Abstract
Neurotransmitters are conventionally viewed as nerve-secreted substances that mediate the stimulatory or inhibitory neuronal functions through binding to their respective receptors. In the past decades, many novel discoveries come to light elucidating the regulatory roles of neurotransmitters in the physiological and pathological functions of tissues and organs. Notably, emerging data suggest that cancer cells take advantage of the neurotransmitters-initiated signaling pathway to activate uncontrolled proliferation and dissemination. In addition, neurotransmitters can affect immune cells and endothelial cells in the tumor microenvironment to promote tumor progression. Therefore, a better understanding of the mechanisms underlying neurotransmitter function in tumorigenesis, angiogenesis, and inflammation is expected to enable the development of the next generation of antitumor therapies. Here, we summarize the recent important studies on the different neurotransmitters, their respective receptors, target cells, as well as pro/antitumor activity of specific neurotransmitter/receptor axis in cancers and provide perspectives and insights regarding the rationales and strategies of targeting neurotransmitter system to cancer treatment.
Similar content being viewed by others
Introduction
Neurotransmitters released from peripheral and autonomic nerves play a very wide spectrum of activities in the signaling from the cells of the nervous system to target cells through binding to their respective receptors. Based on their specific chemical structure, neurotransmitters are divided into three categories: (1) amino acids, including acetylcholine (Ach), glutamate, glycine, and gamma-aminobutyric acid (GABA); (2) biogenic amines, including dopamine, norepinephrine (NE), epinephrine (E), and serotonin; (3) peptidergic neurotransmitters termed neuropeptide, including but not limited to substance P (SP), neuropeptide Y (NPY), opioids, calcitonin gene related peptide (CGRP), vasoactive intestinal polypeptide (VIP), bombasin, neurotensin, and many others.
In recent years, neurotransmitters emerged as an essential microenvironmental component in regulating tissue homeostasis and influencing diverse malignant phenotypes of human cancers [1, 2]. Neurotransmitters can not only be released by autonomic nervous system from the brain, peripheral plexuses, ganglia, and adrenal medulla, but also be produced by cancer cells and immune cells. Thus, neurotransmitters might affect cancer cells and immune cells in an autocrine/paracrine manner. Similar to the processes of neoangiogenesis and lymphangiogenesis, growing evidence provide the possibility of formation of new nerve endings within the tumors, a phenomenon termed as neoneurogenesis [1, 3]. Nerve fibers-derived neurotransmitters liberated in the tumor microenvironment activate tumor cells through binding specific neurotransmitter receptors [4]. This process further expanded our knowledge of the complex network of neurotransmitters related to tumor progression. In addition, immune cells and endothelial cells infiltrated in the tumor microenvironment likewise express diverse neurotransmitter receptors and react with neurotransmitters, and are known to have a strong impact on the outcome of human cancers [5]. Notably, many neurotransmitters and/or their analogs or antagonist/agonist for their receptors have medicinal properties are served as drugs for various diseases including cancers. In the following section, we will describe the implication of several classical neurotransmitters and neuropeptides on the cancer and the tumor microenvironment, and we will also discuss the possibility of their inhibition as potential therapy.
Epinephrine and norepinephrine
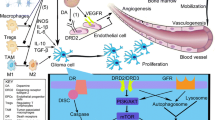
Clinical and epidemiological studies have extensively identified stress and chronic depression as cancer risk factors. Growing evidence supports a longstanding hypothesis that chronic stress can affect tumor initiation and progression [6,7,8]. Catecholamines, including dopamine, E, and NE, are also known as stress neurohormones because of their circulation levels are remarkably increased during psychological stress. E and NE, derived from the amino acid tyrosine and released primarily from the adrenal medulla and the sympathetic nerves, are the best-characterized and well-studied neurotransmitters. The effects of E and NE are mediated by interactions with alpha (α)- and beta (β)-adrenergic receptors, which are 7-transmembrane G-protein-coupled receptors and widely expressed in most of mammalian tissues. E and NE are profoundly implicated in multiple biological behaviors of cancers, including cancer cell survival, proliferation, resistance to apoptosis, invasion, metastasis, angiogenesis, and stromal compartments in the tumor microenvironment [9,10,11] (Fig. 1a). The tumor growth and angiogenesis induced by chronic stress can be mimicked by a β-adrenergic agonist, isoproterenol, and blocked by its antagonist, propranolol [12]. NE can stimulate endothelial cell metabolism toward the inhibition of oxidative phosphorylation and the induction of an angiogenic switch that fuels cancer progression [13, 14]. There are several mechanisms underlying the tumor-promoting roles of E and NE. Activation of β2-adrenoceptor (AR) promotes tumor growth and angiogenesis through increasing the expression of vascular endothelial growth factor (VEGF), metalloproteases 2 (MMP2), and MMP9, which further potentiate the angiogenic and metastatic processes in overian cancer, lung cancer, and breast cancer [12]. These effects are largely mediated by β-AR-dependent increase in cAMP levels and subsequent activation of PKA, which executes relevant functional regulations through phosphorylating downstream targets, such as cAMP response element binding protein (CREB), nuclear factor kappa B, and activator protein 1 [15]. Through transactivation of extracellular signal-regulated kinase (ERK)/cyclooxygenase 2 (COX2) signaling pathway, β-AR facilitates the cell proliferation of esophageal squamous cell carcinoma [16]. In pancreatic cancer, catecholamines promote β-AR-dependent secretion of neurotrophins, which in turn increase NE level and facilitate tumor growth [11]. In addition, we have previously demonstrated that NE induces hepatocellular carcinoma invasion and anoikis resistance through β-AR-mediated EGFR transactivation [17]. Collectively, these and other numerous studies provide solid data that E and/or NE are profoundly implicated in the tumor growth and progression on a variety of cancer types. Recent findings shed light on the impact of nerve fibers-derived autonomic neurotransmitters on cancer cells. However, the contribution and clinical relevance of circulating NE and E in cancers is largely unknown. Further preclinical and clinical studies in this aspect will help to fully uncover the molecular mechanism of autonomic neurotransmitters at both system and microenvironmental level.
