Abstract
Background
The importance of neonatal resuscitator resistance is currently unknown. In this study we investigated peak flows and pressure stability resulting from differences in imposed resistance during positive pressure ventilation(PPV) and simulated spontaneous breathing (SSB) between the r-PAP, low-resistance resuscitator, and Neopuff™, high-resistance resuscitator.
Methods
In a bench test, 20 inflations during PPV and 20 breaths during SSB were analysed on breath-by-breath basis to determine peak flow and pressure stability using the Neopuff™ with bias gas flow of 8, 12 or 15 L/min and the r-PAP with total gas flow of 15 L/min.
Results
Imposed resistance of the Neopuff™ was significantly reduced when the bias gas flow was increased from 8 to 15 L/min, which resulted in higher peak flows during PPV and SSB. Peak flows in the r-PAP were, however, significantly higher and fluctuations in CPAP during SSB were significantly smaller in the r-PAP compared to the Neopuff™ for all bias gas flow levels. During PPV, a pressure overshoot of 3.2 cmH2O was observed in the r-PAP.
Conclusions
The r-PAP seemed to have a lower resistance than the Neopuff™ even when bias gas flows were increased. This resulted in more stable CPAP pressures with higher peak flows when using the r-PAP.
Impact
-
The traditional T-piece system (Neopuff™) has a higher imposed resistance compared to a new neonatal resuscitator (r-PAP).
-
This study shows that reducing imposed resistance leads to smaller CPAP fluctuations and higher inspiratory and expiratory peak flows.
-
High peak flows might negatively affect lung function and/or cause lung injury in preterm infants at birth. This study will form the rationale for further studies investigating these effects.
-
A possible compromise might be to use the traditional T-piece system with a higher bias gas flow (12 L/min), thereby reducing the imposed resistance and generating more stable PEEP/CPAP pressures, while limiting potentially harmful peak flows.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
During neonatal stabilisation at birth, the most frequently used respiratory support device for providing non-invasive ventilation (e.g., continuous positive airway pressure (CPAP) or intermittent positive pressure ventilation (iPPV)) is a T-piece resuscitator. The T-piece generates positive end expiratory pressure (PEEP) or CPAP by providing resistance to the bias gas flow through the expiratory outlet of the T-piece (called the PEEP valve). It then generates positive inflation pressures (PIP) when the PEEP valve is occluded.1,2 The resistance of the PEEP valve is regulated by altering the area of the aperture to achieve a certain CPAP level, but the required area (thus resistance) to achieve this CPAP level depends on the bias gas flow. To achieve the same PEEP/CPAP level, a low bias gas flow (e.g. 6–8 L/min) requires a small aperture with high resistance, whereas higher bias gas flows (e.g. 12–15 L/min) require a larger aperture with a lower resistance.1,3,4 As the infant expires through the PEEP valve, a reduced valve resistance will reduce the expiratory resistance imposed on the infant and thereby increase expiratory flows. When expiration is active, this is thought to reduce the imposed work of breathing (iWOB) (e.g., breathing effort and energy cost).3,4 When a T-piece is compared to other CPAP methods in a simulated setting with an active expiration, it generates a substantially higher iWOB due to the relatively high resistance of the PEEP valve.3,4
Recently, a new low imposed resistance resuscitator with a low iWOB for neonatal stabilisation was introduced using dual flow (e.g., bias flow and jet flow). This device, now commercially available as r-PAP, generates PEEP/CPAP using the jet flow. The turbulence created in the angulated tube opposes expiration and supports inspiration, a phenomenon that is called a fluidic flip. During iPPV the system is occluded in the same manner as a T-piece and the bias flow added to achieve a sufficient total gas flow for an acceptable inspiratory rise time. In this way, the dual flow system is able to generate the same PEEP/CPAP levels as a T-piece, but with a lower imposed resistance during active expiration.5 In addition, the dual flow system is able to generate more stable PEEP level across a range of different lung compliances during iPPV.2 A recently performed multicentre randomised controlled trial6 comparing this dual flow system with a traditional T-piece system (e.g., Neopuff™) in preterm infants reported less delivery room intubation when the dual flow system was used. However, this was an unblinded study and the reason for the differences in intubation rates have not been explained. It could be related to the interface (face mask vs nasal prongs) or other differences such as imposed resistance or more stable CPAP and PEEP levels.
