Abstract
Background
Retinopathy of prematurity (ROP) is one of the leading cause of child blindness. Preterm newborns of very low gestational age (GA) and very low birth weight are at the greatest risk. Our objective was to evaluate the role of genetic variants associated with ROP risk and its comorbidities in an Argentinian sample of premature infants.
Methods
A sample of 437 preterm infants <33 weeks GA, born at a maternity hospital in Tucumán, Argentina, 2005–2010, was analyzed. Environmental factors, perinatal outcomes, and fourteen single nucleotide polymorphisms associated with ROP were evaluated, comparing ROP with non-ROP newborns. A lasso logistic regression was performed to select variables; then, a conditional logistic regression was used to identify ROP maternal and perinatal risk factors adjusting by maternal and gestational ages, respectively.
Results
ROP maternal risk factors were alcohol intake, periodontal infections, and severe stress. Respiratory distress, sepsis, and intracranial hemorrhage were the ROP perinatal risk factors. Markers rs186085 of EPAS1 and rs427832 of AGTR1 were significantly associated with ROP newborns.
Conclusion
We identified three maternal and three perinatal risk factors associated with ROP. Genes EPAS1 and AGTR1, involved in angiogenesis and vascularization, were identified to be of risk for ROP.
Impact
-
Genetic and environmental risk factors associated with ROP and its comorbidities are evaluated in a Latin American population.
-
Genes EPAS1 and AGTR1, involved in angiogenesis and vascularization, were identified to be of risk for ROP.
-
Three maternal and three perinatal risk factors associated with ROP were also identified.
-
A matrix of significant relationships among genetic markers and comorbidities is presented.
-
Reported data may help develop more effective preventive measures for ROP in the Latin American region.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is one of the most frequent causes of childhood blindness due to a development impairment of retinal blood vessels in preterm infants, mainly in those of low birth weight.1 Preterm birth affects nearly 10% of worldwide deliveries while ROP, with a 10–30% prevalence in premature newborns, annually leads to blindness or severe visual impairment in around 32,000 neonates. Yearly, 10% of worldwide ROP infants are born in Latin America and the Caribbean region.2,3 Screening and treatment programs have shown that ROP accounts for 5 to 15% of childhood blindness cases.4 Applied protocols vary among countries and may result in different incidence rates and risk factors for ROP.5 Similarly to other countries and in response to the epidemic proportions that ROP reached in 2010, in Argentina, the local public health authorities created a national program for its prevention.4,6
ROP is a multifactorial disease and premature newborns of <33 weeks gestational age (GA) and of very low birth weight (BW ≤ 1500 g) are at the greatest risk.7,8 Factors predisposing to ROP include multiple pregnancy, low Apgar score, respiratory distress, oxygen supplementation, and prolonged mechanical ventilation, while associated conditions are anemia, intraventricular hemorrhage, necrotizing enterocolitis, and sepsis.9,10,11 These factors present regional variations possibly due to population heterogeneity and differences in neonatal care availability.8,12
Based on racial and specific regional risk profiles, several authors have described a genetic predisposition for ROP with an estimated 70% heritability.8,13,14 Bizzarro et al. studied ROP prevalence in monozygotic and dizygotic twin pairs and reported a significant genetic contribution to its development.15 Studies on specific candidate gene polymorphisms, such as those of the vascular endothelial growth factor (VEGF) gene, have also shown to be associated with ROP.16,17,18
The objective of the present study was to evaluate the role of genetic variants associated with the risk of ROP and its comorbidities in a sample of premature newborns of Argentina and to compare them with data from other populations.18
Methods
Study sample
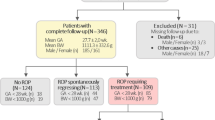
The study sample was a subset of a larger (n = 1068) preterm infants group who, in a cross sectional study, underwent genotyping to identify genetic contributions to preterm delivery; 437 premature infants <33 weeks of GA were selected, 75 had ROP (cases) and 362 did not (controls) (Fig. 1). The infants were born between July 2005 and December 2009 at the Instituto de Maternidad y Ginecología Nuestra Señora de Las Mercedes (IMGNSLM), in Tucumán, a northwestern Argentine province with a population of mostly Amerindian and Latin European ancestry.19,20,21,22,23 Demographic information and newborns’ phenotype data were collected through an interview with the mother and through a medical chart review. At the IMGNSLM, eye fundus examination of preterm infants was performed by experienced ophthalmologists following national and international guidelines for ROP prevention, according to the newborns’ GA.1,24,25,26 Infants of 24–26 weeks were evaluated at 7, 6, or 5 completed weeks postnatal age, respectively, or at 31 weeks postmenstrual age (PMA). For infants of ≥27 to 30 weeks, evaluation was performed at 4 weeks postnatal age and for those of ≥31 weeks, at 2 weeks postnatal age. When ROP screening yielded normal findings, the evaluation was repeated every 15 days until total retinal vascularization. If pathologic changes were identified, the follow up depended on each particular case.25,26
Biological samples of the babies (cord blood) and their parents (peripheral blood or saliva) were obtained for DNA studies.
