Abstract
Our aim was to compare the work of breathing (WOB) during synchronised nasal intermittent positive pressure ventilation (SNIPPV) and heated humidified high flow nasal cannula (HHHFNC) when used as post-extubation support in preterm infants. A randomised crossover study was undertaken of nine infants with a median gestational age of 27 (range 24–31) weeks and post-natal age of 7 (range 2–50) days. Infants were randomised to either SNIPPV or HHHFNC immediately following extubation. They were studied for 2 h on one mode and then switched to the other modality and studied for a further 2-h period. The work of breathing, assessed by measuring the pressure time product of the diaphragm (PTPdi), and thoracoabdominal asynchrony (TAA) were determined at the end of each 2-h period. The infants’ inspired oxygen requirement, oxygen saturation, heart rate and respiratory rate were also recorded. The median PTPdi was lower on SNIPPV than on HHHFNC (232 (range 130–352) versus 365 (range 136–449) cmH2O s/min, p = 0.0077), and there was less thoracoabdominal asynchrony (13.4 (range 8.5–41.6) versus 36.1 (range 4.3–50.4) degrees, p = 0.038).
Conclusion: In prematurely born infants, SNIPPV compared to HHHFNC post-extubation reduced the work of breathing and thoracoabdominal asynchrony.
What is Known: • The work of breathing and extubation failure are not significantly different in prematurely-born infants supported by HHHFNC or nCPAP. • SNIPPV reduces inspiratory effort and increases tidal volume and carbon dioxide exchange compared to nCPAP in prematurely born infants. | |
What is New: • SNIPPV, as compared to HHHFNC, reduced the work of breathing in prematurely-born infants studied post-extubation. • SNIPPV, as compared to HHHFNC, reduced thoracoabdominal asynchrony in prematurely born infants studied post-extubation. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mechanical ventilation can be life-saving in prematurely born infants, but prolonged ventilation is associated with the development of bronchopulmonary dysplasia (BPD) [3]. Post-extubation, practitioners use non-invasive respiratory support, but it is not clear which modality is most effective.
Synchronised nasal intermittent positive pressure ventilation (SNIPPV) is a non-invasive mode of ventilation. During the delivery of SNIPPV, the aim is to synchronise mechanical inflations with the infant’s breathing efforts. Synchronisation can be achieved either with a sensor that detects abdominal movement, a proximal flow sensor, or more recently by using the diaphragm electromyogram detected with an oesophageal catheter [11]. SNIPPV delivers positive pressure cycles over a continuous distending pressure and has been shown to increase the tidal volume and decrease the work of breathing in infants when compared with non-synchronised NIPPV and nasal continuous positive airway pressure (CPAP) [1, 15]. HHHFNC delivers heated and humidified air or oxygen at a flow rate higher than 2 L/min [14]. The proposed advantages of HHFNC include washout of anatomical deadspace, reduction in work of breathing and provision of a positive distending pressure [2, 8]. A Cochrane review demonstrated that HHHFNC was comparable to CPAP in reducing rates of extubation failure but had the advantage that nasal trauma occurred less frequently. It concluded, however, that further studies were needed to compare HHHFNC with other forms of respiratory support [22].
The work of breathing is particularly important in the post-extubation period, as common reasons for extubation failure include increased oxygen requirements and the development of a respiratory acidosis [6]. As a consequence, a lower work of breathing is an important consideration when choosing which non-invasive mode should be used to support preterm infants immediately after extubation. There are studies comparing work of breathing between HHHFNC and CPAP [9, 20] and SNIPPV and CPAP [1, 12]. Increasingly, however, practitioners are using HHHFNC in preference to CPAP as post-extubation support [21], but there are no studies which compare the work of breathing between SNIPPV and HHHFNC. The aim of this study, therefore, was to compare the work of breathing and thoracoabdominal asynchrony in prematurely born infants receiving SNIPPV and HHHFNC as post-extubation support.
Methods
Infants born at less than or equal to 32 weeks gestation at King’s College Hospital NHS Foundation Trust, London, who had been mechanically ventilated for at least 48 h and deemed by the clinical team to require non-invasive respiratory support post-extubation were eligible for the study. Those with major congenital abnormalities or any contraindications to SNIPPV or HHHFNC were excluded. Contraindications included nasal trauma and gastrointestinal surgery within the previous 7 days. The study was approved by the London Riverside Research Ethics Committee and parents gave informed, written consent for their infant to take part in the study.
