Abstract
Background/Objectives:
Coffee is one of the most popularly consumed beverages worldwide. Many epidemiological studies have investigated the association between coffee consumption and lung cancer risk, but the results are inconsistent. Hence, we conducted a systematic analysis of relevant population-based studies to examine this association and derive a more precise estimation.
Subjects/Methods:
The Cochrane library, PubMed and Embase databases were searched to identify studies published through Mar 2015 that met the predetermined inclusion criterion. Seventeen studies (5 cohort and 12 case–control studies) involving 12 276 cases and 102 516 controls were included.
Results:
The summary odds ratio (OR) of lung cancer was 1.17 (95% confidence interval (CI): 1.03–1.33) for coffee drinkers compared with nondrinkers and 1.31 (95% CI: 1.11–1.55) for the highest category of coffee consumption compared with the lowest category. Compared with nondrinkers, the pooled ORs for lung cancer were 1.10 (95% CI: 0.92–1.31) for ⩽1 cup per day, 1.10 (95% CI: 0.93–1.30) for 2–3 cups per day and 1.20 (95% CI: 1.02–1.39) for ⩾3 cups per day. Further analysis showed that the ORs for hospital-based case–control studies, population-based case–control studies and prospective cohort studies were 1.36 (95% CI: 1.10–1.69), 0.99 (95% CI: 0.77–1.28) and 1.59 (95% CI: 1.26–2.00), respectively. Significant associations for high coffee intake with increased risk of lung cancer were observed in men (OR=1.41 95% CI: 1.21–1.63), but not in women (OR=1.16, 95% CI: 0.86–1.56), in American (OR=1.34 95% CI: 1.08–1.65) and Asian populations (OR=1.49 95% CI: 1.28–1.74), but not in European populations (OR=1.12, 95% CI: 0.74–1.67), and in smokers (OR=1.24, 95% CI: 1.00–1.54), but not in nonsmokers (OR=0.85, 95% CI: 0.64–1.11). Particularly over the last 5 years, studies have consistently indicated that lung cancer risk is significantly increased by 47% in the population with the highest category intake of coffee compared with that with the lowest category intake (OR=1.47, 95% CI: 1.21–1.79).
Conclusion:
The present study suggested that coffee intake was associated with an increased risk of lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is one of the most prevalent malignancies and is the leading cause of cancer death in the United States, in both men and women.1 The incidence and mortality rate of lung cancer are continuing to increase worldwide, particularly in developing countries. As reported by World Health Organization, the steadily increasing proportion of elderly people in the world will result in ~50% increase in new cancer cases over the next 20 years. If the current smoking levels and the adoption of unhealthy lifestyles persist, the increase will be even greater. Epidemiological studies and systematic analyses have showed the intimate association between dietary factors and the risk of lung cancer. The high consumption of saturated fat may increase the risk of lung cancer, whereas the consumption of vegetables, fruit and carotene may decrease the risk of lung cancer.2, 3, 4, 5 Therefore, the identification of modifiable risk factors, particularly in the diet, for lung cancer is of importance because it may lead to potential prevention opportunities.
Coffee is one of the most widely consumed beverages in the world. Because of its popularity, even small potentially unhealthy or beneficial properties could have important public health consequences. In addition to caffeine, coffee has been reported to contain more than a thousand different chemical compounds with many bioactivities, such as anti-oxidative,6 anti-inflammatory7 and insulin-sensitizing8 effects. Coffee contains complex mixtures of biochemically active components that have been hypothesized to impact the etiology of certain diseases ranging from carcinogenesis and cancer progression to cellular apoptosis, oxidative stress and inflammatory diseases.9, 10, 11, 12 Thus, it is important to elucidate the association between coffee consumption and the risk of cancers. In fact, extensive epidemiological studies have been performed to estimate the relationship between coffee consumption and various types of cancer, including lung cancer. However, these studies have reported inconsistent findings for coffee consumption and lung cancer risk. To derive a more precise estimation of this relationship, we performed a meta-analysis to summarize the available evidence from prospective and case–control studies.