For sample homogenization, exclusion criteria were stillborn, multiple gestation, major birth defect, maternal age under 16 years, known cervix/placental origin of prematurity (cervix incompetence, placental abruption, uterine structural anomaly), and medically induced preterm birth (Fig. 1).
Before enrollment, signed informed consent (IRB 199911068, 200411759, and 200506792) was obtained from all participating families.
Exposure variables
Maternal socio-demographic characteristics
Maternal age (years), multigravidity (>3), low maternal and paternal education (<7 years of schooling), marital status (unmarried yes/no), short cohabitation with current partner (<1 year), and change of partner between current and previous pregnancy.
Lifestyle features during pregnancy
The following variables (yes/no) were considered: tobacco smoking, alcohol intake, coffee intake, maternal employment, and stress due to severe life events.
Pregnancy care and complications
Few prenatal visits (<5), vaginal bleeding, urinary and/or periodontal infections, and low body mass index (BMI < 18.5).
Perinatal outcomes
Gestational age (weeks), birth weight (grams), sex, hypotonia, anemia, intracranial hemorrhage, hyperbilirubinemia, sepsis, patent ductus arteriosus, hyperglycemia, necrotizing enterocolitis, respiratory distress syndrome, oxygen supply, hypocalcemia, polycythemia, heart failure, seizures, and clinical subtypes of preterm birth (PTB) [Idiopathic (PTB-I); Premature rupture of membranes (PTB-PPROM).27
Respiratory distress syndrome was defined when preterm newborns presented signs and symptoms of increased work of breathing together with pulmonary surfactant administration requirement.28,29 Late-onset sepsis due to pathogenic bacteria was diagnosed by positive culture of blood or cerebrospinal fluid samples obtained at 3 postnatal days.30 Intraventricular hemorrhage was defined as the presence of subependymal germinal matrix hemorrhage, intraventricular hemorrhage, or parenchymal hemorrhage.31 The four grades of intraventricular hemorrhages (I-II-III-IV) were classified according to Papile.32
Genotyping
The selected candidate genes were the nine that presented significant differences in the work of Mohamed et al. plus two VEGF single nucleotide polymorphisms (SNP).18
Fourteen SNP were used: EPAS-1 (Endothelial PAD domain protein 1) (rs1867785; rs1868085), CFH (Complement factor H) (rs529825, rs800292, rs1061170), IHH (Indian Hedgehog Signaling Molecule) (rs3099), AGTR-1 (Angiotensin II Receptor Type 1) (rs427832), VEGF (rs69994, rs2010963), FZD4 (Frizzled 4) (rs713065, rs7925666), TBX5 (T-Box Transcription Factor 5) (rs1895602), CETP (Cholesteryl Ester Transfer Protein) (rs289747), and GP1BA (Glycoprotein Ib Platelet Subunit Alpha) (rs2243093).
Allelic variation was determined using the TaqMan genotyping system (Applied Biosystems, Foster City, CA). Genotyping quality controls were performed before completing the statistical analysis (e.g., missing genotype rate by individual and by SNP minor allele frequency (0.05), Hardy–Weinberg equilibrium failures for each SNP, and Mendelian error rate).
Statistical analysis
Mean and standard deviation were calculated for continuous variables and percentage and Chi square test were used for discrete variables. The Mann Whitney test was applied to compare differences between cases and controls.