Protocol
At the point of extubation, infants were randomised to either SNIPPV or HHHFNC for 2 h and then switched to the other mode for a further 2 h. A blocked random number sequence was generated using an online random number generator [19]. Allocations were concealed in order in consecutively numbered, sealed opaque envelopes by an independent researcher not associated with the study. During the study, infants were monitored continuously and observations (heart rate, respiratory rate, oxygen saturations and inspired oxygen fraction) were recorded at 10-min intervals by a member of the research team. During the last 5 min of each 2-h period, the work of breathing and thoracoabdominal asynchrony was assessed.
SNIPPV was delivered using a Giulia neonatal ventilator via nasal prongs (Ginevri Medical Technologies, Rome, Italy). Synchronisation was achieved via a flow sensor contained at the y piece, just proximal to the nasal prongs. The largest possible prongs were used to minimise nasal leak as per the manufacturer’s recommendation. Aside from provision of a pacifier, if the nurse caring for the infant felt it was appropriate, we did not attempt to affect leakage from the mouth. SNIPPV was delivered at peak inspiratory pressure (PIP)/positive end expiratory pressure (PEEP) of 14/5 cmH2O for infants weighing less than 1 kg and PIP/PEEP 16/5 cmH2O for infants weighing 1 kg or more as per unit protocol. The back-up rate was set at the same rate as the back-up rate during invasive ventilation prior to extubation. The trigger level was set at 0.1 L/min for all infants.
HHHFNC was delivered either by an Optiflow system (Fisher and Paykel Healthcare Limited, Auckland, New Zealand) or by the SLE 6000 ventilator (SLE Limited, Croydon, UK). Optiflow nasal prongs (Fisher and Paykel Healthcare Limited, Auckland, New Zealand) were used with both systems. The flow rate was set at 6 L/min for infants weighing less than 1 kg and 8 L/min for those weighing 1 kg or more. During both modes, the inspired oxygen fraction (FiO2) was titrated to maintain oxygen saturations between 92 and 96%.
Infants were positioned either prone or supine but remained in the same position throughout the study. Each infant acted as their own control; hence, the position of study did not bias whether one mode was better than another in an individual. Feeds and “cares” were carried out at least 30 min before each episode of recording.
Measurements
To assess the work of breathing, oesophageal and gastric pressures (Poes and Pgas) were measured using a dual-pressure transducer-tipped catheter (Gaeltec, Dunvegan, UK). To assess thoracoabdominal asynchrony (TAA), abdominal and rib cage movements were measured using uncalibrated respiratory inductance plethysmography (RIP) (Respitrace Model 10.9230, Ambulatory Monitoring, NY, USA). The catheter was inserted, and the RIP bands applied prior to extubation whilst the infant was still ventilated. During the last 5 min of each 2-h period, Poes, Pgas, abdominal movement (AB) and rib cage movement (RC) were recorded using Spectra software (Grove Medical, London, UK). Transdiaphragmatic pressure (Pdi) was calculated by digital subtraction of Poes from Pgas. The transdiaphragmatic pressure time product (PTPdi) was calculated using the area subtended by the Pdi waveform during inspiration, multiplied by the individual respiratory rate for each breath. The initial rise in Pdi was used to mark the beginning of inspiration whilst the end of inspiration was determined from the subsequent fall in rib cage movement as measured by uncalibrated RIP (Fig. 1). The data were exported and analysed using Labchart software (V.7.3.7 27, Powerlab 16SP, ADInstruments, Sydney, Australia). The mean PTPdi of the first 20 artefact-free breaths was calculated.
The upper trace is thoracic motion (rib cage movement) as documented using RIP. The lower trace is the transdiaphragmatic pressure (Pdi) signal which was obtained from digital subtraction of POES from Pgas. The PTPdi was calculated using the shaded area; that is the area subtended by the Pdi waveform during inspiration. The initial rise in Pdi was used to mark the beginning of inspiration and the end of inspiration, the subsequent fall in rib cage movement
TAA was calculated by plotting a Lissajous figure using the RC and AB movements in the first five artefact-free breaths of the final 5-min period on each mode. The criteria for breath selection were there were no artefacts from movement or peristalsis. Asynchrony between RC and AB movements were quantified by determining the phase angle comparing the difference between inspiratory and expiratory abdominal positions at mid-RC excursion (ABdiff) with the maximum abdominal excursion (ABmax). The phase angle θ was calculated as sin θ = ABdiff/ABmax. Respiratory rate, heart rate, oxygen saturation and the fraction of the inspired oxygen (FiO2) were recorded at 10-min intervals throughout the 2-h, and the results expressed as an average of each 2-h period. Any episode of desaturation, i.e., an SaO2 < 88% at any time was recorded.
