Abstract
Background/objectives:
Plant stanol esters lower serum low-density lipoprotein (LDL)-cholesterol (LDL-C), but responses between individuals vary widely. As the ability of subjects to respond to acute dietary challenges may reflect the flexibility to adapt to changes on the longer term, we related subjects' acute postprandial metabolic changes to changes in serum lipoproteins after chronic intake of plant stanol esters.
Subjects/methods:
In a double-blind crossover design, 20 healthy subjects received in random order a high-fat shake enriched with or without plant stanol esters (4 g). Blood samples were taken during 4 h to examine lipid, glucose and lipoprotein profiles. Two subjects dropped out. For the 3 weeks after this postprandial test, the subjects who received the shake with plant stanol esters continued the consumption of plant stanol-enriched (3g/day) margarine and subjects receiving the control shake in the postprandial test consumed for the next 3 weeks a control margarine. After the washout period, subjects received the other shake and margarines.
Results:
The margarine enriched with plant stanol esters lowered concentrations of total cholesterol by 7.3% (P<0.01), LDL-C by 9.5% (P<0.01) and apoB100 by 8.6% (P<0.01). Furthermore, particle concentrations of total very low-density lipoprotein (VLDL), small VLDL and large LDL were reduced by 26.6% (P=0.02), 27.6% (P=0.02) and 12.3% (P=0.04), respectively. Plant stanol esters did not affect parameters related to lipid and glucose metabolism during the postprandial phase. However, the incremental area under the curve (iAUC) of the postprandial glucose concentration after consuming the control shake correlated positively with changes in fasting concentrations of total cholesterol, LDL-C, apoB100, total VLDL, small VLDL and intermediate-density lipoprotein after 3 weeks.
Conclusions:
A single dose of plant stanol esters does not change postprandial lipid and lipoprotein profiles. However, postprandial glucose responses may predict the effects of chronic plant stanol ester consumption.
Similar content being viewed by others
Introduction
The cholesterol-lowering effect of plant sterols was already observed in 1950. Numerous studies later, it is generally accepted that a daily intake of 2.5 g of plant sterols or stanols lowers serum low-density lipoprotein (LDL)-C concentrations up to 10%.1 However, the mechanism underlying this effect is still under debate. As recently reviewed,2 the earliest explanations suggested an effect on mixed micelle composition, whereas more recent theories suggest involvement of several intestinal transporter molecules or activation of the recently described transintestinal cholesterol excretion pathway.3 Moreover, although serum LDL-C concentrations decrease in most individuals after consumption of plant sterol of stanol ester-enriched foods, a large inter-individual variation exists.4 Identification of factors related to this variability may help to identify responsive (sub)populations and add to a better understanding of the underlying mechanisms.5 In this respect, postprandial challenge studies may be helpful. During the postprandial phase, not only glucose and insulin concentrations change profoundly, but also those of the intestine-derived cholesterol-containing chylomicrons concentrations. Until now, only a few studies have evaluated the effects of components that interfere with intestinal cholesterol absorption on postprandial glucose and lipoprotein metabolism. Bozzetto et al.6 have shown that in type 2 diabetic patients with hypertriglyceridemia, 6 weeks of ezetimibe treatment lowered the incremental area under the curve (iAUC) for apoB48 concentrations in the chylomicron fraction, but not the iAUC for chylomicron cholesterol or triacylglycerol (TAG) concentrations. In line with these findings, Relas and colleagues7 have shown that in normolipidemic men, an acute intake of plant stanol esters did not lower postprandial TAG or cholesterol concentrations in the chylomicron fraction. In these studies, relations between postprandial changes in lipid and lipoprotein or glucose metabolism to changes in fasting serum lipid and lipoprotein concentrations after longer-term consumption were not examined. This is unfortunate, as the ability of subjects to respond to acute dietary challenges may reflect the flexibility to adapt to changes on the longer term.8 Therefore, we evaluated whether it is possible to predict individual responses to chronic consumption of plant stanol esters based on results obtained during a postprandial test at baseline. In addition, comparing the effects of an acute dietary challenge with and without plant stanol esters on postprandial lipid metabolism might also provide information on the cholesterol-lowering mechanism of plant stanols. We deliberately choose to compare the acute effects of a fat load containing no or 4 g of plant stanols as their fatty acid esters after subjects had followed for 1 week a plant sterol and stanol-poor diet. In this way, possible interference of day-to-day and inter-individual differences in plant sterol and stanol intake from the background diet was reduced, while an optimal contrast in intake was created.