Due to the large number of analyzed variables in relation with the sample size, a lasso logistic regression was performed to exclude variables whose impact on the model was null or almost null.33 The lasso models use a penalty factor Lambda (ƛ) that allows to include a significant number of variables on a reduced study sample. Variables present in less than five ROP newborns were excluded. After this selection, a conditional logistic regression was used to identify maternal risk factors for ROP, comparing ROP with non-ROP newborns, adjusting by maternal age. The dependent variable was ROP (yes/no) and the independent variables were maternal characteristics, lifestyle features, and pregnancy care and complications. A second conditional logistic regression was performed to analyze perinatal outcomes adjusted by GA in weeks. In both analyses, odds ratios and 95% confidence intervals were estimated. The significance level was set at p < 0.05 and the study power test was 80% for a risk factor prevalence <10% in the population to detect a minimal OR > 2.5, for a minimum sample size of N = 70 (ROP cases) and N = 280 (non-ROP controls).
The Transmission Disequilibrium Test (TDT), within the family-based association study approach, was used for the analysis of candidate genes. Family-based designs are an alternative strategy to study associations that are not easily affected by confounding due to population structure and/or recent admixture; they are based on triads (mother, father, newborn) that involve the assessment of both parents and the affected child.34
Given the exploratory nature of the present study, the Bonferroni correction test was not applied.
Finally, the association between SPN and newborns’ comorbidities was analyzed only in ROP newborns; events were characterized as dichotomous: present (1) or absent (0). A double entry table where each cell showed the number of ROP cases sharing a pair of events was created (Supplementary Appendix 1). The expected values for each pair of events were calculated as if they were independent, multiplying their marginal probabilities. The deviation of the observed from the expected values was evaluated with Fisher’s exact test. Odds ratios between pairs of events were calculated; associations with p-values less than 0.10 were represented in a network graph (Fig. 2).
Results
Of the 75 ROP cases 42 were males and 33 females. The average maternal age was 24.7 ± 6.3 and 25.2 ± 6.6 (p = 0.525) for cases and controls, respectively, and gravidity was 2.0 ± 1.8 and 1.8 ± 2.3 (p = 0.832); the differences were not significant. Tables 1 and 2 show the maternal and perinatal risk factors for ROP, respectively.
Maternal environmental risk factors
A univariate analysis of maternal environmental risk factors showed that infants had a greater risk for ROP when their mothers presented low body mass index, alcohol intake during pregnancy, stress due to a severe event, and periodontal infections.
After lasso selection, three of the four variables were incorporated into the final model as the best predictors for ROP: alcohol intake (OR = 2.52, 95% CI: 1.26–5.03, p = 0.009), severe stress events (OR = 1.60, 95% CI: 0.92–2.17, p = 0.063), and periodontal infections (OR = 1.58, 95% CI: 0.92–2.73, p = 0.095).
ROP and perinatal outcomes
ROP newborns had a significantly shorter mean GA (29.4 ± 1.9) than the non-ROP control group (30.5 ± 2.4) (t = 3.48, p < 0.001) and a significantly lower birth weight (1164 g ± 266 vs. 1404 g ± 399), (t = 4.97, p < 0.001).
All ROP newborns were hospitalized and presented associated complications. A univariate analysis showed that ROP newborns had a higher risk of seizures, intracranial hemorrhage, hyperbilirubinemia, respiratory distress, sepsis, patent ductus arteriosus, hyperglycemia, and necrotizing enterocolitis than the control group. After Lasso selection and adjustment for GA, the variables respiratory distress, sepsis, and intracranial hemorrhage showed a significantly higher risk in ROP cases than in the control group (Table 2).
Genetic susceptibility for ROP
In a first analysis, marker rs186085 of EPAS1 was significantly associated with ROP newborns <33 weeks GA (Table 3). When ROP newborns <30 weeks GA were re-analyzed, markers rs427832 of AGTR1 and rs1868085 of EPAS1 were both significantly associated with ROP (Table 3).