Schematic representation of different neurotransmitters, their respective receptors, target cells as well as pro/antitumor activity of specific neurotransmitter/ receptor axis. a Norepinephrine (NE) and Epinephrine (E) activate their β-adrenoreceptors (ARs) expressed on cancer cells and immune cells to promote tumor malignancies and inflammation. Moreover, NE/E can directly induce endothelial cell (EC) metabolic switch to via β-AR to increase tumor vascularization. b Dopamine exhibits a conflicting effect regarding the influence of dopamine receptors (DRs) activation on cancers indicative of the tumor-specific roles of DRs in cancers. Both D1-like DR and D2-like DR are expressed by immune cells and endothelial cells to inhibit pro-tumor immune response and angiogenesis. c Activation of GABAA receptors stimulates tumor cell proliferation and migration as well as migration, while activation of GABAB receptor leads to inhibitory effects on tumor development. Activation of GABAA receptors also regulates macrophage recruitment and T cell-dependent cytotoxicity in cancers. d Diverse 5-HT receptors expressed by cancer cells, immune cells, and endothelial cells finally accelerate tumor growth, angiogenesis, as well as metastasis dissemination in many human cancers. e Both the nicotinic acetylcholine receptors (nAchRs) and muscarinic receptors (mAchRs) are expressed by cancer cells and endothelial cells and their activation are sufficient to promote oncogenic activities and angiogenesis. Activation of mAchRs in immune cells can promote macrophage recruitment and induce an anti-inflammatory response. f Activation of metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs) in cancer cells and endothelial cells contributes to increased cancer cell proliferation, migration, invasion, differentiation, and angiogenesis in several human cancers. In addition, mGluRs is expressed by myeloid-derived suppressor cells (MDSC) and is essential to its immune-suppressive effect
Escape from immune surveillance is one of the most critical steps that ensure proper establishment and growth of the formed tumor. Apart from function as physiological or pharmacological stimuli to form a tumor-promoting character in the tumor microenvironment, E and NE have been shown to influence inflammatory immune cells in cancers. β-ARs are present in both helper and T suppressor lymphocytes, B lymphocytes, NK cells, macrophage, and dendritic cells [5]. NE can increase the production of proinflammatory cytokines IL-8, which in turn stimulate the growth of ovarian cancer [18]. Endogenous E together with prostaglandins can decrease NK cell activities and reduction of antitumor resistance and thereby promote leukemia progression [19]. In addition, β-AR-mediated hormone signaling reduces the deformability of macrophages [20] and regulates integrin activation of human antigen-specific T cells [21]. Activation of β-AR signaling is sufficient to increase the infiltration of CD11b(+) F4/80(+) macrophages into primary tumor parenchyma and thereby induce a prometastatic gene expression signature accompanied by indications of M2 macrophage differentiation [22]. Interestingly, enriched environment enhances NK cell activity and promotes infiltration of NK cells in the tumor microenvironment; blocking β-AR signaling or chemical sympathectomy effectively abolishes the effects of enriched environment on NK cells and attenuates the antitumor effect of enriched environment [23]. Collectively, these findings suggest the emerging roles of β-AR signaling in modulating tumor immunity. Meanwhile, targeting the β-AR signaling in immune cells may serve for new therapeutic avenues to improve T-cell or NK cell eradication of cancer.
Epidemiological studies support the hypothesis that cardiovascular patients treated with β-AR antagonists (β-blockers) have reduced incidence of cancer. A large case–control study among patients with cardiovascular disease revealed that β-blockers were correlated with a reduction in cancer occurrence [24, 25]. In the different classes of antihypertensives, only β-blockers had a significant association with lower risk of prostate cancer. Furthermore, exposure to β-blockers may reduce tumor progression in established cancers. In breast cancer, cardiovascular patients receiving β-blockers have a remarkable reduction in metastasis development, tumor recurrence, and cancer-related mortality [26,27,28,29]. In addition, β-blockers also improve the relapse-free survival in patients with breast cancer and melanoma [30, 31]. However, conflicting findings have been reported. Several clinical studies pointed out that treatment of β-blockers had no beneficial effects on patients with lung, breast, and colorectal cancer, and even produced adverse effects on the overall survival in patients with prostate and pancreatic cancer [32]. The cardioselective β1-blockers, such as metoprolol, bisoprolol, and atenolol, showed no significant associations with cancer incidence and mortality [33]. From the prevention and therapeutic point of view, it is interesting to consider the potential application of β-blockers in cancer therapy because of β-blockers are clinically well characterized and have been safely administered as therapeutics for cardiovascular diseases. Although controversial conclusions are present for the therapeutic potential of β-blockers in cancers, some important issues should be addressed by future researches, including but not confined to β-ARs expression, tumor types and stages, blocker sensitivity, and microenvironmental factors. In addition, it is time to launch a well-designed and multicenter clinical trial to confirm the exact role of β-blockers in cancer patients and to test β-blockers in adjuvant treatment of relevant cancers.
Dopamine
Dopamine is a precursor for the synthesis of E and NE. It is also an important neurotransmitter in the regulation of several key functions, such as behavior, control of movement, endocrine regulation, and cardiovascular functions. Dopamine exerts its function via binding to five different seven-transmembrane G-protein-coupled receptors, which are divided into two classes: D1-like dopamine receptors (DRs) and D2-like DRs. The D1-like family (D1 and D5 receptors) is coupled to Gs receptors and the D2-like family (D2, D3, and D4 receptors) is comprised of Gi/o receptors. Dopamine or DR agonists seem to exhibit inhibitory effect on tumor growth, including breast cancer, gastric cancer, and sarcoma [34]. However, dopamine fails to diminish the proliferation and invasion of breast and colon cancer cells [35], indicating that difference in outcome might be the consequence of different tumor types, DRs expressed, and doses used. A main mechanism of tumor-suppressive effect is associated with decreased angiogenesis [36]. In an elegant study dopamine was shown to inhibit endothelial progenitor cell mobilization from the bone marrow through restraining the VEGFA-mediated ERK1/2 phosphorylation [37]. Recently, several studies reported the oncogenic activities of DRs with regard to migration or other effects on cancer cells. Activation of DRD2 inhibits proliferation, clonogenic ability, and invasiveness of these cells in non-small cell lung cancer [38]. In glioblastoma, inhibition of DRD4 impedes autophagic flux, proliferation, and survival of cancer stem cells [39]. However, inhibition of DRD2 reduces pancreatic cancer cell proliferation and migration, and slows growth of xenograft tumors in mice [40]. Activation of the DRD1/cGMP/PKG pathway induces growth arrest in vitro and causes tumor shrinkage and reduced bone metastasis in breast cancer [41]. There is conflicting evidence regarding the influence of DR activation on cancers, suggesting that the roles of DRs in cancers might be tumor specific (Fig. 1b). Interestingly, dopamine is involved in antagonizing the carcinogenic action of adrenergic system, whether β-AR signaling is affected by dopamine in cancer cells and the detailed molecular mechanism warrants further investigations. Taken together, all these findings would make dopamine and its receptors as the potential targets for cancer diagnosis and therapy.
Dopamine is critically involved in the neural-immune communication by acting through its receptors present in immune cells in an autocrine/paracrine manner [42]. The antitumoral effects of dopamine can be manifested by regulation of diverse immune competent cells within the tumor microenvironment. Dopamine is able to inhibit the function of Gr-1+CD115+ myeloid-derived suppressor cells through D1-like receptors and enhance antitumor immunity [43]. Moreover, dopamine can regulate peritoneal macrophages and CD4+CD25+ regulatory T lymphocytes (Tregs) to promote tumor progression [44]. Therefore, the inhibitory role of dopamine on Treg function supports dopaminergic pathways as a druggable target to develop innovative antitumor strategies [45].
