Abstract
Published data on the association of vascular endothelial growth factor (VEGF) gene polymorphisms with retinopathy of prematurity (ROP) are inconclusive. The aim of the study was to assess the association by using meta-analysis. Data were collected from the following electronic databases: PubMed, Elsevier Science Direct, Excerpta Medica Database, Cochrane Library and China National Knowledge Infrastructure, with the last report up to 30 April, 2012. The odds ratio (OR) and its 95 % confidence interval (95 %CI) were used to assess the strength of the association. Meta-analysis was performed in a fixed/random effect model by using the software Review Manager 4.2. A total of 7 studies based on the search criteria were involved in this meta-analysis. Meta-analysis was performed for four VEGF gene polymorphisms (−634G/C, −460T/C, −2578C/A and 936C/T). Significant association was found for −460T/C polymorphism (C vs T: OR = 0.74, 95 %CI = 0.57–0.95, P = 0.02; TC+CC vs TT: OR = 0.75, 95 %CI = 0.47–1.21, P = 0.24; CC vs TT+TC: OR = 0.45, 95 %CI = 0.26–0.76, P = 0.003; CC vs TT: OR = 0.45, 95 %CI = 0.24–0.84, P = 0.01; TC vs TT: OR = 0.96, 95 %CI = 0.59–1.57, P = 0.87) in the VEGF gene, but not for other polymorphisms (−634G/C, −2578C/A and 936C/T). This meta-analysis demonstrates that advanced ROP is associated with VEGF gene −460T/C polymorphism, but not −634G/C, −2578C/A and 936C/T.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a major cause of visual impairment in premature infants, and is thought to be caused by disorganized growth of retinal blood vessels which may result in scarring and retinal detachment [1]. The pathogenesis of ROP includes two phases [2, 3]. The first phase of ROP is due to the sudden postnatal increase of tissue oxygen tension, and, as a result, the normal development of retinal vasculature ceases in utero. In the second phase, precipitated by the increasing metabolic demand of the developing retina with a compromised vascular supply is characterized by relative hypoxia, which leads to pathologic neovascularization that extends into the vitreous. Globally at least 50,000 children are blind from ROP which is now a significant cause of blindness in many middle income countries in Latin American as well as Eastern Europe, and ROP is also being reported from the emerging economies of India and China [4].
ROP can be mild and may resolve spontaneously, but it may lead to blindness in advanced cases. An understanding of the causes of advanced ROP is an area of intense interest. Unfortunately, the etiology of pathogenesis of advanced ROP is currently unknown. Research with candidate gene approach, higher concordance rate in monozygotic twins and other clinical and experimental animal studies, suggest a strong genetic predisposition to advanced ROP besides environmental factors [5]. Vascular endothelial growth factor (VEGF) is important in physiological growth of retinal vessels, and the presence of it is necessary for normal retinal angiogenesis in utero. Recently, a number of experimental and clinical data suggested that VEGF may play a causative role in the development of ROP [6]. The retinal synthesis of VEGF decreases in the first phase of ROP, and the inadequate retinal oxygenation triggers abnormal angiogenesis and acts as a survival factor for newly formed abnormal retinal vessels in the second phase of ROP [7]. Thus, the VEGF gene is a good candidate for genetics studies on ROP.
The VEGF gene is located on chromosome 6p21.3, and consists of eight exons exhibiting alternate splicing to form a family of proteins [8, 9]. In the past few decades, many polymorphisms have been identified in the VEGF gene, and a number of studies have investigated the association of these polymorphisms with ROP, but findings are not always consistent [10–17]. Meta-analysis is a statistical procedure for combining the results of several studies to produce a single estimate of the major effect with enhanced precision [18]. The aim of the present study is to perform a comprehensive meta-analysis to evaluate the association of VEGF gene polymorphisms with advanced ROP.
