Number 82200588 to H.W.
Author information
Authors and Affiliations
Contributions
A.T. supervised the project. H.W., H.Z., and A.T. conceived and designed this project. H.W., S.K., Y.L., S.W., and H.Z. drafted the manuscript. H.Z. polished the language. H.W. and H.H. helped review the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Kim, S.J., Lei, Y. et al. Neutrophil extracellular traps in homeostasis and disease. Sig Transduct Target Ther 9, 235 (2024). https://doi.org/10.1038/s41392-024-01933-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01933-x
- Springer Nature Limited