While a resuscitation device that provides a low imposed resistance might provide advantages, there might be benefits from increasing resistance and limiting expiratory flows. Peak flows >500 L/min (equivalent to >~6 L/kg/min) during coughing produced shear stress in airway walls in adults.7 Therefore, reducing peak flows by using a T-piece system could potentially lead to less shear stress and airway epithelial cell damage, particularly in the less compliant immature lung of preterm infants at birth. Limiting flows during resuscitation and stabilisation have not been investigated for iPPV or spontaneous breathing.
A possible compromise might be to use the traditional T-piece system with a higher bias gas flow (e.g., 12 L/min), thereby reducing the imposed resistance and generating more stable PEEP/CPAP pressures, while limiting potentially harmful peak flows. Indeed, we recently investigated the differential effects of a bias gas flow of 12 L/min and 8 L/min in spontaneous breathing preterm infants at birth and showed that increased bias gas flow resulted in a lower inspiratory and expiratory resistance with less inspiratory CPAP fluctuations.1
As resuscitators providing either a low imposed resistance (e.g., r-PAP) or a higher imposed resistance (e.g., Neopuff™), both provide advantages and disadvantages, we aim to investigate i) the differences in peak flows and pressure stability resulting from differences in resistance during intermittent positive pressure ventilation (iPPV) and simulated spontaneous breathing between the r-PAP and Neopuff™.
Methods
An experimental study was performed at The Ritchie Centre, Hudson Institute of Medical Research in Melbourne, Australia to compare a low imposed resistance resuscitator, Inspire r-PAP (Inspiration Healthcare Group, Steenderen, The Netherlands), to a higher imposed resistance resuscitator, Neopuff™ T-Piece resuscitator (Neopuff™ Infant Resuscitator, Fisher & Paykel Healthcare Ltd., Auckland, New Zealand) with various bias gas flows.
The test setting is shown in Fig. 1. A custom made test-lung (Fig. 1), with a FRC of 35 mL, was connected to the respiratory tubing of the Neopuff™ or r-PAP. A disposable Avea Varflex Flow transducer (Carefusion, CA) was placed between the end of the test-lung’s trachea and the respiratory tubing. Three pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) were connected to (1) the intrathoracic space, (2) the lung and (3) the respiratory tubing at the end of the test-lung’s trachea. Flows were recorded digitally using the Polybench physiological software (Applied Biosignals, Weener, Germany) by connecting the flow transducer to a respiratory function monitor (Advanced Life Diagnostics, Weener, Germany). Pressures were recorded digitally using the LabChart data acquisition system via a Bridge Amp (Powerlab, ADInstruments, Sydney, Australia). The Neopuff’s bias gas flow was set to 8, 12 and 15 L/min and the CPAP level was set to 8 cm H2O. For the r-PAP, the GE Giraffe Stand-alone Infant Resuscitation System (GE Healthcare, Rydalmere, Australia) was used to set the bias gas flow and jet flow (as also described in a previous study6). The jet flow was titrated to achieve a CPAP/PEEP level of 8 cm H2O and bias flow was then added to reach a total fresh gas flow of 15 L/min.
Test-lung (system compliance: 7 mL/cm H2O), which consists of a Draeger test-lung (a) within a Sealed Perspex cylinder with two 3D printed rubber lids (b), which is partly filled with polystyrene foam (c). The test-lung ends in a simulated trachea (d), which is connected to a flow sensor (e) and then to either the Neopuff™ or r-PAP respiratory support system (f). On the other side of the cylinder a suction tube with T-piece (g) is inserted through the lid. niPPV was simulated by occluding and opening the aperture of the respiratory support system whilst spontaneous breathing was simulated by occluding and opening the T-piece of the suction tube. Pressures where measured in the lung (h), the cylinder (i) and the simulated trachea (j).