ROP genetic markers and comorbidities relationships
A co-occurrence matrix of comorbidities and/or genetic markers observed in ROP newborns was created (Supplementary Appendix 1). The significant relationships among the SNP of the analyzed candidate genes as well as the relationships among these markers and ROP comorbidities are shown in Fig. 2. Marker VEGF rs201093 appeared as the main node, relating a cascade of genes (AGTR1, IHH, CEPT, and EPAS1) with comorbidities (necrotizing enterocolitis, seizures, sepsis, and hyperglycemia), mediated by respiratory distress while, on the other hand, the association between VEGF and cranial hemorrhage related through CFH markers (Fig. 2).
Discussion
After adjusting for maternal age, the variables severe stress events, periodontal infections, and -specially- alcohol intake during pregnancy showed an association with the ROP cases. These three risk factors have already been associated with prematurity and low birth weight.35,36 Gauthier et al. have reported alcohol prenatal exposure during the first trimester in 34% of very low birth weight (VLBW) newborns,37 while Taylor et al. have related acute or chronic stressful events with VLBW among other adverse outcomes.38 It could therefore be hypothesized that the identified maternal risk factors actually result in prematurity and low birth weight and that they are not directly related to the ROP pathway.
The risk of having respiratory distress, sepsis, and intracranial hemorrhage was significantly higher in ROP than in non-ROP newborns. These comorbidities have already been widely reported in association with ROP; genetic susceptibility and candidate genes have been described for them as well as for other comorbidities.8,39,40
Using the family-based analysis strategy to control the population substructure of our Argentinian sample, we identified the same relationship between ROP and SNP EPAS1 rs1868085 as Mohamed et al.18 Other authors have also reported this association supporting a possible causal relationship.41,42
EPAS1 and AGTR1, shown to be of risk for ROP in our study, are involved in the processes of angiogenesis and vascularization.18,41 EPAS1 includes a gene for erythropoietin (EPO), which regulates red blood cell production, and a gene for vascular endothelial growth factor (VEGF).41 VEGF, a hypoxia-induced potent angiogenic factor,18 is a primary mediator of retinal angiogenesis; it increases vascular permeability and local tissue oxygenation. EPAS1 could represent an important regulator of vascularization, perhaps involving the regulation of endothelial cell gene expression in response to hypoxia. The importance of VEGF in the development of ROP is supported by a number of experimental and clinical data.15,43,44,45,46 Decreased VEGF production was demonstrated during the first phase of ROP while it increased during the proliferative second phase.43,44 The VEGF gene has more than 70 known loci and previous studies have identified several SNP, particularly rs2010963 also analyzed in our study, as directly related with the risk of ROP progression.15,45,46 In our study, the matrix of associations showed that the SNP of VEGF were significantly associated with sepsis, respiratory distress, seizures, and necrotizing enterocolitis. Such associations have also been previously described.47,48,49,50
In a recent work, Ilguy et al. (2021) have analyzed the association of ROP with SNP in VEGF-A and EPAS1, among other genes, and their relationship with ROP severity.51 The authors have identified eight significant SNP, three of which were analyzed in our work, and they showed that VEGF-A rs2010963 was associated with severe retinopathy while EPAS1 rs1867785 and rs1868085 were associated with milder cases.
Rathi et al. (2017) observed strong associations of ROP with variants in CFH (Complement factor H), among other genes (CEPT, CFB, CXCR4, and FBLN5).The vitreous of ROP infants presented increased levels of proteins in the extracellular matrix and the complement pathways; the authors concluded that increased retina/vitreous complement activation, in turn, activated microglia leading to inflammation.52 In our study, the matrix of associations showed that the SNP of CFH were significantly associated with inflammatory related conditions such as intracranial hemorrhage. Previous research works have described such associations.53,54
Furthermore, SNP rs427832 of the AGTR1 gene, identified in the work of Mohamed et al.18 was also recognized in the present study as associated with ROP in newborns of <30 weeks GA. AGTR1 encodes for angiotensin II type I receptor which has vasopressor effects and regulates aldosterone secretion.16,55 These mechanisms have been shown to be present in the retina.56 In a cattle experimental model, angiotensin II was found to act as a stimulant of VEGF-induced endothelial cell proliferation, mediated through the KDR/Flk-1 VEGF receptor; upregulation of this receptor was transcriptionally regulated by the type I angiotensin receptor.57 Studies have also shown a role in angiogenesis for hedgehog proteins, such as Indian Hedgehog (IHH) and Sonic Hedgehog (SHH).