Sample size
A sample size of 18 infants allowed detection of a difference in the WOB results of one standard deviation with 80% power and 5% significance between the two respiratory support modes. In a previous study [20], the standard deviation was 43 cmH2O s/min.
Statistics
The Wilcoxon signed rank test was used to compare the respiratory measures within infants by the two modes of respiratory support. Statistical analysis was carried out using IBM SPSS Statistics 14.
Results
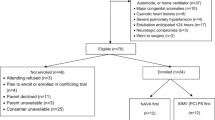
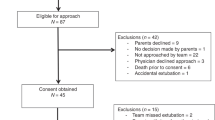
Parents of 32 eligible infants were approached to take part in the study, 21 agreed to recruitment and 9 were subsequently included in the study. Of those who consented but were not included, two infants self-extubated, two were excluded as the consultant in charge felt non-invasive respiratory support post-extubation was not appropriate, three infants were transferred back to their local hospitals whilst still ventilated, three infants were extubated when the research team were unavailable and two infants died before being extubated. Infants who were and were not included in the study did not differ significantly with regard to their maturity at birth (median 24 (range 23–27) weeks versus 27 (24–31) weeks (p = 0.058)) or birth weight (744 (455–1100) g versus 864 (617–1638) g (p = 0.148)).
After assessing nine infants, the researcher who analysed the traces was aware that all nine infants had a lower PTPdi (primary outcome) when supported by SNIPPV. The clinical team considered that the trial should stop, and an interim statistical analysis was conducted. This showed a highly significant difference (p = 0.0077) between the two modes of support in favour of SNIPPV, and hence, the study was terminated. Six (67%) of the nine infants were females, and all been exposed to at least one dose of antenatal corticosteroids and post-natal surfactant. All were receiving caffeine at the time of study. They were assessed at a median post-natal age of 7 (range 2–50) days and post-menstrual age of 30 (range 25–32) weeks. Four infants were studied first on SNIPPV and five first on HHHFNC; there were no significant differences between those first studied on SNIPPV or HHHFNC (Table 1). The PTPdi was significantly lower during SNIPPV than during HHHFNC (p = 0.0077), as was the TAA (p = 0.038). There were no significant differences in the FiO2, oxygen saturation, heart rate or respiratory rate between the two groups (Table 2).
Discussion
We have demonstrated that the work of breathing post-extubation in infants supported by SNIPPV was significantly less than that when using HHHFNC. It has previously been demonstrated that SNIPPV provided superior support to nCPAP post-extubation as indicated by higher tidal volumes, yet smaller oesophageal pressure deflections [13]. A crossover study, however, found no significant difference in the PTPdi between HHHFNC and nCPAP [11]. Our study adds to the literature by providing a comparison of SNIPPV and HHFNC. To our knowledge, this is the first study which has made such a comparison. Our results provide proof of principle that during SNIPPS “unloading” occurs. If the work of breathing is reduced because of unloading, then in preterm infants who are prone to chest wall distortion there would be a lower phase angle of the thoracic and abdominal component. Indeed, we demonstrated less TAA. There were no significant differences in the inspired oxygen concentration or oxygen saturation levels, but infants were studied for only 2 h on each mode.
Previous studies have reported difficulties using flow sensors in preterm neonates because of leak [4]. In this study, flow was measured by a pressure transducer contained within the y piece of the nasal prongs and interpreted by software which the manufacturer states stabilises the flow signal despite variation in air leak from the baby’s nose and mouth (Giulia neonatal ventilator, Ginevri, Italy). The reliability of the flow sensor contained in the Giulia has been assessed on a simulated neonatal model [13]. In that study, the flow sensor was reported to detect 100% of simulated spontaneous breaths in the presence of up to 46% leak from the prongs [13]. The device has been used in subsequent studies without issues regarding synchronisation being reported [5, 18]. Gizzi et al. highlighted that SNIPPV using the Guilia was superior to non-synchronised NIPPV in reducing desaturations, bradycardias and apnoeas in preterm infants [5]. Other studies have emphasised the importance of synchronised rather than non-synchronised NIPPV. Huang et al. found that synchronised NIPPV reduced respiratory effort and improved gas exchange when compared with non-synchronised NIPPV [7]. Furthermore, in a study researching the effects of NIPPV on the infant’s breathing, NIPPV was shown to only increase the tidal volume of individual breaths when the onset of the inflation coincided with spontaneous inspiration [17]. The provision, therefore, of inflations synchronised to the infants’ respiratory efforts, maybe responsible for the reduced work of breathing during SNIPPV. We were, however, unable to assess this, as the infants were studied when extubated, and thus, it was not possible to make measurements from the airway. An alternative explanation is that SNIPPV may have provided a higher distending pressure than HHHFNC.