During iPPV, a positive end expiratory pressure (PEEP) of 8 cm H2O and positive inflation pressures (PIP) of 20 cm H2O were set. During simulated spontaneous breaths, an intrathoracic pressure gradient of 30 cm H2O was generated by occluding the lateral opening of the suction device simulating an active inspiration, while a passive expiration was simulated by re-opening the lateral opening of the suction device. For each group, 20 inflation breaths over 60 s and 20 spontaneous breaths over 60 s were simulated by the resuscitator manually. All pressures were set based on the pressure measurements visible on the RFM.
All inflation breaths and spontaneous breaths were analysed on a breath-by-breath basis to determine PEEP/CPAP level, PIP level, peak inspiratory flow rate (PIFR), PEFR, inspiratory tidal volume (Vti) and the resistance of the T-piece valve (Rv). During simulated spontaneous breathing inspiratory and expiratory CPAP fluctuations (ΔCPAPinsp, ΔCPAPexp) were also assessed. All pressures were measured by the pressure sensor in line (Fig. 1j).
The valve resistance was calculated using a derivation of Ohm’s law (Formula 1, Fig. 2).
As the r-PAP uses a dual flow system and generates CPAP by a jet of fresh gas instead of a resistor, we were unable to calculate the imposed resistance during ventilation or spontaneous breathing for this device. However, it is known that systems resembling the r-PAP, such as infant flow and the benveniste valve, have a much lower expiratory resistance than a T-piece, even with a bias gas flow of 15 L/min.4
In addition, we measured the CPAP fluctuations during spontaneous breaths in both devices. During inspiration less flow travels through the expiratory outlet resulting in a pressure below the set CPAP (formula 2), while during expiration the flow through the expiratory outlet increases resulting in a pressure above the set CPAP (formula 3).
The local institutional Research Ethics Committee of the LUMC approved the study protocol and issued a statement of no objection for performing this study.
All statistical analyses were performed with IBM SPSS Statistics version 25 (IBM Software, Chicago, Illinois, 2016). Continuous data are presented as median (IQR) or mean ± SD. Normality was judged based on the inspections of histograms. The Kruskal–Wallis test was used to compare all four groups (r-PAP, NP 8 L/min, NP 12 L/min and NP 15 L/min). In addition, the Mann–Whitney test was used to compare the r-PAP to each group of the Neopuff™. A p-value < 0.05 was considered statistically significant.
Results
Intermittent positive pressure ventilation
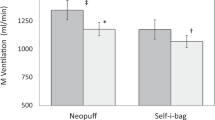
The valve resistance of the T-piece was significantly reduced when the bias gas flow in the Neopuff™ was increased from 8 to 15 L/min (NP8 0.86 ± 0.01 vs. NP12 0.62 ± 0.01 vs. NP15 0.46 ± 0.01 cm H2O/L/min, p < 0.001) . As a result, the PIFR and PEFR increased in the Neopuff™, but the peak flows were still significantly lower compared to the peak flows observed in the r-PAP (PIFR: NP8 3.8 ± 0.06 vs. NP12 3.9 ± 0.06 vs. NP15 4.0 ± 0.05, r-PAP 6.0 ± 0.26 L/min, p < 0.001; PEFR NP8 2.4 ± 0.08 vs NP12 3.0 ± 0.02 vs. NP15 3.5 ± 0.06 vs r-PAP 4.6 ± 0.19 L/min, p < 0.001). During iPPV, a pressure overshoot of 3.2 cm H2O was observed in the r-PAP (Table 1 and Fig. 3).