Methods
Identification of eligible studies
A systematic search was conducted using a retrieving query formulation “(retinopathy of prematurity OR ROP) AND (gene OR allele OR polymorphism OR variation) AND (vascular endothelial growth factor OR VEGF)” in PubMed, Elsevier Science Direct, Excerpta Medica Database (EMBASE), Cochrane Library and China National Knowledge Infrastructure (CNKI) (last search updated on 30 April, 2012). Advanced ROP was defined as ROP with stage 2+ or more. Control was infants with mild ROP or no disease. Eligible studies were selected according to the following explicit inclusion criteria: (a) Study was designed using the methodology of a case–control study. (b) The association between VEGF gene polymorphisms and advanced ROP was explored. (c) There was sufficient data for the computation of odds ratio (OR) and 95 % confidence interval (95 %CI). Moreover, all references cited in the retrieved articles were reviewed to identify other relevant publications. Review articles were also inspected to find additional publications. No language restrictions were applied. Two investigators independently searched the electronic databases. Where discrepancies occurred, a third investigator did additional assessment.
Data extraction
Two investigators independently reviewed the articles and extracted the data from all eligible publications according to the criteria listed above. The following information were recorded for each study: first author, year of publication, ethnicity, polymorphisms, number of cases and controls, number of cases and controls by allele and genotype. Any discrepancies between the two investigators were resolved by discussion and consultation with a third investigator. If original genotype frequency data were unavailable in relevant articles, a request for additional data were sent to the corresponding author.
Meta-analysis methods
The strength of the association between VEGF gene polymorphisms and advanced ROP was evaluated by the OR with 95 %CI. The heterogeneity between the studies was assessed by the Chi square-test based Q-statistic, and a significant Q-statistic (P < 0.10) indicated heterogeneity across studies [19]. Additionally, we assessed the effect of heterogeneity by another measure, I 2 = 100 % × (Q-df)/Q [20]. The pooled OR was calculated by a fixed effect model (using the Mantel–Haenszel method) or a random effect model (using the DerSimonian–Laird method) according to the heterogeneity among studies [21, 22]. The significance of the pooled OR was determined by the Z-test. The pooled OR was calculated for the heterozygote comparison, homozygote comparison, dominant model, recessive model and allele comparison, respectively. Chi square-test was used to determine if observed frequencies of genotypes conformed to Hardy–Weinberg equilibrium (HWE) expectations.
Evaluation of publication bias
Funnel plot and Egger’s regression test were used to investigate publication bias. The potential publication bias was assessed with funnel plots of the effect sizes versus the standard errors. The funnel plot should be asymmetric when there is a publication bias. Funnel plot asymmetry was further assessed by the method of Egger’s regression test [23].
Analyses were performed using the software Review Manager 4.2 (Cochrane Collaboration, http://www.cc-ims.net/RevMan/relnotes.htm/) and the software Stata version 10 (StataCorp LP, College Station, TX, USA). A P value less than 0.05 was considered statistically significant. All the P values were two sided.
Results
Description of studies (Table 1)
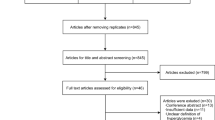
The characteristics of the studies investigating the association of VEGF gene polymorphisms with advanced ROP are listed in Table 1 [10–17]. A total of 1,605 potential publications were relevant to the search words (PubMed: 77; Elsevier Science Direct: 1043; EMBASE: 127; Cochrane: 0; CNKI: 358). There were 1,454 potentially relevant articles after duplicates removed. Through the step of screening abstract or full text, 1,446 of these articles were excluded (225 were review; 591 did not conducted in human; 438 were not case–control study; 114 did not explore VEGF gene polymorphisms; 78 did not conducted in patients with ROP), leaving 8 studies [10–17] for detailed assessment. Of these studies, 1 was excluded due to unavailable data [11]. Thus, a total of 7 studies [10, 12–17] were included in the current meta-analysis. The study selection process is shown in Fig. 1.
The 7 studies explored six different polymorphisms (702C/T, 936C/T, 1612G/A, −2578C/A, −460T/C and −634G/C) in the VEGF gene. Four of these polymorphisms (−634G/C, −460T/C, −2578C/A and 936C/T) were investigated in more than two studies. Thus, meta-analysis was performed for four polymorphisms (−634G/C [13, 14, 16, 17], −460T/C [13, 14, 16], −2578C/A [12, 15] and 936C/T [10, 17]). Only allele frequency of the VEGF gene −460T/C polymorphism was extracted from the study by Shastry et al. [14]. The distribution of the genotype in total control population were in HWE for the four polymorphisms (P > 0.05).