The existence of different subtypes of DRs in the cancer cells and immune effector cells suggest the roles of dopamine in the regulation of tumor development. Agonists/antagonists for DRs hold considerable promise as therapeutic drugs in cancer treatments. The DRD2 receptor agonist is available in clinical use for the treatment of hypertension. Therefore, these safe and effective with manageable side effects can be taken consideration into future clinical trials for the treatment of cancer.
Gamma-aminobutyric acid
GABA is the major inhibitory neurotransmitter for cells of the central nervous system (CNS) in adult mammals. Three different types of receptors for GABA (A, B, and C) have been identified: the ionotropic GABAA and GABAC receptors and the metabotropic GABAB receptor. The oligomeric chloride channel GABAA and GABAC are heteromeric complexes composed of five subunits, while the GABAB receptor is a member of the serpentine coupled to adenylyl cyclase. GABA receptors have been detected in many tumor tissues and exert regulative effects in cancer cell proliferation and migration [46,47,48] (Fig. 1c). GABA is mainly derived from cancer cells and GABA content is increased in several types of human tumors, including glioma, gastric cancer, ovarian cancer, and breast cancer [47, 49,50,51,52]. GABAA receptor is upregulated in prostate cancer, breast cancer, and pancreatic cancer [53,54,55], while GABAB receptor is downregulated in liver cancer and pancreatic cancer [56, 57]. In most cases, GABA stimulates cancer cell proliferation through the GABAA receptor pathway and inhibits cancer cell growth through the GABAB receptor [48]. The GABAA receptor agonist muscimol increases cell proliferation of gastric cancer cells by activating mitogen-activated protein kinases (MAPK). Similarly, GABA through overexpressing a subunit of GABAA, GABRP, increases intracellular Ca2+ levels and MAPK/ERK cascade, and stimulates pancreatic cancer growth [58]. Adversely, activation of GABAB receptors strongly inhibits isoproterenol-induced cAMP, p-CREB, cAMP response element-luciferase activity, and ERK1/2 phosphorylation, and effectively blocks DNA synthesis and cell migration [59]. However, GABA or GABAB agonist baclofen has been reported to promote the invasive ability of prostate cancer cells through increasing EGFR transactivation [60]. These findings suggest that different effects of GABA activation on cancer growth/migration might be cancer-specific or GABA receptor type-dependent. Different from the mechanism mentioned above, our recent study showed that the π subunit of GABAA receptor promotes pancreatic cancer progression through tuning KCNN4-mediated Ca2+ in a GABA-independent manner [54]. Intriguingly, GABA is present in the tumor microenvironment raises the possibility that GABA might modulate the inflammatory response by targeting the infiltrated immune cells [54].
The GABAergic signaling system is critically implicated in the immune system in response to diverse inflammatory diseases and affects a variety of functional properties of the immune cells like antigen-induced T-cell proliferation, LPS-induced cytokine release, the cytotoxicity of effector T-cells activity, and chemotaxis [61, 62]. GABA also modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits [63]. Recently, our findings revealed that GABRP regulates macrophage recruitment in pancreatic cancer by upregulating expression of CXCL5 and CCL20 [54]. Nevertheless, much remains to be identified, as little is known about the roles and mechanisms underlying GABA and the GABA signaling system in the immune cells with the tumor microenvironment.
These findings above demonstrate that GABAergic system could reflect an antitumor activity by modulation of cancer cells and inflammatory response, and therefore point towards the GABAergic system as potential therapeutic target. Actually, GABA is widely used in medicine as a hypotension inducer, tranquilizer, and antidiabetic agent [64]. In addition, some GABA receptor agents can be used for addiction treatment and sedation [65]. However, observational epidemiological studies showed benzodiazepine use increased the risk of breast cancer, brain cancer, esophagus cancer, renal cancer, prostate cancer, liver cancer, gastric cancer, pancreatic cancer, and lung cancer in a dose-dependent manner, and no significant association was found in ovarian cancer, malignant melanoma, and colon cancer [66]. Therefore, the consideration of GABAergic agents for translational application in cancer therapy warrants further investigations.
Serotonin
Serotonin (5-Hydroxytryptamine, 5-HT) is a neurotransmitter synthesized in the serotonergic neurons in the brain and in the enterochromaffin cells of the intestine. Enterochromaffin cells contributes to more than 90% of the body’s 5-HT and is the main source of peripheral 5-HT, which is then stored at platelets. 5-HT plays critical cognitive and behavioral functions in humans, including memory, mood, emotions, wakefulness, sleep, appetite, and temperature. 5-HT serves numerous important peripheral functions, such as platelet aggregation, immune response, bone development, insulin secretion, and systemic energy homeostasis [67]. 5-HT exerts its multiple functions through interaction with a multiplicity of receptors coupled to various signaling pathway. To date, seven different subtypes of receptors (5-HT1–7) have been identified. Except for 5-HT3, a ligand-gated ion channel, all of the 5-HT receptors belong to the family of G-protein-coupled receptors. Specially, 5-HT1 and 5-HT5 receptors are Gi/o coupled to adenylyl cyclase and downregulate cAMP. 5-HT2 receptors are Gq/11 coupled to phospholipase C and upregulate diacylglycerol (DAG) and inositol triphosphate, resulting in intracellular Ca2+ release. The 5-HT5 receptor is a pseudogene. 5-HT4, 5-HT6, and 5-HT7 receptors are Gs coupled to adenylyl cyclase and upregulate cAMP. In addition to its known functions as a neurotransmitter, 5-HT is identified as a potent mitogenic factor for many types of tumor cells and nontumoral cells, such as fibroblasts, smooth muscle cells, osteoblasts, mesangial cells, and endothelial cells [68, 69]. Dysregulation of epithelial homeostatic systems is responsible for the initiation and development of cancers. 5-HT is known to regulate epithelial homeostasis of the mammary, lung, pancreas, liver, and prostate. Dysregulated 5-HT signaling is frequently observed in epithelial tumors [70, 71]. Emerging data have elucidated the tumor biology of 5-HT. 5-HT was also found to promote cell proliferation in prostate cancer, breast cancer, and melanoma via different 5-HT receptors [72,73,74]. In liver cancer, 5-HT promotes tumor growth by inhibiting autophagy and inhibition of 5-HT signaling by targeting 5-HT2B receptor consistently impairs tumor growth [75]. Recently, we demonstrated that human pancreatic cancer tissues have increased levels of 5-HT, and pancreatic cancer cells increase expression of its receptor, HTR2B. These increases allow for tumor glycolysis under metabolic stress and promote growth of pancreatic cancer [76]. Furthermore, platelet-derived 5-HT also promotes tumor angiogenesis, tumor growth, and the metastatic potential of cancer cells [77]. Notably, depletion of 5-HT and selective inhibition of 5-HT2B receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and p-ERK1/2 [78]. These investigations suggest that 5-HT signaling is critically involved in the development and progression of cancers (Fig. 1d). Notably, 5-HT is a substrate for protein posttranslational modification, known as serotonylation [79]. Therefore, the serotonylation process might be a novel molecular mechanism underlying 5-HT-mediated oncogenic functions.