Simulated spontaneous breathing
When increasing the bias gas flow from 8 to 15 L/min in the Neopuff™, the valve resistance of the T-piece was significantly lower (NP8 0.94 ± 0.01 vs. NP12 0.67 ± 0.01 vs. NP15 0.50 ± 0.01 cm H2O/L/min, p < 0.001). This was consistent with the finding that both the PIFR and PEFR significantly increased following the increase in bias gas flow (PIFR: NP8 3.1 ± 0.06 vs. NP12 3.2 ± 0.06 vs. NP15 3.4 ± 0.06 L/min, p < 0.001; PEFR: NP8 4.9 ± 0.07 vs. NP12 5.2 ± 0.08 vs. NP15 5.7 ± 0.12 L/min, p < 0.001), whereas the inspiratory and expiratory CPAP fluctuation significantly decreased (ΔCPAPinsp: NP8 5.7 ± 0.17 vs. NP12 4.7 ± 0.19 vs. NP15 4.4 ± 0.16 cm H2O, p < 0.001; ΔCPAPexp: NP8 8.8 ± 0.19 vs. NP12 7.2 ± 0.21 vs. NP15 6.3 ± 0.21 cm H2O, p < 0.001)) (Table 2).
Compared to the Neopuff™, the r-PAP device resulted in significantly higher PIFR and PEFR and had significantly less inspiratory and expiratory CPAP fluctuations (PIFR NP15 3.4 ± 0.06 vs. r-PAP 4.5 ± 0.10 L/min, p < 0.001; PEFR NP15 5.7 ± 0.12 vs. r-PAP 7.3 ± 0.14 L/min, p < 0.001); ΔCPAPinsp: NP15 4.4 ± 0.16 vs. r-PAP 1.1 ± 0.16 cm H2O, p < 0.001; ΔCPAPexp: NP15 6.3 ± 0.21 vs. r-PAP 1.9 ± 0.20 cm H2O, p < 0.001) (Table 2).
Discussion
In this study, we found that increasing the bias gas flow in the Neopuff™ by a factor of 1.88 (i.e., from 8 to 15 L/min) reduced the resistance of the valve by the same magnitude during simulated spontaneous breathing while maintaining the same CPAP level. Reducing imposed resistance led to smaller CPAP fluctuations and higher inspiratory and expiratory peak flows. However, even with a bias gas flow of 15 L/min, the Neopuff™ still had a higher imposed resistance compared to the r-PAP device as well as larger CPAP fluctuations and lower inspiratory and expiratory peak flows. In addition, a pressure overshoot was observed when using the r-PAP during positive pressure ventilation.
This study confirms the results of our previous retrospective study investigating the effect of a higher bias gas flow on valve resistance and imposed expiratory resistance during spontaneous breathing in preterm infants at birth. In the retrospective study, we also observed that increasing the bias gas flow by a factor of 1.5, reduced the imposed inspiratory and expiratory resistance by the same factor.1 However, the retrospective study reported a lower PIFR with similar PEFR, while we observed higher peak flows in an experimental setting during which airway resistance and pulmonary pressures remained similar during the study period. This is also confirmed by Huckstadt et al.,8 who reported an increase in PIFR and PEFR in preterm infants admitted to the NICU when a device administering CPAP with a high expiratory resistance (Babylog 8000) was compared to a low-resistance CPAP device (Infant Flow). The observed differences between in vitro and in vivo measurements at birth might be explained by the fact that airway resistance and pulmonary pressures markedly change during the transition at birth.