Meta-analysis (Table 2)
The summary of meta-analysis for VEGF gene polymorphisms and advanced ROP risk is shown in Table 2.
Analysis for VEGF gene −634G/C polymorphism
Four studies (296 cases and 392 controls) explored the association of VEGF gene −634G/C polymorphism with advanced ROP. The Q-test of heterogeneity was almost significant and we conducted analyses using the random effect models except in the contrast of GC versus GG. We found no association between VEGF gene −634G/C polymorphism and advanced ROP (C vs G: OR = 1.08, 95 %CI = 0.69–1.69, P = 0.72; GC+CC vs GG: OR = 0.99, 95 %CI = 0.58–1.68, P = 0.96; CC vs GG+GC: OR = 1.44, 95 %CI = 0.69–3.00, P = 0.33; CC vs GG: OR = 1.36, 95 %CI = 0.53–3.48, P = 0.53; GC vs GG: OR = 0.90, 95 %CI = 0.65–1.24, P = 0.52).
Analysis for VEGF gene −460T/C polymorphism
The association of VEGF gene −460T/C polymorphism with advanced ROP was investigated in 3 studies with a total of 212 cases and 297 controls. The Q-test of heterogeneity was not significant and we conducted analyses using fixed effect models. An association of VEGF gene −460T/C polymorphism with advanced ROP was found (C vs T: OR = 0.74, 95 %CI = 0.57–0.95, P = 0.02; TC+CC vs TT: OR = 0.75, 95 %CI = 0.47–1.21, P = 0.24; CC vs TT+TC: OR = 0.45, 95 %CI = 0.26–0.76, P = 0.003; CC vs TT: OR = 0.45, 95 %CI = 0.24–0.84, P = 0.01; TC vs TT: OR = 0.96, 95 %CI = 0.59–1.57, P = 0.87).
Analysis for VEGF gene −2578C/A polymorphism
The association of VEGF gene −2578C/A polymorphism with advanced ROP was investigated in 2 studies with a total of 190 cases and 210 controls. We found no significant between-study heterogeneity in the contrasts of CA+AA versus CC as well as CA versus CC, and we conducted analyses using fixed effect models. But we detected significant between-study heterogeneity in the contrasts of A versus C, AA versus CC+CA as well as AA versus CC, and we conducted analyses using the random effect models. We did not detect a significant association between VEGF gene −2578C/A polymorphism and advanced ROP (A vs C: OR = 1.04, 95 %CI = 0.51–2.14, P = 0.91; CA+AA vs CC: OR = 1.01, 95 %CI = 0.66–1.54, P = 0.96; AA vs CC+CA: OR = 1.38, 95 %CI = 0.19–9.90, P = 0.75; AA vs CC: OR = 1.34, 95 %CI = 0.16–11.41, P = 0.79; CA vs CC: OR = 0.97, 95 %CI = 0.62–1.50, P = 0.87).
Analysis for VEGF gene 936C/T polymorphism
The association of VEGF gene 936T/C polymorphism with advanced ROP was investigated in 2 studies (129 cases and 146 controls). The Q-test of heterogeneity was not significant and we conducted analyses using fixed effect models. We did not detect a significant association of VEGF gene 936T/C polymorphism with advanced ROP (T vs C: OR = 0.65, 95 %CI = 0.42–1.01, P = 0.06; CT+TT vs CC: OR = 0.64, 95 %CI = 0.38–1.07, P = 0.09; TT vs CC+CT: OR = 0.54, 95 %CI = 0.18–1.61, P = 0.27; TT vs CC: OR = 0.50, 95 %CI = 0.16–1.51, P = 0.22; CT vs CC: OR = 0.67, 95 %CI = 0.39–1.17, P = 0.16).