5-HT is a versatile molecule in modulating immunological functions [80]. It regulates diverse immune processes, such as chemotaxis, leukocyte activation, proliferation, cytokine secretion, and anergy. These mechanisms are cell-specific and depend on three major components of 5-HT system: (1) membrane receptors that regulate the response to 5-HT, such as SERT and 5-HTR; (2) downstream transduction signals; and (3) enzymes responsible for 5-HT metabolism, such as indoleamine 2,3-Dioxygenase 1 and monoamine oxidase, which can generate biologically active catabolites, including kynurenines and kynurenamines. However, the role of serotonergic system in tumor microenvironment is largely unexplored. In mouse models of melanoma, the antidepressants selective serotonin reuptake inhibitor (SSRI) treatment exhibits an anticancer effect by influencing cytokine secretion [81]. The SSRI fluoxetine treatment increases the number of breast cancer brain metastases, an effect accompanied by proinflammatory changes in the brain [82]. In addition, 5-HT promotes angiogenesis by influencing MMP-12 expression in tumor-infiltrating macrophages, thereby affecting the production of circulating angiostatin [83]. Although further investigations are required, these findings suggest that the 5-HT system can impact cancer cells directly or indirectly through its immunomodulatory functions.
As accumulating evidences have demonstrated the role of 5-HT signaling in cancers, therapeutic approaches targeting 5-HT signaling are of great translational significance [70]. Diverse agonists and antagonists are available for 5-HT signaling. Drugs used in neurological disorders, such as paliperidone, pimozide, and risperidone, the potent and well-tolerated inhibitors at 5-HT7, are under investigation for glioblastoma treatment [84]. Moreover, cyproheptadine, a 5-HT antagonist, has recently been reported to exhibit antitumor activity in urothelial carcinoma cells by targeting GSK3β to suppress mTOR and β-catenin signaling pathways [85]. Notably, our group has demonstrated that SB204741, a specific antagonist for HTR2B, showed significantly inhibitory effects on tumor growth of pancreatic cancer by suppressing Warburg effect [76]. Thus, pharmacological regulation of the 5-HT system may represent a promising antitumor avenue through inhibiting the respective 5-HT tumorigenic actions, and provide therapeutic alternatives for these deadly diseases.
Acetylcholine
Ach, synthesized from choline and acetylCoA by the choline acetyltransferase enzyme, is a well-known neurotransmitter of the cholinergic system. Besides neurons, Ach synthesis has been found in a variety of nonneuronal cells, including epithelial (airways, alimentary tract, urogenital tract, and epidermis), mesothelial (pleura and pericardium), endothelial cell, adipocytes, fibroblasts, immune cells, and cancer cells [86]. The nonneuronal Ach is known to participate in cell proliferation, migration, differentiation, apoptosis, angiogenesis, secretion, cytoskeletal, and immune functions [87] (Fig. 1e). Therefore, tumor cells-derived Ach can act as an autocrine growth factor to promote tumor progression through binding to their receptors. Two classes of Ach receptors, the nicotinic acetylcholine receptors (nAchRs), and muscarinic receptors (mAchRs), have been identified. The nAchR, figured by different alpha (α2–10) and beta (β2–4) subunits, is a Ca2+ or Na+ ion channel comprised of five homolog transmembrane proteins symmetrically arranged from a central cation-selective pore. The mAchR belongs to the superfamily of GPCRs that activates second messenger pathways. Five subtypes of mAchR have been identified, M1–M5. The M1, M3, and M5 subtypes of mAchRs belong to Gq/11 family, whereas the M2 and M4 subtypes are related to Gi/o family.
A great number of researches have documented that 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, a tobacco-specific nitrosamine) act as a carcinogen in promoting the initiation and progression of various cancers through nAchRs, such as lung cancer, gastric cancer, pancreatic cancer, and breast cancer [88, 89]. Activation of nAchR results in Ca2+ flows inside the cell and contributes to cell proliferation, differentiation, epithelial-mesenchymal transition, angiogenesis, migration, and invasion [90, 91]. The activation of nAchR is also able to crosstalk with other neurotransmitter receptors and in turn induces the activation of multiple cascades including PKC/ERK1/2, COX2, PGE2, CREB, SRC, AKT, Ras-RAF1, and the MAPK cascade, which ultimately contributed diverse malignant phenotypes in cancers [92]. Specifically, nAchRs can inhibit drug-induced apoptosis through upregulating survivin, X-liked inhibitor of apoptosis protein, BCL-2, and NF-κB in pleural mesothelioma and breast cancer. Moreover, dysregulation of mAchRs is profoundly involved in the progression of different cancers. Ach can stimulate NSCLC cell proliferation through M3R-mediated activation of Akt and MAPK [93]. Administration of a muscarinic agonist suppresses pancreatic cancer tumorigenesis through inhibition of MAPK and PI3K/AKT signaling [94]. In gastric cancer, Dclk1+ tuft cells and nerve fibers-released Ach activates M3R to promote tumor progression by stimulating EGFR signaling [95] and Wnt and YAP pathways [96]. Consistently, activation of M3R can facilitate tumor invasion and metastasis by upregulation of MMPs in colon cancer [93]. These studies suggest a therapeutic potential for targeting M3R in cancers. Indeed, pharmacological inhibition or genetic knockout of the M3R in gastric cancer leads to inhibition of Wnt signaling and suppression of stem cell expansion [97].
Ach is profoundly involved in the modulation of inflammatory response [98]. Actually, elegant studies have defined the “cholinergic anti-inflammatory pathway” that highlights a unique role for the vagus nerve to inhibit the proinflammatory cytokine production [99]. Mast cells, macrophages, dendritic cells, mononuclear lymphocytes, neutrophils, and eosinophils are endowed with mAChRs and nAChRs, which can be activated via both autocrine and paracrine mechanisms [98]. The implications of cholinergic system in immune cell have been broadly demonstrated. However, limited evidence exists regarding the role of Ach on immune response at the level of tumor microenvironment. In a mouse model of pancreatic cancer, treatment with bethanechol suppresses TNFα levels in the spleen and circulation and reduces number of CD11b+ myeloid cells in the pancreata, suggesting that enhanced cholinergic signaling contributes to an antitumor immune microenvironment in pancreatic cancer [94].