In conclusion, these markers (AGTR1 rs427832, IHH rs3099, CEPT rs289747, EPAS1 rs1868085, and VEGF rs2010963) could interact and, in association with angiogenic factors, hypoxia, vascularization, and inflammatory processes, be linked to the development of ROP and to its comorbidities.
Strengths and pitfalls
This exploratory study is part of a larger project developed in collaboration with the Department of Genetics at the University of Iowa. It has been conducted in a single maternity hospital in Argentina that serves an ethnically and socially homogeneous population (mainly Amerindians of low and medium socioeconomic level).
Some aspects should be considered when reviewing studies that try to assess genetic variants related to ROP and its severity. ROP is associated with the grade of prematurity and both short GA and low birth weight are related to an increased ROP severity. Therefore, in order to differentiate genetic variants affecting prematurity and/or ROP, we adjusted the comparison by GA.
The construction of a matrix of associations was not intended to assess interactions or determine ROP causal pathways but to interpret the relationships among the involved genes as well as to provide information on shared risks among genes and comorbidities; this could help in the selection of candidate genes, clinical orientation, and inclusion of better predictor models for ROP.
The main limitation of the present study was its small sample size. For this reason, we decided to consider it as exploratory, with lax levels of statistical significance and to report associations instead of defining interactions that could be analyzed in future studies involving larger sample sizes.
We are aware that the frequency of exposure to environmental factors that affect ROP may have changed over time but, as a limitation of the study, detailed information on ROP-infants was only available for the 2005–2010 period.
Furthermore, information on ROP severity, which would have allowed us more precision when defining associations, was not available. Finally, the study sample was recruited from a single maternity hospital, limiting the generalization of the current findings.
Conclusion
In the present work, we were able to identify two candidate genes for ROP (EPAS1 and AGTR1) and replicate findings of a previous study. We also found association pathways involving Endothelial PAS Domain Protein 1 (EPAS1), Vascular Endothelial Growth Factor (VEGF), and Complement Factor H, as well as their relationship with comorbidities. The role of candidate genes and their association with other genes, maternal risk factors, and ROP comorbidities are shown, all of which could lead to hypotheses for further studies aimed at investigating these relationships.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fierson, W. M. American Academy of Pediatrics, Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity [published correction appears in Pediatrics. 2019 Mar;143(3):]. Pediatrics 142, e20183061 (2018).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Preterm Birth 1: Epidemiology and Causes of Preterm Birth. Obstet. Anesth. Dig. 29, 6–7 (2009).
Blencowe, H., Lawn, J. E., Vazquez, T., Fielder, A. & Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res 74, 35–49 (2013).
Bouzas, L. et al. Retinopathy of prematurity in the XXI century in a developing country: an emergency that should be resolved. Pediatrics 66, 551–558 (2007).
van Sorge, A. J. et al. Nationwide inventory of risk factors for retinopathy of prematurity in the Netherlands. J. Pediatr. 164, 494–498 (2014).
Ministry of Health of Argentina, National Program for the Prevention of Blindness in Childhood due to Retinopathy of Premature. Resolution 1613/2010. https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-1613-2010-172868 (2010). Last accessed March 23 (2022).
Dammann, O., Rivera, J. C. & Chemtob, S. The prenatal phase of retinopathy of prematurity. Acta Paediatr. 110, 2521–2528 (2021).
Kim, S. J. et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv. Ophthalmol. 63, 618–637 (2018).
Fortes Filho, J. B. et al. Is being small for gestational age a risk factor for retinopathy of prematurity? A study with 345 very low birth weight preterm infants. J. Pediatr. 85, 48–54 (2009).
Gonçalves, E. et al. Incidence and risk factors for retinopathy of prematurity in a Brazilian reference service. Sao Paulo Med. J. 132, 85–91 (2014).
Binenbaum, G. Algorithms for the prediction of retinopathy of prematurity based on postnatal weight gain. Clin. Perinatol. 40, 261–270 (2013).