MATERIALS AND Methods
Search strategy
We conducted a systematic search of the literature published on 1 March 2015 using the Cochrane, PubMed and Embase databases. The following search terms were used: ‘coffee’, ‘beverages’, ‘diet’, ‘lifestyle’ and ‘lung cancer’. We also performed a manual search via reference lists. Only full-length journal articles with a prospective cohort or case–control study design were considered.
Study selection
Articles were eligible for the present meta-analysis if they conformed to the following criteria: (i) the study design was a population-based study, including cohort or case–control study; (ii) a relatively complete assessment of coffee intake was performed; (iii) the association of coffee intake with lung cancer risk was specifically evaluated; and (iv) the relative risk (RR), hazard ratio or odds ratio (OR) and the corresponding 95% confidence interval (95% CI) values were available. In cases in which duplicate reports from the same study were identified, we chose the most recent one.
Data extraction
The data from each paper fulfilling the inclusion criteria were extracted carefully by two independent reviewers. The following information from each study was recorded: (i) the first author’s name, publication year and country or city of origin; (ii) the study design (prospective cohort study, population-based case–control study or hospital-based case–control study); (iii) the mean follow-up time used in the study; (iv) the population (numbers of cases and controls); (v) coffee consumption; (vi) the relative risk, hazard ratio or OR values from the most fully adjusted model and their 95% CI values; and (vii) the listed confounders adjusted for in the multivariate analysis. In addition, because of the low incidence of lung cancer, the OR was assumed to be the same as the hazard ratio and relative risk, and the summary results were reported as OR for simplicity.13
Statistical analysis
The summary ORs and corresponding 95% CIs of the included studies were used as a measure to assess the association of coffee consumption with lung cancer risk. The statistical heterogeneity among studies was assessed using the Q test and I2 statistics. If a statistical difference in heterogeneity existed (P<0.10 or I2>50%), a random-effects model was selected to pool the data; otherwise, a fixed-effects model was used. When statistical heterogeneity was detected, a sensitivity analysis was performed to explore potential sources of heterogeneity, both in the overall pooled estimate and within the subgroups. The potential publication bias was examined by the funnel plot and Egger’s test (P<0.10). All of the analyses were performed using STATA version 11.0 (Stata Corp, College Station, TX, USA). A P-value <0.05 was considered to be statistically significant unless otherwise specified.
Results
Characteristics of the included studies
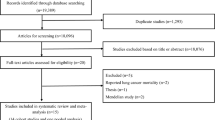
As shown in Figure 1, the systematic search of the literature identified a total of 2658 studies. After excluding 2625 irrelevant titles and/or abstracts, the remaining 33 full-text articles were subjected to a more detailed evaluation. Among those articles, 16 were excluded as irrelevant or because they did not meet the inclusion criteria. In the end, 17 studies relevant to the role of coffee intake in the risk of lung cancer were included in the present meta-analysis.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
The characteristics of these studies are presented in Table 1. The studies included in the final analysis included 12 276 cases and 1 02 516 controls. The selected studies were published between 1986 and 2014, which is a period that spans 28 years. One of the studies was published in Japanese, and the others were published in English. Among these 17 studies, five were prospective cohort studies, four were population-based case–control studies and eight were hospital-based case–control studies. In addition, five were conducted in America (two in USA, one in Canada and one in Uruguay), five were conducted in Europe (two in Sweden, one in Norway, one in France and one in Czech) and seven were conducted in Asia (three in Japan, one in Hong Kong, one in Korea, one in India and one in Pakistani). Two studies did not have any adjustments, two studies only adjusted for age and smoking, and the other 13 studies adjusted for a wide range of potential confounders of lung cancer, such as age, smoking, occupation, radon index, alcohol, education, residence, socioeconomic status, and intake of fruit and vegetables.