For cancer pharmacotherapy, both the nicotinic and mAchR antagonists are under investigation [100]. Different AChRs have been identified in specific cancer cell types. The α7-nAChR and M3-mAChR are the most common Ach receptors in cancers. Different approaches have been developed to inhibit or modulate α7-nAChR activity including gene silencing, antagonists (D-tubocurarine and α-bungarotoxin), and allosteric drugs (SLURP1) [101]. And all these methods are sufficient to block the nAChRs-downstream signaling. Despite the presence of solid basis to exploit α7-nAChR antagonists for cancer treatment, more investigations are needed before potential clinical utilization, especially its pharmacological efficiency in preclinical models. Meanwhile, inhibition of M3-mAChR by antisense or antagonists (darifenacin, tiotropium, and others) reduces cell proliferation of colon cancer [102]. However, several antagonists targeting M3-mAChR show similar equilibrium binding affinities to other mAChRs. Therefore, new drugs displaying M3 kinetic selectivity may potentiate the treatment of a large variety of severe clinical diseases, especially cancers [102]. Taken together, targeting AChRs should have salient translational implications.
Glutamate
Glutamate is the most important excitatory neurotransmitter in the mammalian CNS, involved in affective, sensory, and motor function, as well as learning, memory, and synaptic plasticity. Glutamate is also actively involved in biosynthetic, bioenergetics, metabolic, and oncogenic signaling pathways [103, 104]. Glutamate receptors are divided into two main groups: the metabotropic glutamate receptors (mGluRs), which belong to the superfamily of GPCRs, and ionotropic glutamate receptors (iGluRs), which form ligand-gated ion channels. Based on sequence homology, pharmacological and intracellular signaling mechanisms, mGluRs are further categorized into three groups. Group I mGluRs: mGluR1 and mGluR5 are coupled to the Gq proteins and their activation stimulates PLC. In contrast, mGluRs of groups II (mGluR2 and mGluR 3) and III (mGluR4, mGluR6, mGluR7, and mGluR8) are all negatively coupled to adenylate cyclase. Similarly, the iGluRs comprise three subgroups based on structural similarities and named according to the type of synthetic agonist that activates them: N-methyl-D-aspartate receptors, amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors, and 2-carboxy-3-carboxymethyl-4-iso-propenylpyrrolidine (kainate) receptors.
In addition to synaptic transmission, aberrant glutamate signaling has been demonstrated to participate in the initiation and progression of a broad spectrum of cancers, including glioma, melanoma, breast cancer, and prostate cancer, suggesting the oncogenic functions of glutamate signaling in cancers [103,104,105,106,107]. For instance, triple-negative breast cancer cells secrete glutamate, which is both necessary and sufficient for the paracrine induction of HIF1α, resulting in tumorigenesis [105]. The glutamate-mGluR axis can activate phosphoinositide 3-kinase through phosphorylation of p110β to facilitate prostate cancer progression [107]. A tremendous amount of work has been emerged on the role of GluRs in the tumor biology (Fig. 1f). Although we will not focus on the relevance of mGluRs and iGluRs in cancer pathogenesis including expression pattern, signaling pathway, and therapeutic strategies, this issue has been addressed in detail in two recent review articles [103, 104]. In particular, glutamate affects the immune activity and GluRs are expressed in various immune cells; and the abnormal expression and function of the receptors in immune cells in many diseases, such as multiple sclerosis and amyotrophic lateral sclerosis, but not cancers [108]. Noteworthy, our knowledge achieved so far the opens up great opportunities for developing therapeutic agents targeting glutamate signaling, but further investigations are deserved to confirm the roles of glutamate signaling in immune microenvironment in tumors, which is crucial to the outcome of patients suffered from cancers.
Neuropeptides
Neuropeptides are small protein-like molecules used by neurons to communicate with each other. Different neuropeptides are involved in a wide range of brain functions, including analgesia, reward, food intake, metabolism, reproduction, social behaviors, learning, and memory. Neuropeptides can also function peripherally as paracrine and endocrine factors to regulate diverse physiologic processes, such as exocrine and endocrine secretion, smooth muscle contraction, pain transmission, fluid homeostasis, blood pressure, and inflammation [109]. Apart from these traditional roles, the tumor-promoting roles of neuropeptides are widely reported [1, 110]. Several neuropeptides have been well studied in cancers, especially SP and NPY [111, 112]. In most cases, the receptors of neuropeptides are members of the superfamily of GPCRs. For example, the biological action of SP is mainly mediated by the neurokinin-1 (NK-1) receptor, which is coupled to Gq family of G proteins and its activation leads to the formation of two-second messengers: inositol 1,4,5-triphosphate (IP3) and DAG [113].
The SP/NK-1 system is frequently dysregulated and widely involved in the pathogenesis of cancers. SP has been implicated in cell proliferation, apoptosis, angiogenesis, migration, and invasion of many cancers, including glioma, melanoma, osteosarcoma, colon, pancreatic, gastric, larynx, and lung carcinoma [111, 113]. Both in vitro and in vivo observations have demonstrated that pharmacological inhibition of NK-1 receptor with specific antagonists (aprepitant, fosaprepitant, L-732,138, and L-733,060) results in pronounced antitumor effects [114, 115]. Another neuropeptide, NPY, has been implicated as a growth-promoting factor in various cancers, including neuroblastoma, Ewing sarcoma, breast, and prostate cancer [112, 116]. In addition to the role of NPY on tumor growth and vascularization, emerging studies provide insight into the potential role of NPY in the metastatic and chemoresistant phenotype of cancer cells [117, 118]. Of particular importance are interactions of the NPY system with the tumor microenvironment. NPY released from tumor cells acts on its receptors expressed on the endothelial cells or immune cells, altering tumor-associated angiogenesis and inflammation and ultimately promoting tumor progression. Except for SP and NPY, neuropeptides including but not limited to opioids, CGRP, VIP, bombasin, and neurotensin have been broadly reported to be involved in cancer development [59, 92, 119,120,121,122]. While the investigations on deciphering the mechanisms by which neuropeptides modulate oncogenic processes underlying tumor progression is still in progress, the existing evidence eloquently support the promise of the neuropeptides and its receptors as therapeutic targets in oncology.
Neurotransmitter effects on the tumor microenvironment
The tumor microenvironment is crucial to tumor development and progression. Aside from cancer cells and immune cells, the regulation of endothelial and stromal cell by neurotransmitters may also constitute a mechanism for tumor progression (Fig. 2). It is well documented that angiogenesis or neovascularization is essential for the growth and metastasis of malignant cancers. There is extensive evidence that endothelial cells express neurotransmitter receptors and can be stimulated by exogenous neurotransmitters to formation of neovessel [37]. For instance, dopamine can mobilize endothelial progenitor cells from the bone marrow and their subsequent participation contribute to neovascularization and tumor growth. Sympathetic adrenergic nerve-derived NE can stimulate β-AR signaling in endothelial cells to drive tumor angiogenesis [13]. Stromal cells are common constituent of the tumor microenvironment and endorse the carcinogenic process in multiple ways. Neurotransmitters also play a role on the stromal cells. In a landmark paper, Magnon et al. have demonstrated that activation of β2- and β3-adrenergic receptors expressed on stromal cells promotes the survival of prostate cancer cells and the tumor initiation [10]. Thus, the influences of neurotransmitters in tumor development and progression encompass a direct role on tumor growth and metastasis, not only through cancer cells and stromal cells, but also via the endothelial cells and immune cells to promote angiogenesis, lymphangiogenesis and inflammatory response. However, more studies are encouraged to fully uncover the networks of cellular and molecular interactions involved.