Publication bias
Funnel plot was performed to estimate the publication bias of literatures. We further assessed funnel plot asymmetry by the method of Egger’s regression test. The results of Egger’s regression test showed that there was no publication bias for some comparisons (−634G/C: C vs G P = 0.905, GC+CC vs GG P = 0.907, CC vs GG+GC P = 0.757, CC vs GG P = 0.813, GC vs GG P = 0.983; −460T/C: C vs T P = 0.549). However, Egger’s regression test was not applied in some comparisons due to the small number of studies (−460T/C: TC+CC vs TT, CC vs TT+TC, CC vs TT, TC vs TT; −2578C/A: A vs C, CA+AA vs CC, AA vs CC+CA, AA vs CC, CA vs CC; 936C/T: T vs C, CT+TT vs CC, TT vs CC+CT, TT vs CC, CT vs CC).
Discussion
Major risk factors of ROP are prematurity and oxygen toxicity, and, while, as not each infant from the same developmental cohort with the same clinical condition develops ROP, there is also an individual susceptibility to the disease [24, 25]. Recent evidences have supported an important role for genetics in determining risk for ROP [5]. However, small sample sized association studies lack statistical power and result in apparently contradicting findings [26]. Meta-analysis become important in complex human diseases genetics because of rapid increases in the number and size of datasets. In the current meta-analysis, we retrieved 7 studies to evaluate the association of VEGF gene polymorphisms with advanced ROP. Meta-analysis was performed for four polymorphisms (−634G/C, −460T/C, −2578C/A and 936C/T). Results of our meta-analysis indicate that advanced ROP is associated with VEGF gene −460T/C polymorphism, but not −634G/C, −2578C/A and 936C/T. To our knowledge, the present meta-analysis is the first to assess the association between VEGF gene polymorphisms and advanced ROP.
Our results demonstrated that the VEGF gene −460T/C polymorphism could be a protective factor for advanced ROP. The T allele carriers have a higher risk than infants carrying C allele. Growth factors including VEGF, growth hormone and insulin-like growth factor-1 have been found to involve in the pathogenesis of ROP [27]. VEGF is essential for neovascularization. Abnormal and progressive neovascularization is the most important pathophysiological mechanism in the development of ROP. Retinal diseases might share the disease process of vasculopathy with ROP, and recent studies indicated that VEGF gene polymorphisms were associated with proliferative diabetic retinopathy and age-related degeneration [28, 29]. Dysregulated VEGF expression is implicated in a number of disease pathologies, and increased VEGF expression resulting in inappropriate VEGF-induced angiogenesis is linked with tumor, rheumatoid arthritis and diabetic retinopathy [30–33]. Recently, it was also reported that increased VEGF expression was associated with both avascular retina and intravitreous neovascularization in a model of ROP [34]. The VEGF gene −460T/C polymorphism is located within the promoter region. In patients with psoriasis, the evidence showed that the TT genotype was associated with significantly higher production of VEGF in comparison to the CC genotype [35]. Therefore, we speculate that the VEGF gene −460T/C polymorphism may decrease production of VEGF, resulting in a decreased susceptibility to advanced ROP. But the detailed mechanisms need further study.
Several considerations should be discussed for our study. A first consideration is that significant between-study heterogeneity was found in some comparisons, and may be distorting the meta-analysis. A second consideration is that we could not construct Egger’s regression test for all comparisons due to small number of studies, and publication bias may be present. A third consideration is that our results are based on unadjusted estimates, and a more precise analysis stratified by clinical characteristics could be performed if individual data were available. Finally, although we did not detect the association of VEGF gene −634G/C, −2578C/A and 936C/T polymorphisms with advanced ROP, the result should be interpreted with caution because the number of studies was relatively small.
In conclusion, our meta-analysis demonstrates that advanced ROP is associated with VEGF gene −460T/C polymorphism, but not −634G/C, −2578C/A and 936C/T. To reach a definitive conclusion, further studies based on larger sample size are still needed.