Coffee consumption and lung cancer risk
The summary OR values of lung cancer included in the studies were calculated using fixed- or random-effects models depending on the heterogeneities. As shown in Figure 2a, the pooled OR of lung cancer from the combination of the included studies was 1.17 (95% CI: 1.03–1.33) for coffee drinkers compared with nondrinkers, indicating that the risk of lung cancer is significantly increased in coffee drinkers. In addition, the pooled OR is higher in magnitude when it is estimated as the highest category intake of coffee versus the lowest category (OR=1.31, 95% CI: 1.11–1.55; Figure 2b). Nevertheless, substantial heterogeneities existed across these studies. Hence, we estimated ORs for different contrasts for coffee consumption. As shown in Figures 2c, d and e, the pooled OR of lung cancer for the population with ⩽1 cup per day, 2–3 cups per day and ⩾3 cups per day coffee consumption compared with nondrinkers were 1.10 (95% CI: 0.92–1.31), 1.10 (95% CI: 0.93–1.30) and 1.20 (95% CI: 1.02–1.39), respectively. No significant heterogeneities were observed in these studies. The data suggested that an increase in coffee consumption was associated with an increased risk of lung cancer and that the consumption of more than three cups of coffee per day might significantly increase lung cancer risk.
Forest plots of investigating association for various categories of coffee consumption with lung cancer risk. (a) Coffee drinkers versus nondrinkers. Coffee consumption <2 times per week or ⩽1 cups per week was also defined as nondrinkers. (b) The highest level of coffee consumption in each included study versus the lowest level of intake. We also estimated ORs for different contrasts for coffee consumption. (c) Less than or equal to 1 cups per day coffee consumption versus nondrinkers. (d) Two to 3 cups per day coffee consumption versus nondrinkers. (e) More than or equal to 3 cups per day coffee consumption versus nondrinkers.
We then stratified the included studies by design, sex, population, smoking and publication time. As shown in Table 2, the pooled ORs for hospital-based case–control studies, population-based case–control studies and prospective cohort studies were 1.36 (95% CI: 1.10–1.69), 0.99 (95% CI: 0.77–1.28) and 1.59 (95% CI: 1.26–2.00), respectively. The prospective cohort studies revealed a significant positive association for lung cancer risk with coffee intake, and no substantial heterogeneity existed across these studies. Next, a significant association for high coffee intake with increased risk of lung cancer was observed in men (OR=1.41 95% CI: 1.21–1.63) but not in women (OR=1.16, 95% CI: 0.86–1.56). Statistical heterogeneity existed across the studies conducted in women but not in those conducted in men. Furthermore, statistically significant associations between high coffee consumption and lung cancer risk were observed among studies conducted in American (OR=1.34 95% CI: 1.08–1.65) and Asian populations (OR=1.49 95% CI: 1.28–1.74) but not in European populations (OR=1.12, 95% CI: 0.74–1.67). In addition, a positive association was observed in smokers (OR=1.24, 95% CI: 1.00–1.54) but not in nonsmokers (OR=0.85, 95% CI: 0.64–1.11). Last, when the various studies were stratified by publication time, a positive association between coffee intake and lung cancer risk was shown among the studies published before 2000 (OR=1.45, 95% CI: 1.19–1.76) and after 2010 (OR=1.47, 95% CI: 1.21–1.79), but not in those published in the period from 2000 to 2009 (OR=1.09, 95% CI: 0.84–1.43). In particular, the studies published over the last 5 years consistently revealed that the risk of lung cancer was significantly increased by 47% in the population with the highest category intake of coffee versus the lowest category.
Sensitivity analysis
Statistical heterogeneities existed across studies in the overall pooled estimate. We performed sensitivity analysis to evaluate the stability of the results, in which individual studies were sequentially dropped. The analysis excluded any single study in turn and pooled the OR of the remaining included studies. The overall summary OR did not substantially change, with a range from 1.14 (95% CI: 1.01–1.30) to 1.21 (95% CI: 1.06–1.37) for coffee drinkers compared with nondrinkers, and 1.27 (95% CI: 1.08–1.50) to 1.38 (95% CI: 1.18–1.61) for the highest category compared with the lowest. To exclude the residual confounding by smoking, we also omitted three studies with no adjustment for smoking and pooled the OR of the remaining included studies. The ORs were reduced but did not apparently change, which were 1.15 (95% CI: 1.01–1.32) for coffee drinkers versus nondrinkers and 1.29 (95% CI: 1.08–1.54) for the highest category versus the lowest category.