Neurotransmitters are an active component of the tumor microenvironment. Neurotransmitters released by neuro-endocrine-immune system and other tissues or cells can promote cancer cell proliferation, migration, and invasion through the stimulation of specific membrane receptors. Moreover, neurotransmitters can affect tumor angiogenesis and inflammation by targeting immune cells and endothelial and stromal cells infiltrated in the tumor microenvironment. NE Norepinephrine, E Epinephrine, Ach Acetylcholine, 5-HT serotonin, GABA Gamma-aminobutyric acid
Conclusions and future perspectives
Our knowledge regarding the regulatory role neurotransmitter system in tumor initiation and progression is constantly evolving. The neurotransmitters differentially regulate a plethora of activities of cancer cells, endothelial cells, and immune cells in many types of human cancers. The understanding of this expanded role of neurotransmitter system in tumor biology and the tumor microenvironment opens up new opportunities for developing targeted therapies for cancerous disease. In translational terms, many classical drugs related to neurotransmitters such as β-AR antagonists, serotonin receptor antagonists, AChR antagonists, and DR agonist might have clinical implications in cancer treatment and act as promising candidates for combined drug therapy. Moreover, targeting neurotrophic signaling to prevent neoneurogenesis and surgical or chemical denervation should be further explored as therapeutic approaches for cancers. Interestingly, recent findings suggest that several neurotransmitters (5-HT, dopamine, NE, and histamine) might serve as substrates to participate protein posttranslational modification, such as the known histone serotonylation [123, 124]. Therefore, SSRIs or other small molecules acting on biogenic amines or transglutaminase may prove to be an innovative therapy in oncology. However, further investigations are warranted to consolidate the inclusion of these medicines in the arsenal of cancer therapy and to avoid side effects.
References
Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–54.
Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–73.
Entschladen F, Palm D, Niggemann B, Zaenker KS. The cancer’s nervous tooth: considering the neuronal crosstalk within tumors. Semin Cancer Biol. 2008;18:171–5.
Entschladen F, Drell TLt, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5:254–8.
Sarkar C, Chakroborty D, Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. J NeuroImmune Pharmacol. 2013;8:7–14.
Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–28.
Nilsson MB, Sun H, Diao L, Tong P, Liu D, Li L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: implications for combinations with beta-blockers. Sci Transl Med. 2017;9:eaao4307.
Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634.
Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Investig. 2010;120:1515–23.
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361.
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. Beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90 e7.
Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44.
Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6.
Hondermarck H, Jobling P. The sympathetic nervous system drives tumor angiogenesis. Trends Cancer. 2018;4:93–4.
Coelho M, Soares-Silva C, Brandao D, Marino F, Cosentino M, Ribeiro L. Beta-adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017;143:275–91.
Liu X, Wu WK, Yu L, Sung JJ, Srivastava G, Zhang ST, et al. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem. 2008;105:53–60.
Li J, Yang XM, Wang YH, Feng MX, Liu XJ, Zhang YL, et al. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014;60:1225–34.
Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–70.
Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS ONE. 2011;6:e19246.
Kim TH, Ly C, Christodoulides A, Nowell CJ, Gunning PW, Sloan EK, et al. Stress hormone signaling through beta-adrenergic receptors regulates macrophage mechanotype and function. FASEB J. 2019;33:3997–4006.
Dimitrov S, Lange T, Gouttefangeas C, Jensen ATR, Szczepanski M, Lehnnolz J, et al. Galphas-coupled receptor signaling and sleep regulate integrin activation of human antigen-specific T cells. J Exp Med. 2019;216:517–26.
Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52.
Song Y, Gan Y, Wang Q, Meng Z, Li G, Shen Y, et al. Enriching the housing environment for mice enhances their NK cell antitumor immunity via sympathetic nerve-dependent regulation of NKG2D and CCR5. Cancer Res. 2017;77:1611–22.
Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–41.
Santala EE, Rannikko A, Murtola TJ. Antihypertensive drugs and prostate cancer survival after radical prostatectomy in Finland-A nationwide cohort study. Int J Cancer. 2019;144:440–7.
Spera G, Fresco R, Fung H, Dyck JRB, Pituskin E, Paterson I, et al. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol. 2017;28:1836–41.
Sorensen GV, Ganz PA, Cole SW, Pedersen LA, Sorensen HT, Cronin-Fenton DP, et al. Use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol. 2013;31:2265–72.
Powe DG, Entschladen F. Targeted therapies: using beta-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol. 2011;8:511–2.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44.
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52.
De Giorgi V, Grazzini M, Benemei S, Marchionni N, Botteri E, Pennacchioli E, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
Na Z, Qiao X, Hao X, Fan L, Xiao Y, Shao Y, et al. The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. Onco Targets Ther. 2018;11:4913–44.
Jansen L, Below J, Chang-Claude J, Brenner H, Hoffmeister M. Beta blocker use and colorectal cancer risk: population-based case-control study. Cancer. 2012;118:3911–9.
Roney MSI, Park SK. Antipsychotic dopamine receptor antagonists, cancer, and cancer stem cells. Arch Pharm Res. 2018;41:384–408.
Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–10.
Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–56.
Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Investig. 2008;118:1380–9.
Wu XY, Zhang CX, Deng LC, Xiao J, Yuan X, Zhang B, et al. Overexpressed D2 dopamine receptor inhibits non-small cell lung cancer progression through inhibiting NF-kappaB signaling pathway. Cell Physiol Biochem. 2018;48:2258–72.
Dolma S, Selvadurai HJ, Lan X, Lee L, Kushida M, Voisin V, et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29:859–73.
Jandaghi P, Najafabadi HS, Bauer AS, Papadakis AI, Fassan M, Hall A, et al. Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology. 2016;151:1218–31.
Minami K, Liu S, Liu Y, Chen A, Wan Q, Na S, et al. Inhibitory effects of dopamine receptor D1 agonist on mammary tumor and bone metastasis. Sci Rep. 2017;7:45686.
Pinoli M, Marino F, Cosentino M. Dopaminergic regulation of innate immunity: a review. J NeuroImmune Pharmacol. 2017;12:602–23.
Wu J, Zhang R, Tang N, Gong Z, Zhou J, Chen Y, et al. Dopamine inhibits the function of Gr-1+CD115+ myeloid-derived suppressor cells through D1-like receptors and enhances anti-tumor immunity. J Leukoc Biol. 2015;97:191–200.
Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–42.