Whilst we have demonstrated a lower work of breathing on SNIPPV compared to HHFNC, but access to SNIPPV may be limited. In a survey of UK practice in 2008, only 37% of tertiary NICUs were using synchronised SNIPPV [16]. Subsequently, there have been 10 RCTs or quasi-randomised trials involving 1431 infants post-extubation. The systemic review demonstrated NIPPV was associated with a significant reduction in need for reintubation and air leaks [10]. In addition, ten trials have examined early NIPPV versus CPAP in the first 6 h after birth and demonstrated a significantly reduced risk of requiring intubation [10]. Those data and ours suggest neonatal practitioners should consider NIPPV in their suite of respiratory modes.
There are strengths and some limitations to this study. It is the first study to compare the work of breathing during SNIPPV to that on HHHFNC. Our protocol did not include assessment of carbon dioxide or blood pressure levels, but we did demonstrate no significant differences in the number of desaturations between the two groups. The study was terminated when only half the planned sample size had been studied, as in all nine of those infants the WOB were lower during SNIPPV than during HHHFNC. The researcher who analysed the WOB and TAA traces was aware of the modalities and that previous studies had been terminated half way through when consistently one modality proved superior to the other. This may have introduced bias, but there are no significant differences in the demographics according to order of mode studies and infants were studied on both modalities. It is also possible that the finding is, therefore, a type I error, but statistically that is very unlikely with such a small p value (0.0077) for the primary outcome. Thirty-two parents were approached, and 21 agreed for their infants to take part. Those not included compared to those included did not differ significantly with regard to their birth weight or gestational age. We do not present long-term outcome data, but the lower work of breathing on SNIPPV is likely to be advantageous.
In conclusion, we have demonstrated that SNIPPV compared to HHHFNC significantly reduced the work of breathing and TAA in preterm infants studied immediately post-extubation.
Abbreviations
- AB:
-
Abdominal movement
- ABdiff:
-
Difference between inspiratory and expiratory abdominal positions at mid-RC excursion
- ABmax:
-
Maximum abdominal excursion
- BPD:
-
Bronchopulmonary dysplasia
- CPAP:
-
Continuous positive airway pressure
- FiO2 :
-
Inspired oxygen fraction
- HHHFNC:
-
Heated humidified high flow nasal cannula
- PdI:
-
Transdiaphragmatic pressure
- PEEP:
-
Positive end expiratory pressure
- Pgas:
-
Gastric pressures
- PIP:
-
Peak inspiratory pressures
- Poes:
-
Oesophageal pressure
- PTPdi :
-
Pressure time product of the diaphragm
- RC:
-
Rib cage movement
- RIP:
-
Respiratory inductance plethysmography
- SNIPPV:
-
Synchronised nasal intermittent positive pressure ventilation
- TAA:
-
Thoracoabdominal asynchrony
- WOB:
-
Work of breathing
References
Chang HY, Claure N, D’ugard C, Torres J, Nwajei P, Bancalari E (2011) Effects of synchronization during nasal ventilation in clinically stable preterm infants. Pediatr Res 69:84–89
Dysart K, Miller TL, Wolfson MR, Shaffer TH (2009) Research in high flow therapy: mechanisms of action. Respir Med 103:1400–1405
Fischer H, Buehrer C (2013) Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 132:e1351–e1360
Fischer HS, Roehr CC, Proquitte H, Hammer H, Wauer RR, Schmalisch G (2009) Is volume and leak monitoring feasible during nasopharyngeal continuous positive airway pressure in neonates? Intensive Care Med 35:1934–1941
Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, Papoff P, Moretti C, Agostino R (2015) Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)?. A randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed 100:F17–F23
Hermeto F, Martins BM, Ramos JR, Bhering CA, Sant'Anna GM (2009) Incidence and main risk factors associated with extubation failure in newborns with birth weight< 1,250 grams. J Pediatr 85:397–402
Huang L, Mendler MR, Waitz M, Schmid M, Hassan MA, Hummler HD (2015) Effects of synchronization during noninvasive intermittent mandatory ventilation in preterm infants with respiratory distress syndrome immediately after extubation. Neonatology 108:108–114