References
Ng YK, Fielder AR, Shaw DE, Levene MI (1988) Epidemiology of retinopathy of prematurity. Lancet 2:1235–1238
Patz A, Eastham A, Higginbotham DH, Kleh T (1953) Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol 36:1511–1522
Chen ML, Guo L, Smith LE, Dammann CE, Dammann O (2010) High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics 125:e1483–e1492
Gilbert C (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 84:77–82
Shastry BS (2010) Genetic susceptibility to advanced retinopathy of prematurity (ROP). J Biomed Sci 17:69
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27:331–371
Pierce EA, Foley ED, Smith LE (1996) Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 114:1219–1228
Vincenti V, Cassano C, Rocchi M, Persico G (1996) Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 93:1493–1495
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266:11947–11954
Gismondi D, Ndoja L, Qu X, Shastry BS (2012) Lack of association of VEGF gene 3′- UTR polymorphisms (C702T, C936T and G1612A) and the risk of developing advanced retinopathy of prematurity (ROP). Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-011-1913-4
Kusuda T, Hikino S, Ohga S, Kinjo T, Ochiai M, Takahata Y, Tokunaga S, Ihara K, Hata Y, Hara T (2011) Genetic variation of vascular endothelial growth factor pathway does not correlate with the severity of retinopathy of prematurity. J Perinatol 31:246–250
Shastry BS (2009) Lack of association of VEGF (-2578 C– > A) and ANG 2 (-35 G– > C) gene polymorphisms with the progression of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 247:859–860
Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Pietrzyk JJ (2008) The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 246:1467–1475
Shastry BS, Qu X (2007) Lack of association of the VEGF gene promoter (−634 G– > C and -460 C– > T) polymorphism and the risk of advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 245:741–743
Bányász I, Bokodi G, Vannay A, Szebeni B, Treszl A, Vásárhelyi B, Tulassay T, Szabó A (2006) Genetic polymorphisms of vascular endothelial growth factor and angiopoietin 2 in retinopathy of prematurity. Curr Eye Res 31:685–690
Vannay A, Dunai G, Bányász I, Szabó M, Vámos R, Treszl A, Hajdú J, Tulassay T, Vásárhelyi B (2005) Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 57:396–398
Cooke RW, Drury JA, Mountford R, Clark D (2004) Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci 45:1712–1715
Munafo MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20:439–444
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315:629–634
Karna P, Muttineni J, Angell L, Karmaus W (2005) Retinopathy of prematurity and risk factors: a prospective cohort study. BMC Pediatr 5:18
Dunai G, Vásárhelyi B, Szabó M, Hajdú J, Mészáros G, Tulassay T, Treszl A (2008) Published genetic variants in retinopathy of prematurity: random forest analysis suggests a negligible contribution to risk and severity. Curr Eye Res 33:501–505
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN (2003) Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33:177–182
Chen J, Smith LE (2007) Retinopathy of prematurity. Angiogenesis 10:133–140
Errera FI, Canani LH, Silva ME, Yeh E, Takahashi W, Santos KG, Souto KE, Tschiedel B, Roisenberg I, Gross JL, Passos-Bueno MR (2007) Functional vascular endothelial growth factor -634G > C SNP is associated with proliferative diabetic retinopathy: a case-control study in a Brazilian population of European ancestry. Diabetes Care 30:275–279
Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, Tsai CH, Tseng SH, Tsai FJ (2008) Vascular endothelial growth factor gene polymorphisms in age-related macular degeneration. Am J Ophthalmol 145:1045–1051
Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE (2000) Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 12:1232–1235
Claffey KP, Robinson GS (1996) Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev 15:165–176
Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N (1994) Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol 152:4149–4156
Miller JW, Adamis AP, Aiello LP (1997) Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 13:37–50
Budd SJ, Hartnett ME (2010) Increased angiogenic factors associated with peripheral avascular retina and intravitreous neovascularization: a model of retinopathy of prematurity. Arch Ophthalmol 128:589–595
Young HS, Summers AM, Read IR, Fairhurst DA, Plant DJ, Campalani E, Smith CH, Barker JN, Detmar MJ, Brenchley PE, Griffiths CE (2006) Interaction between genetic control of vascular endothelial growth factor production and retinoid responsiveness in psoriasis. J Invest Dermatol 126:453–459
Acknowledgments
We thank all the people who give the help for this study. This work was supported by grants from 2011 Guangdong Province Medical Foundation (A2011550).
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, P., Wu, D., Zhou, W. et al. Association of VEGF gene polymorphisms with advanced retinopathy of prematurity: a meta-analysis. Mol Biol Rep 39, 10731–10737 (2012). https://doi.org/10.1007/s11033-012-1964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1964-6