Although higher inspiratory and expiratory peak flows were observed in the low imposed resistance resuscitator compared to a higher imposed resistance resuscitator, these peak flows remained <6 L/kg/min when assuming that a FRC of 35 mL correlates to a birthweight of 2 kg.9 However, it should be kept in mind that the driving force for inspiratory and expiratory flow is the pressure gradient between inside and outside of the lung when flow is zero. During simulated spontaneous breathing, this was the difference between the internal pulmonary pressure (~–30 cm H2O) at peak inspiration and the external CPAP level (~8 cm H2O). During iPPV, this was the difference between PIP (~20 cm H2O) and PEEP (~8 cm H2O). As the average pressures generated during the first breaths at birth10 have been recorded at –70 cm H2O and PIP > 20 cm H2O can be used during neonatal stabilisation, the peak flows that occur naturally in infants at birth might be higher than what we observed in this study. Indeed, in our retrospective study1 there were occasional peak flows of >~6 L/kg/min observed during spontaneous breaths on CPAP when the higher imposed resistance resuscitator, Neopuff™, was used. As the peak flow in the low imposed resistance resuscitator will be higher, the incidence of spontaneous breaths or ventilated breaths with peak flows >~6 L/kg/min might be as well. In addition, higher peak expiratory flows increase the deflation rate, which could lead to a loss of FRC due to the lung’s higher momentum as it approaches FRC.1 This effect would even be higher when air accumulates in the abdomen as it would increase abdominal pressure and apply an upward displacement on the diaphragm leading to higher peak flows. This risks increasing the momentum of the lung during deflation, which would cause the lung to deflate to a greater degree, leading to a reduction in FRC. As such, the lower the expiratory resistance, the greater the effect that abdominal air accumulation will have. Future animal studies will be necessary to investigate whether lower peak flows as seen with T-piece systems positively affect FRC and/or reduce shear stress and subsequent airway epithelial cell damage in the fragile lungs of preterm infants at birth. In the meantime, a possible compromise might be to use the traditional T-piece system with a higher bias gas flow, thereby reducing the imposed resistance and generating more stable PEEP/CPAP pressures, while limiting potentially harmful peak flows.
The pressure overshoot observed when using the low imposed resistance resuscitator is in line with results reported in previous studies and related to the pressure release valve in the GE driver. These studies reported spikes above set PIP for both T-piece and r-PAP, ranging from 0.6 to 9.46 cm H2O, in GE and Draeger systems, depending on lung compliance and the set PIP.2,11 As these spikes increase pulmonary pressures above the set PIP and are not obvious unless a respiratory function monitor is used, caregivers using these devices should be aware that they might be providing higher PIP levels than intended. Although the clinical significance of these pressure spikes are unknown, they might be harmful for preterm infants at birth. Indeed, the spikes shown in Fig. 3 are ~200 ms in duration and so when using higher inflation rates (e.g., at 60 inflations per minute) these pressure spikes will form a highly significant component of the inflation time. It is important to note that the pressure spikes above PIP were not observed with the r-PAP device when the designated driver was used.
As this study was a bench test with the purpose to generate rationale, the results should be translated with the appropriate caution to clinical practice. In this study, all variables are controlled, while during the transition at birth airway resistance and pulmonary pressures markedly change. As the r-PAP uses a dual flow system, we were unable to measure the imposed resuscitator resistance of the r-PAP, which made it harder to compare the results with the Neopuff™. However, looking at the pressure and flow values and resistance reported in other papers,3,4,12 the imposed resuscitator resistance of the r-PAP is lower than the imposed resuscitator resistance of the Neopuff™. In addition, as we recorded values at 200 Hz, our measure of PEFR (and the size of the pressure spike) may be an under-estimation, despite the variability between measurements being extremely low.
Conclusion
In a bench test, it seemed that the r-PAP had a lower resistance than the T-piece system (e.g., Neopuff™) even when bias gas flows in the T-piece system were increased to compensate. This resulted in more stable CPAP pressures with higher peak flows when using the low imposed resistance resuscitator. The lower peak flows observed in higher imposed resistance systems such as the Neopuff™ might have advantages by increasing lung function and reducing lung injury and this needs to be further investigated.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kuypers, K. L. A. M. et al. The effect of a higher bias gas flow on imposed T-piece resistance and breathing in preterm infants at birth. Front. Pediatr. 10, 817010 (2022).
Hinder, M., McEwan, A., Drevhammer, T., Donaldson, S. & Tracy, M. B. T-piece resuscitators: how do they compare? Arch. Dis. Child Fetal Neonatal Ed. 104, F122–F7 (2019).