Carrion, J. Z., Fortes Filho, J. B., Tartarella, M. B., Zin, A. & Jornada, I. D. Jr Prevalence of retinopathy of prematurity in Latin America. Clin. Ophthalmol. 5, 1687–1695 (2011).
Darlow, B. A. et al. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 115, 990–996 (2005).
Hartnett, M. E. et al. Genetic variants associated with severe retinopathy of prematurity in extremely low birth weight infants. Invest. Ophthalmol. Vis. Sci. 55, 6194–6203 (2014).
Bizzarro, M. J. et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics 118, 1858–1863 (2006).
Cooke, R. W., Drury, J. A., Mountford, R. & Clark, D. Genetic polymorphisms and retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 45, 1712–1715 (2004).
Vannay, A. et al. Association of genetic polymorphisms of vascular endotelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr. Res. 57, 396–398 (2005).
Mohamed, S. et al. Genetic contributions to the development of retinopathy of prematurity. Pediatr. Res. 65, 193–197 (2009).
Gimenez, L. G. et al. Maternal and neonatal epidemiological features in clinical subtypes of preterm birth. J. Matern. Fetal Neonatal Med. 29, 3153–3161 (2016).
Kaluarachchi, D. C. et al. Polymorphisms in NR5A2, gene encoding liver receptor homolog-1 are associated with preterm birth. Pediatr. Res. 79, 776–780 (2016).
Gimenez, L. G. et al. Association of candidate gene polymorphisms with clinical subtypes of preterm birth in a Latin American population. Pediatr. Res. 82, 554–559 (2017).
Elias, D. et al. Preterm birth and genitourinary tract infections: assessing gene-environment interaction. Pediatr. Res. 90, 678–683 (2021).
Elias, D. et al. Preterm birth etiological pathways: a Bayesian networks and mediation analysis approach. Pediatr. Res. 91, 1882–1889 (2022).
Hutchinson, A. K., Saunders, R. A., O’Neil, J. W., Lovering, A. & Wilson, M. E. Timing of initial screening examinations for retinopathy of prematurity. Arch. Ophthalmol. 116, 608–612 (1998).
Grupo ROP Argentina. Ministerio de Salud. Guía de Práctica Clínica para la prevención, diagnóstico y tratamiento de la retinopatía del prematuro (ROP). (Buenos Aires, Ministerio de Salud, 2015).
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 123, 991–999 (2005).
Krupitzki, H. B. et al. Environmental risk factors and perinatal outcomes in preterm newborns, according to family recurrence of prematurity. Am. J. Perinatol. 30, 451–461 (2013).
Bahadue, F. L. & Soll, R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 11, CD001456 (2012).
Sweet, D. G. et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2019 Update. Neonatology 115, 432–450 (2019).
Flannery, D. D., Edwards, E. M., Coggins, S. A., Horbar, J. D. & Puopolo, K. M. Late-Onset Sepsis Among Very Preterm Infants. Pediatrics 150, e2022058813 (2022).
Maller, V. V. & Cohen, H. L. Neurosonography: Assessing the Premature Infant. Pediatr. Radiol. 47, 1031–1045 (2017).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatrics 92, 529–534 (1978).
Lim, M. & Hastie, T. Learning interactions via hierarchical group-lasso regularization. J. Comput. Graph. Stat. 24, 627–654 (2015).
Spielman, R. S., McGinnis, R. E. & Ewens, W. J. Transmission test for linkage disequilibrium: the insulin gene region and insulindependent diabetes mellitus (IDDM). Am. J. Hum. Genet. 52, 506–516 (1993).
Nykjaer, C. et al. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J. Epidemiol. Community Health 68, 542–549 (2014).
Ikehara, S. et al. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: The Japan Environment and Children’s Study. BJOG 126, 1448–1454 (2019).
Gauthier, T. W., Guidot, D. M., Kelleman, M. S., McCracken, C. E. & Brown, L. A. Maternal Alcohol Use During Pregnancy and Associated Morbidities in Very Low Birth Weight Newborns. Am. J. Med. Sci. 352, 368–375 (2016).
Traylor, C. S., Johnson, J. D., Kimmel, M. C. & Manuck, T. A. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: an expert review. Am. J. Obstet. Gynecol. Mfm. 2, 100229 (2020).