Nasi G, Ahmed T, Rasini E, Fenoglio D, Marino F, Filaci G, et al. Dopamine inhibits human CD8+ Treg function through D1-like dopaminergic receptors. J Neuroimmunol. 2019;332:233–41.
Kanbara K, Otsuki Y, Watanabe M, Yokoe S, Mori Y, Asahi M, et al. GABAB receptor regulates proliferation in the high-grade chondrosarcoma cell line OUMS-27 via apoptotic pathways. BMC Cancer. 2018;18:263.
Sung HY, Yang SD, Ju W, Ahn JH. Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Exp Mol Med. 2017;49:e335.
Zhang X, Du Z, Liu J, He J. Gamma-aminobutyric acid receptors affect the progression and migration of tumor cells. J Recept Signal Transduct Res. 2014;34:431–9.
Hujber Z, Horvath G, Petovari G, Krencz I, Danko T, Meszaros K, et al. GABA, glutamine, glutamate oxidation and succinic semialdehyde dehydrogenase expression in human gliomas. J Exp Clin cancer Res. 2018;37:271.
Maemura K, Shiraishi N, Sakagami K, Kawakami K, Inoue T, Murano M, et al. Proliferative effects of gamma-aminobutyric acid on the gastric cancer cell line are associated with extracellular signal-regulated kinase 1/2 activation. J Gastroenterol Hepatol. 2009;24:688–96.
Zhang D, Li X, Yao Z, Wei C, Ning N, Li J. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett. 2014;348:100–8.
Blanchart A, Fernando R, Haring M, Assaife-Lopes N, Romanov RA, Andang M, et al. Endogenous GABAA receptor activity suppresses glioma growth. Oncogene. 2017;36:777–86.
Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:10715.
Jiang SH, Zhu LL, Zhang M, Li RK, Yang Q, Yan JY, et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca(2+) signalling in a GABA-independent manner. Gut. 2019. https://doi.org/10.1136/gutjnl-2018-317479.
Abdul M, McCray SD, Hoosein NM. Expression of gamma-aminobutyric acid receptor (subtype A) in prostate cancer. Acta Oncol. 2008;47:1546–50.
Schuller HM. Regulatory role of G protein-coupled receptors in pancreatic cancer development and progression. Curr Med Chem. 2018;25:2566–75.
Wang T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 2008;82:536–41.
Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, et al. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704–12.
Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer drugs. 2008;19:655–71.
Xia S, He C, Zhu Y, Wang S, Li H, Zhang Z, et al. GABABR-Induced EGFR transactivation promotes migration of human prostate cancer cells. Mol Pharm. 2017;92:265–77.
Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol Lett. 2003;90:165–72.
Lang K, Bastian P. Neurotransmitter effects on tumor cells and leukocytes. Prog Exp Tumor Res. 2007;39:99–121.
Bergeret M, Khrestchatisky M, Tremblay E, Bernard A, Gregoire A, Chany C. GABA modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits. Biomed Pharmacother. 1998;52:214–9.
Wang Q, Ren L, Wan Y, Prud’homme GJ. GABAergic regulation of pancreatic islet cells: physiology and antidiabetic effects. J Cell Physiol. 2019. https://doi.org/10.1002/jcp.28214.
Rolland B, Labreuche J, Duhamel A, Deheul S, Gautier S, Auffret M, et al. Baclofen for alcohol dependence: relationships between baclofen and alcohol dosing and the occurrence of major sedation. Eur Neuropsychopharmacol. 2015;25:1631–6.
Kim HB, Myung SK, Park YC, Park B. Use of benzodiazepine and risk of cancer: a meta-analysis of observational studies. Int J Cancer. 2017;140:513–25.
Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66.
Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–8.
Chabbi-Achengli Y, Coudert AE, Callebert J, Geoffroy V, Cote F, Collet C, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci USA. 2012;109:2567–72.
Sarrouilhe D, Mesnil M. Serotonin and human cancer: a critical view. Biochimie. 2019;161:46–50.
Sarrouilhe D, Clarhaut J, Defamie N, Mesnil M. Serotonin and cancer: what is the link? Curr Mol Med. 2015;15:62–77.
Liu Y, Zhang H, Wang Z, Wu P, Gong W. 5-Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. Eur J Cancer. 2019;114:8–24.
Del Bello F, Bonifazi A, Giorgioni G, Quaglia W, Amantini C, Morelli MB, et al. Chemical manipulations on the 1,4-dioxane ring of 5-HT1A receptor agonists lead to antagonists endowed with antitumor activity in prostate cancer cells. Eur J Med Chem. 2019;168:461–73.
Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H, et al. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15:75.
Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–54.
Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM, et al. Increased serotonin signaling contributes to the Warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;153:277–91 e19.
Mammadova-Bach E, Mauler M, Braun A, Duerschmied D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets. 2018;29:541–8.
Asada M, Ebihara S, Yamanda S, Niu K, Okazaki T, Sora I, et al. Depletion of serotonin and selective inhibition of 2B receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and extracellular signal-regulated kinase 1/2 phosphorylation. Neoplasia. 2009;11:408–17.
Muma NA, Mi Z. Serotonylation and transamidation of other monoamines. ACS Chem Neurosci. 2015;6:961–9.
Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velazquez MA, Garces-Alvarez ME, Hurtado-Alvarado G, et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957.
Grygier B, Arteta B, Kubera M, Basta-Kaim A, Budziszewska B, Leskiewicz M, et al. Inhibitory effect of antidepressants on B16F10 melanoma tumor growth. Pharmacol Rep. 2013;65:672–81.
Shapovalov Y, Zettel M, Spielman SC, Amico-Ruvio SA, Kelly EA, Sipe GO, et al. Fluoxetine modulates breast cancer metastasis to the brain in a murine model. BMC Cancer. 2014;14:598.
Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–8.
Kast RE. Glioblastoma chemotherapy adjunct via potent serotonin receptor-7 inhibition using currently marketed high-affinity antipsychotic medicines. Br J Pharm. 2010;161:481–7.
Hsieh HY, Shen CH, Lin RI, Feng YM, Huang SY, Wang YH, et al. Cyproheptadine exhibits antitumor activity in urothelial carcinoma cells by targeting GSK3beta to suppress mTOR and beta-catenin signaling pathways. Cancer Lett. 2016;370:56–65.
Reijmen E, Vannucci L, De Couck M, De Greve J, Gidron Y. Therapeutic potential of the vagus nerve in cancer. Immunol Lett. 2018;202:38–43.
Wessler IK, Kirkpatrick CJ. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J Neurochem. 2017;142Suppl 2:144–50.
Nimmakayala RK, Seshacharyulu P, Lakshmanan I, Rachagani S, Chugh S, Karmakar S, et al. Cigarette smoke induces stem cell features of pancreatic cancer cells via PAF1. Gastroenterology. 2018;155:892–908 e6.
Elisia I, Cho B, Hay M, Li MY, Hofs E, Lam V, et al. The effect of diet and exercise on tobacco carcinogen-induced lung cancer. Carcinogenesis. 2019;40:448–60.