Publication bias
We performed Begg’s funnel plots and Egger’s tests to assess the publication bias in the included studies. As shown in Figure 3, the shape of the funnel plot did not reveal any evidence of obvious asymmetry. Egger’s test, which provides statistical evidence of the funnel plot symmetry, indicated little evidence of publication bias. Therefore, no significant publication bias was observed in these studies.
Discussion
Cigarette smoking has been well established as a major risk factor in the carcinogenesis and progression of lung cancer. In addition to smoking, some other potential risk factors also have been considered, including occupational and non-occupational pollutants. The major occupational exposures occur in workers who are engaged in the smelting and refining of metals or in the production of pesticides, pigments, dyes, glass, semiconductors, wood/cotton products and various pharmaceutical substances.31, 32, 33 Non-occupational exposures mainly include outdoor and indoor air pollution, arsenic and chlorinated by-products in drinking water, asbestos, dioxins and electromagnetic fields.34, 35, 36 Some dietary components may also exert significant effects on the carcinogenesis and development of lung cancer. The identification of these modifiable risk factors in the diet is important for cancer prevention.
Coffee is a popular beverage worldwide, and its potentially unhealthy and beneficial bioactivities have been extensively investigated in epidemiological and experimental studies. However, the role of coffee consumption in the development of various types of cancer remains unclear. A previous meta-analysis conducted in 2010 indicated a significant positive association between the highest coffee intake category and lung cancer (OR=1.27, 95% CI: 1.04–1.54),37 but substantial heterogeneities and controversy existed across the studies included in the analysis. Importantly, the previous study only included 13 studies involving 5347 lung cancer cases. In fact, the association between lung cancer with coffee consumption, either the data were too inconsistent or the number of studies and cases were too few to allow conclusions to be reached.30 Thus, in recent years, several population-based studies were conducted to further examine the association between coffee consumption and lung cancer risk. Notably, a large French population-based case–control study, the ICARE study (investigation of occupational and environmental causes of respiratory cancers), including a total of 5926 lung cancer cases, suggested that coffee consumption is not associated to the risk of lung cancer. Therefore, it is necessary and important to provide an updated meta-analysis and derive a more precise estimation. The present study included 17 studies involving 12 276 cases, which was more than twice the number of cases in the previous meta-analysis. Although the large French study recently showed that coffee consumption is not associated to the risk of lung cancer (OR=1.11, 95% CI: 0.72–1.72; for ⩾5 cups per day versus never). The present meta-analysis still indicated that an increase in coffee consumption was associated with an increased risk of lung cancer, and that consumption of more than three cups of coffee per day might significantly increase lung cancer risk. Furthermore, the positive association between coffee consumption and lung cancer is particularly notable and consistent in prospective cohort studies. In case–control studies, it is difficult to ascertain the ‘typical’ coffee consumption patterns among the cases, who likely changed their habits near the time of their diagnosis, which would contribute to differential recall bias. Therefore, data from prospective cohort studies should exhibit more reliability and consistency. Given this, more carefully designed cohort studies would be performed to assess the association of coffee consumption with lung cancer.
Although the present study indicated the association of coffee intake with an increased risk of lung cancer, it should be noted that several recently updated meta-analysis have reported that coffee consumption is associated with a reduction in the risk of various types of cancers, including prostate cancer, bladder cancer, colorectal cancer and liver cancer.38, 39, 40, 41 A recent meta-analysis showed that coffee consumption was not statistically significantly associated with total cancer mortality.42 Certainly, residual confounding by smoking is always a great concern in studies of lung cancer. The association between coffee consumption and lung cancer risk is strongly confounded by smoking. Because the residual confounding influences of smoking or other risk factors may still exist, the result of the present study should be interpreted with caution.