Mehmet, S. et al. One-year experience in the retinopathy of prematurity: frequency and risk factors, short-term results and follow-up. Int. J. Ophthalmol. 4, 634–640 (2011).
Hellström, A., Smith, L. E. & Dammann, O. Retinopathy of prematurity. Lancet 382, 1445–1457 (2013).
Tian, H., McKnight, S. L. & Russell, D. W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11, 72–82 (1997).
Liu, P. et al. Association of VEGF gene polymorphisms with advanced retinopathy of prematurity: a meta-analysis. Mol. Biol. Rep. 39, 10731–10737 (2012).
Alon, T. et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1, 1024–1028 (1995).
Naug, H. L., Browning, J., Gole, G. A. & Gobe, G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin. Exp. Ophthalmol. 28, 48–52 (2000).
Watson, C. J., Webb, N. J., Bottomley, M. J. & Brenchley, P. E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 12, 1232–1235 (2000).
Aiken, J. W. & Vane, J. R. Intrarenal prostaglandin release attenuates the renal vasoconstrictor activity of angiotensin. J. Pharmacol. Exp. Ther. 184, 678–687 (1973).
Bányász, I. et al. Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur. Cytokine Netw. 17, 266–270 (2006).
Sabnis, A. et al. Intestinal vascular endothelial growth factor is decreased in necrotizing enterocolitis. Neonatology 107, 191–198 (2015).
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal microcirculation and necrotizing enterocolitis: The vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Gao, X. et al. Association of VEGFA polymorphisms with necrotizing enterocolitis in Chinese Han population. Pediatr. Neonatol. 60, 129–134 (2019).
Ilguy, S. et al. The relationship of retinopathy of prematurity with brain-derivated neurotrophic factor, vascular endothelial growth factor-A, endothelial PAD domain protein 1 and nitric oxide synthase 3 gene polymorphisms. Ophthalmic Genet. 42, 725–731 (2021).
Rathi, S. et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 8, 1868 (2017).
Appelboom, G. et al. Complement factor H Y402H polymorphism is associated with an increased risk of mortality after intracerebral hemorrhage. J. Clin. Neurosci. 18, 1439–1443 (2011).
Guo, H. et al. Genetics of Spontaneous Intracerebral Hemorrhage: Risk and Outcome. Front. Neurosci. 11, 874962 (2022). 16.
Poggi, C. et al. Genetic Contributions to the Development of Complications in Preterm Newborns. PLoS One 10, e0131741 (2015).
Sarlos, S. & Wilkinson-Berka, J. L. The renin-angiotensin system and the developing retinal vasculature. Invest. Ophthalmol. Vis. Sci. 46, 1069–1077 (2005).
Otani, A., Takagi, H., Suzuma, K. & Honda, Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ. Res. 82, 619–628 (1998).
Acknowledgements
The authors would like to thank the health care team at Maternidad Nuestra Señora de la Merced, Tucumán, Argentina., for their hard work and support and to Mariana Piola and Alejandra Mariona at ECLAMC who provided technical support.
Funding
The research program was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT-MINCyT), grant numbers PICT-2018-4275 (PI: López Camelo JS) and PICT-2018-4285 (PI: Lucas G. Gimenez), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and INAGEMP [Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)] grant 465549/2014-4.
Author information
Authors and Affiliations
Contributions
LGG, JAG, and SG made substantial contributions to design, acquisition of data, analysis and interpretation of data, drafting the article and approving the final manuscript as submitted. JSLC, HBK, and JZB made substantial contributions to design, acquisition of data, critical manuscript revision for important intellectual content, and approving of the final version as submitted. DEE, BC, MRS, HC, FAP, SLH, JR, VRC, RU, CS, MN, and MR made contributions to data analysis and interpretation, drafting and editing of the article, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Before enrollment, signed informed consent was obtained from all participating families. Parents provided consent for themselves and for the neonates.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gimenez, L.G., Gili, J.A., Elias, D.E. et al. Genetic susceptibility for retinopathy of prematurity and its associated comorbidities. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03068-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03068-9
- Springer Nature America, Inc.
This article is cited by
-
Genetic risk and retinopathy of prematurity: homing in on a target?
Pediatric Research (2024)