Shimizu R, Ibaragi S, Eguchi T, Kuwajima D, Kodama S, Nishioka T, et al. Nicotine promotes lymph node metastasis and cetuximab resistance in head and neck squamous cell carcinoma. Int J Oncol. 2019;54:283–94.
Schaal CM, Bora-Singhal N, Kumar DM, Chellappan SP. Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer. Mol Cancer. 2018;17:149.
Li ZJ, Cho CH. Neurotransmitters, more than meets the eye–neurotransmitters and their perspectives in cancer development and therapy. Eur J Pharm. 2011;667:17–22.
Patane S. M3 muscarinic acetylcholine receptor in cardiology and oncology. Int J Cardiol. 2014;177:646–9.
Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8:1458–73.
Yu H, Xia H, Tang Q, Xu H, Wei G, Chen Y, et al. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci Rep. 2017;7:40802.
Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34.
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115.
Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Physiological functions of the cholinergic system in immune cells. J Pharm Sci. 2017;134:1–21.
Zila I, Mokra D, Kopincova J, Kolomaznik M, Javorka M, Calkovska A. Vagal-immune interactions involved in cholinergic anti-inflammatory pathway. Physiol Res. 2017;66Suppl 2:S139–S45.
Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14:419–29.
Fujii T, Horiguchi K, Sunaga H, Moriwaki Y, Misawa H, Kasahara T, et al. SLURP-1, an endogenous alpha7 nicotinic acetylcholine receptor allosteric ligand, is expressed in CD205(+) dendritic cells in human tonsils and potentiates lymphocytic cholinergic activity. J Neuroimmunol. 2014;267:43–9.
Russo P, Del Bufalo A, Milic M, Salinaro G, Fini M, Cesario A. Cholinergic receptors as target for cancer therapy in a systems medicine perspective. Curr Mol Med. 2014;14:1126–38.
Ribeiro MP, Custodio JB, Santos AE. Ionotropic glutamate receptor antagonists and cancer therapy: time to think out of the box? Cancer Chemother Pharm. 2017;79:219–25.
Yu LJ, Wall BA, Wangari-Talbot J, Chen S. Metabotropic glutamate receptors in cancer. Neuropharmacology. 2017;115:193–202.
Briggs KJ, Koivunen P, Cao S, Backus KM, Olenchock BA, Patel H, et al. Paracrine Induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell. 2016;166:126–39.
Gelb T, Pshenichkin S, Rodriguez OC, Hathaway HA, Grajkowska E, DiRaddo JO, et al. Metabotropic glutamate receptor 1 acts as a dependence receptor creating a requirement for glutamate to sustain the viability and growth of human melanomas. Oncogene. 2015;34:2711–20.
Palamiuc L, Emerling BM. PSMA brings new flavors to PI3K signaling: a role for glutamate in prostate cancer. J Exp Med. 2018;215:17–9.
Levite M. Glutamate, T cells and multiple sclerosis. J Neural Transm. 2017;124:775–98.
Kaczynska K, Zajac D, Wojciechowski P, Kogut E, Szereda-Przestaszewska M. Neuropeptides and breathing in health and disease. Pulm Pharm Ther. 2018;48:217–24.
Moody TW, Moreno P, Jensen RT. Neuropeptides as lung cancer growth factors. Peptides. 2015;72:106–11.
Covenas R, Munoz M. Cancer progression and substance P. Histol Histopathol. 2014;29:881–90.
Tilan J, Kitlinska J. Neuropeptide Y (NPY) in tumor growth and progression: lessons learned from pediatric oncology. Neuropeptides. 2016;55:55–66.
Munoz M, Rosso M, Covenas R. The NK-1 receptor: a new target in cancer therapy. Curr Drug Targets. 2011;12:909–21.
Munoz M, Covenas R. Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: a new approach. Saudi J Gastroenterol. 2016;22:260–8.
Munoz M, Rosso M, Covenas R. The NK-1 receptor antagonist L-732,138 induces apoptosis in human gastrointestinal cancer cell lines. Pharmacol Rep. 2017;69:696–701.
Li J, Tian Y, Wu A. Neuropeptide Y receptors: a promising target for cancer imaging and therapy. Regen Biomater. 2015;2:215–9.
Galli S, Naranjo A, Van Ryn C, Tilan JU, Trinh E, Yang C, et al. Neuropeptide Y as a biomarker and therapeutic target for neuroblastoma. Am J Pathol. 2016;186:3040–53.
Czarnecka M, Trinh E, Lu C, Kuan-Celarier A, Galli S, Hong SH, et al. Neuropeptide Y receptor Y5 as an inducible pro-survival factor in neuroblastoma: implications for tumor chemoresistance. Oncogene. 2015;34:3131–43.
Ondrovics M, Hoelbl-Kovacic A, Fux DA. Opioids: modulators of angiogenesis in wound healing and cancer. Oncotarget. 2017;8:25783–96.
Moody TW, Nuche-Berenguer B, Jensen RT. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23:38–47.
Ouyang Q, Zhou J, Yang W, Cui H, Xu M, Yi L. Oncogenic role of neurotensin and neurotensin receptors in various cancers. Clin Exp Pharmacol Physiol. 2017;44:841–6.
Griffin N, Faulkner S, Jobling P, Hondermarck H. Targeting neurotrophin signaling in cancer: the renaissance. Pharm Res. 2018;135:12–7.
Anastas JN, Shi Y. Histone serotonylation: can the brain have “Happy” chromatin? Mol Cell. 2019;74:418–20.
Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV, et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–9.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81701945, 81672358, 81871923, 81802890, and 81872242), the Natural Science Foundation of Shanghai (18ZR1436900), Shanghai Sailing Program (19YF1445700), China Postdoctoral Science Foundation (2018M640403), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20181708), Program of Shanghai Academic/Technology Research Leader (19XD1403400), Science and Technology Commission of Shanghai Municipality (18410721000), and Shanghai Municipal Health Bureau (2018BR32).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, SH., Hu, LP., Wang, X. et al. Neurotransmitters: emerging targets in cancer. Oncogene 39, 503–515 (2020). https://doi.org/10.1038/s41388-019-1006-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1006-0
- Springer Nature Limited
This article is cited by
-
Heat-killed Prevotella intermedia promotes the progression of oral squamous cell carcinoma by inhibiting the expression of tumor suppressors and affecting the tumor microenvironment
Experimental Hematology & Oncology (2024)
-
Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis
Signal Transduction and Targeted Therapy (2024)
-
Peripheral Mechanism of Cancer-Induced Bone Pain
Neuroscience Bulletin (2024)
-
TRPV1 inhibition suppresses non-small cell lung cancer progression by inhibiting tumour growth and enhancing the immune response
Cellular Oncology (2024)
-
Crosstalk Between the Nervous System and Colorectal Cancer
Neuroscience Bulletin (2024)