Lampland AL, Plumm B, Meyers P, Worwa CT, Mammel MC (2009) Observational study of humidified high-flow nasal cannula compared with nasal continuous positive airway pressure. J Pediatr 154:177–182
Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, Mosca F, Dellacà RL (2014) Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch Dis Child Fetal Neonatal Ed 99:F315–F320
Leymyre B, Davis PG, De Paoli AG, Kirpalani H (2017) Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rec 2:CD003212
Mahmoud RA, Roehr CC, Schmalisch G (2011) Current methods of non-invasive ventilatory support for neonates. Paediatr Respir Rev 12:196–205
Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, Bucci G (1999) Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Hum Dev 56:167–177
Moretti C, Papoff P, Gizzi C, Montecchia F, Giannini L, Fassi C, Midulla F, Agostino R, Sanchez-Luna M (2013) Flow-synchronized nasal intermittent positive pressure ventilation in the preterm infant: development of a project. JPNIM 2:e020211
Ojha S, Gridley E, Dorling J (2013) Use of heated humidified high-flow nasal cannula oxygen in neonates: a UK wide survey. Acta Paediatr 102:249–253
Owen LS, Manley BJ (2016) Nasal intermittent positive pressure ventilation in preterm infants: equipment, evidence, and synchronization. Sem Fetal Neonatal Med 21:146–153
Owen LS, Morley CJ, Davis PG (2008) Neonatal nasal intermittent positive pressure ventilation: a survey of practice in England. Arch Dis Child Fetal Neonatal Ed 93:F148–F150
Owen LS, Morley CJ, Dawson JA, Davis PG (2011) Effects of non-synchronised nasal intermittent positive pressure ventilation on spontaneous breathing in preterm infants. Arch Dis Child Fetal Neonatal Ed 96:F422–F428
Ramos-Navarro C, Sanchez-Luna M, Sanz-Lopez E, Maderuelo-Rodriguez E, Zamora-Flores E (2016) Effectiveness of synchronized noninvasive ventilation to prevent intubation in preterm infants. AJP Rep 6:e264–e271
S.E. Ltd. Create a blocked randomisation list. 2016. Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists
Shetty S, Hickey A, Rafferty GF, Peacock JL, Greenough A (2016) Work of breathing during CPAP and heated humidified high-flow nasal cannula. Arch Dis Child Fetal Neonatal Ed 101:F404–F407
Shetty S, Sundaresan A, Hunt K, Desai P, Greenough A (2016) Changes in the use of humidified high flow nasal cannula oxygen. Arch Dis Child Fetal Neonatal Ed 101:F371–F372
Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG, Manley BJ (2016) High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev 2:CD006405
Funding
Dr. Hunt was supported by the Charles Wolfson Charitable Trust and additionally by SLE. Dr. Elinor Charles was supported by the Isaac Shapera Fund. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Professor Janet Peacock is an NIHR Senior Investigator. The Guilia ventilated was loaned to us by Gineviri Medical Technologies (see comments re competing interests).
Author information
Authors and Affiliations
Contributions
AG, KAH and GFR designed the study. EC and KAH collected the data. JFP, EC, KAH and AG analysed the data. All authors were involved in the production of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Professor Greenough has held grants from various manufacturers (Abbot Laboratories, MedImmune) and ventilator manufacturers (SLE). Professor Greenough has received honoraria for giving lectures and advising various manufacturers (Abbot Laboratories, MedImmune) and ventilator manufacturers (SLE). Professor Greenough is currently receiving a non-conditional educational grant from SLE. The Guilia ventilator was loaded to us by Ginevri Medical Technologies; they have not been involved in the data collection analysis or production of the manuscript.
Informed consent
Infants whose parents gave informed written consent were enrolled into the study.
Additional information
Communicated by Patrick Van Reempts
Elinor Charles and Katie A Hunt are joint first authors.
Rights and permissions
About this article
Cite this article
Charles, E., Hunt, K.A., Rafferty, G.F. et al. Work of breathing during HHHFNC and synchronised NIPPV following extubation. Eur J Pediatr 178, 105–110 (2019). https://doi.org/10.1007/s00431-018-3254-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-018-3254-3