Drevhammar, T., Nilsson, K., Zetterström, H. & Jonsson, B. Comparison of seven infant continuous positive airway pressure systems using simulated neonatal breathing. Pediatr. Crit. Care Med. 13, e113–e119 (2012).
Wald, M. et al. Variety of expiratory resistance between different continuous positive airway pressure devices for preterm infants. Artif. Organs 35, 22–28 (2011).
Donaldsson, S., Drevhammar, T., Taittonen, L., Klemming, S. & Jonsson, B. Initial stabilisation of preterm infants: a new resuscitation system with low imposed work of breathing for use with face mask or nasal prongs. Arch. Dis. Child. Fetal Neonatal Ed. 102, F203–F207 (2017).
Donaldsson, S. et al. Comparison of respiratory support after delivery in infants born before 28 weeks’ gestational age: The CORSAD Randomized Clinical Trial. JAMA Pediatr. 175, 911–918 (2021).
Basser, P. J., McMahon, T. A. & Griffith, P. The mechanism of mucus clearance in cough. J. Biomech. Eng. 111, 288–297 (1989).
Huckstadt, T., Foitzik, B., Wauer, R. R. & Schmalisch, G. Comparison of two different CPAP systems by tidal breathing parameters. Intensive Care Med. 29, 1134–1140 (2003).
Ferhardt, T., Reifenberg, L., Hehre, D., Feller, R. & Bancalari, E. Functional residual capacity in normal neonates and children up to 5 years of age determined by N2 washout method. Pediatr. Res. 20, 668–671 (1986).
LoMauro, A. & Aliverti, A. Physiology masterclass: extremes of age: newborn and infancy. Breathe (Sheff.) 12, 65–68 (2016).
Hinder, M. K. et al. T-piece resuscitators: can they provide safe ventilation in a low compliant newborn lung? Arch. Dis. Child Fetal Neonatal. Ed. 106, 25–30 (2021).
Drevhammar, T., Berg, N., Nilsson, K., Jonsson, B. & Prahl Wittberg, L. Flows and function of the infant flow neonatal continuous positive airway pressure device investigated with computational fluid dynamics. Acta Paediatr. (Oslo, Nor. : 1992) 110, 811–817 (2021).
Funding
This work was supported by Fisher & Paykel Healthcare Limited by an unrestricted grant. Fisher & Paykel Healthcare Limited had no role in study design nor in the collection, analysis, and interpretation of data, writing of the manuscript and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
K.K.: co-conceived the study, conducted the study, collected, analysed and interpreted the data, wrote the first draft of the manuscript and approved the final version of the manuscript. A.K.: co-conceived the study, conducted the study, collected and interpreted the data, reviewed and edited the manuscript and approved the final version of the manuscript. S.C.: data interpretation, reviewed and edited the manuscript and approved the final version of the manuscript. S.H.: co-conceived the study, supervised the study, interpreted the data, reviewed and edited the manuscript and approved the final version. A.B.t.P.: co-conceived the study, supervised the study, interpreted the data, reviewed and edited the first draft of the manuscript and approved the final version. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
K.L.A.M. Kuypers is the recipient of an unrestricted research grant from Fisher & Paykel Healthcare Limited; they had no role in study design nor in the collection, analysis, and interpretation of data, writing of the report and decision to submit the paper for publication.
Ethics approval and consent to participate
Patient consent was not required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuypers, K.L.A.M., Kashyap, A.J., Cramer, S.J.E. et al. The effect of imposed resistance in neonatal resuscitators on pressure stability and peak flows: a bench test. Pediatr Res 94, 1929–1934 (2023). https://doi.org/10.1038/s41390-023-02715-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02715-x
- Springer Nature America, Inc.
This article is cited by
-
Slowing lung deflation by increasing the expiratory resistance enhances FRC in preterm rabbits
Pediatric Research (2024)