References
Siegel R, Naishadham D, Jemal A . Cancer statistics. CA Cancer J Clin 2013; 63: 11–30.
Gonzalez CA . The European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr 2006; 9: 124–126.
Abdull RAF, Noor NM . Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer Prev 2013; l14: 1565–1570.
Dolara P, Bigagli E, Collins A . Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: a critical commentary. Eur J Nutr 2012; 51: 769–781.
Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr 2008; 88: 372–383.
Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A . Coffee: biochemistry and potential impact on health. Food Funct 2014; 5: 1695–1717.
Shen T, Park YC, Kim SH, Lee J, Cho JY . Nuclear factor-kappaB/signal transducers and activators of transcription-1-mediated inflammatory responses in lipopolysaccharide-activated macrophages are a major inhibitory target of kahweol, a coffee diterpene. Biol Pharm Bull 2010; 33: 1159–1164.
van Dam RM, Feskens EJ . Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002; 360: 1477–1478.
Sapozhnikova Y . Development of liquid chromatography-tandem mass spectrometry method for analysis of polyphenolic compounds in liquid samples of grape juice, green tea and coffee. Food Chem 2014; 150: 87–93.
O'Keefe JH, Bhatti SK, Patil HR, DiNicolantonio JJ, Lucan SC, Lavie CJ . Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J Am Coll Cardiol 2013; 62: 1043–1051.
Bakuradze T, Lang R, Hofmann T, Stiebitz H, Bytof G et al. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol Nutr Food Res 2010; 54: 1734–1743.
Glei M, Kirmse A, Habermann N, Persin C, Pool-Zobel BL . Bread enriched with green coffee extract has chemoprotective and antigenotoxic activities in human cells. Nutr Cancer 2006; 56: 182–192.
Greenland S . Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9: 1–30.
Luqman M, Javed MM, Daud S, Raheem N, Ahmad J, Khan AU . Risk factors for lung cancer in the Pakistani population. Asian Pac J Cancer Prev 2014; 15: 3035–3039.
Ganesh B, Sushama S, Monika S, Suvarna P . A case-control study of risk factors for lung cancer in Mumbai, India. Asian Pac J Cancer Prev 2011; 12: 357–362.
Kubik A, Zatloukal P, Tomasek L, Dolezal J, Syllabova L, Kara J et al. A case-control study of lifestyle and lung cancer associations by histological types. Neoplasma 2008; 55: 192–199.
Baker JA, McCann SE, Reid ME, Nowell S, Beehler GP, Moysich KB . Associations between black tea and coffee consumption and risk of lung cancer among current and former smokers. Nutr Cancer 2005; 52: 15–21.
Takezaki T, Hirose K, Inoue M, Hamajima N, Yatabe Y et al. Dietary factors and lung cancer risk in Japanese: with special reference to fish consumption and adenocarcinomas. Br J Cancer 2001; 84: 1199–1206.
Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio JC, Ronco A . Consumption of tea and coffee and the risk of lung cancer in cigarette-smoking men: a case-control study in Uruguay. Lung Cancer 1998; 19: 101–107.
Axelsson G, Liljeqvist T, Andersson L, Bergman B, Rylander R . Dietary factors and lung cancer among men in west Sweden. Int J Epidemiol 1996; 25: 32–39.
Mettlin C . Milk drinking, other beverage habits, and lung cancer risk. Int J Cancer 1989; 43: 608–612.
Chiu YL, Wang XR, Qiu H, Yu IT . Risk factors for lung cancer: a case-control study in Hong Kong women. Cancer Causes Control 2010; 21: 777–785.
Hu J, Mao Y, Dryer D, White K . Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev 2002; 26: 129–138.
Nyberg F, Agrenius V, Svartengren K, Svensson C, Pershagen G . Dietary factors and risk of lung cancer in never-smokers. Int J Cancer 1998; 78: 430–436.
Bae JM, Li ZM, Shin MH, Kim DH, Lee MS, Ahn YO . Pulmonary tuberculosis and lung cancer risk in current smokers: the Seoul Male Cancer Cohort Study. J Korean Med Sci 2013; 28: 896–900.
Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev 2004; 5: 58–65.
Fu YY, Takezaki T, Tajima K . [Risk factors of lung cancer—follow-up studies in Nagoya Japan]. Zhonghua Liu Xing Bing Xue Za Zhi 1997; 18: 328–330.
Stensvold I, Jacobsen BK . Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control 1994; 5: 401–408.
Nomura A, Heilbrun LK, Stemmermann GN . Prospective study of coffee consumption and the risk of cancer. J Natl Cancer Inst 1986; 76: 587–590.
Sanikini H, Radoï L, Menvielle G, Guida F, Mattei F et al. Coffee consumption and risk of lung cancer: the ICARE study. Eur J Epidemiol 2015; 30: 81–85.
Tomioka K, Saeki K, Obayashi K, Tanaka Y, Kurumatani N . Risk for lung cancer in workers exposed to benzidine and/or beta-naphthylamine: a protocol for systematic review and meta-analysis. Syst Rev 2014; 3: 112.
Edwards JK, McGrath LJ, Buckley JP, Schubauer-Berigan MK, Cole SR, Richardson DB . Occupational radon exposure and lung cancer mortality: estimating intervention effects using the parametric g-formula. Epidemiology 2014; 25: 829–834.
Zendehdel R, Tayefeh-Rahimian R, Kabir A . Chronic exposure to chlorophenol related compounds in the pesticide production workplace and lung cancer: a meta-analysis. Asian Pac J Cancer Prev 2014; 15: 5149–5153.
Cardaba AM, Munoz MMF, Armentia MA, Alonso CM, Carreras VF, Almaraz GA . Health impact assessment of air pollution in Valladolid, Spain. BMJ Open 2014; 4: e005999.
Hong YS, Song KH, Chung JY . Health effects of chronic arsenic exposure. J Prev Med Public Health 2014; 47: 245–252.
Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers Prev 2014; 23: 1529–1538.
Tang N, Wu Y, Ma J, Wang B, Yu R . Coffee consumption and risk of lung cancer: a meta-analysis. Lung Cancer 2010; 67: 17–22.
Sang LX, Chang B, Li XH, Jiang M . Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol 2013; 13: 34.
Tian C, Wang W, Hong Z, Zhang X . Coffee consumption and risk of colorectal cancer: a dose-response analysis of observational studies. Cancer Causes Control 2013; 24: 1265–1268.
Lu Y, Zhai L, Zeng J, Peng Q, Wang J, Deng Y et al. Coffee consumption and prostate cancer risk: an updated meta-analysis. Cancer Causes Control 2014; 25: 591–604.
Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z . Coffee consumption and risk of prostate cancer: a meta-analysis of prospective cohort studies. Carcinogenesis 2014; 35: 256–261.
Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N . Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol 2014; 180: 763–775.
Acknowledgements
This work was supported by a research grant from the National Natural Science Foundation of China (81001197, Su YX, http://isisn.nsfc.gov.cn/egrantindex/funcindex/prjsearch-list) and National Key Specialty Construction of Clinical Projects (#2013-544). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The ethics committee of the Children’s Hospital of Chongqing Medical University approved the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Y., Qin, J., Nan, G. et al. Coffee consumption and the risk of lung cancer: an updated meta-analysis of epidemiological studies. Eur J Clin Nutr 70, 199–206 (2016). https://doi.org/10.1038/ejcn.2015.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.96
- Springer Nature Limited
This article is cited by
-
Causal relationship from coffee consumption to diseases and mortality: a review of observational and Mendelian randomization studies including cardiometabolic diseases, cancer, gallstones and other diseases
European Journal of Nutrition (2022)
-
The investigation of the healing effect of active ingredients in traditional medicinal plants on lung cancer
Medical Oncology (2020)
-
Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population
European Journal of Nutrition (2020)
-
What is the relationship between coffee and lung cancer?
European Journal of Clinical Nutrition (2016)
-
Coffee and cancer risk: A meta-analysis of prospective observational studies
Scientific Reports (2016)