Materials and methods
Study population
Subjects were recruited in Maastricht and surrounding areas through advertisements in local newspapers and via posters in the university and hospital buildings. They were invited for two screening visits if they met the following inclusion criteria: 18–60 years of age, body mass index between 20 and 30 kg/m2, stable body weight (weight gain or loss <2 kg in the previous 3 months), no use of lipid-lowering medication or a prescribed diet, no abuse of alcohol or drugs, no pregnancy or breast feeding, not smoking, not suffering from diabetes, no history of coronary artery disease, no history of gastrointestinal disorders and no participation in another lifestyle or pharmaceutical intervention study for the past 30 days. During the two screening visits, which were separated by at least 3 days, body weight, height and blood pressure were determined and blood was sampled for analysis of serum total cholesterol concentrations. Subjects were excluded if the mean serum total cholesterol concentration was >7.8 mmol/l. Once included, the subjects were asked not to change their dietary habits, level of physical exercise and alcohol intake during the duration of the study. In addition, those subjects regularly taking vitamin supplements were asked to discontinue this at least 1 month before the start of the study and prolonged during the study.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Ethical Committee of the Maastricht University Medical Centre. All participants gave written informed consent before entering the study. The trial was registered on clinicaltrials.gov under study number NCT01574417.
Study design
The study had a randomized, double-blind crossover design and consisted of two intervention periods of 4 weeks separated by a 4-week washout period (Figure 1). During randomization, subjects were stratified for age, gender and body mass index. To examine the acute effects of plant stanol esters on postprandial lipid and lipoprotein metabolism, each intervention period started with a 1-week period during which the subjects were instructed to avoid products relatively rich in plant sterols and stanols. These food items were listed and possible alternatives were discussed with a dietician. Furthermore, subjects were not allowed to consume products enriched with plant sterols/stanols during the study. To further minimize differences between the two postprandial test days, all subjects consumed the day before at dinner a standard lasagne meal, which was provided free of charge. Except for water, they were not allowed to consume any other foods or drinks after dinner, until the morning of the postprandial test. After a 12-h overnight fast, participants visited our department by public transportation or by car avoiding physical activity. After resting for 15 min in a supine position, an intravenous canula was inserted into the anticubital vein and a fasting blood sample was collected (T0). Next, subjects received 1 of the 2 test meals consisting of a slice of white bread with jam and a high-fat shake that was either enriched with plant stanol esters or not. The shake contained 3.3 MJ (797.5 kcal) energy provided by 51.3 g of fat (57.9 energy percent (en%), of which 23.9 en% was saturated fatty acids, 22.9 en% monounsaturated fatty acids and 11.1 en% polyunsaturated fatty acids), 11.7 g protein (5.9 en%) and 66.5 g carbohydrates (33.3 en%). The subjects were requested to consume the bread and shake within 10 min and were not allowed to eat or drink anything else, except water, during the next 4 h. Subsequent blood samples were collected at T=15 min (T15) after meal consumption, T=30 min (T30), T=45 min (T45), T=60 min (T60), T=120 min (T120), T=180 min (T180) and at T=240 min (T240). During the next 3 weeks, subjects consumed a margarine enriched with or without plant stanol esters (3 g/day). Subjects receiving the shake with plant stanol esters continued with the margarine containing plant stanol esters and subjects receiving the control shake in the postprandial test consumed for the next 3 weeks a control margarine. After the washout period, subjects received the other shake and margarines. Plant stanol esters are used as margarine throughout the 3 weeks of chronic consumption. During these 3 weeks, the daily intake was 20 g margarine providing 3 g plant stanols as fatty acid esters per day. From all available literature, we know that 3 g will result in an optimal LDL-cholesterol-lowering effect. The margarines were packed in tubs of 140 g, equivalent to margarine consumption for 7 days. All products were coded with a color label to blind the subjects and the investigators. In addition, the same margarine was used as the ingredient in the shakes that were specifically prepared and developed for this study. During the shake preparation, we used 26.7 g of the margarine as part of the fat source, as such providing 4 g of plant stanols as its fatty acid esters.
At the end of each test period (days 25, 28 and days 81, 85), subjects came to the university twice for taking a fasting blood sample. Body weight was determined at the beginning and at the end of each test period. Participants recorded in dairies any signs of illness, medication used, alcohol consumption, any deviations of the study protocol and any other complaints. They also recorded their food intake during the two test periods by completing food frequency questionnaires to estimate their energy and nutrient intakes. These frequency questionnaires were checked and calculated by a registered dietician.
Blood sampling
Blood was sampled in serum tubes, as well as in EDTA- and NaF-containing vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA). The EDTA and NaF tubes were placed on ice directly after sampling and centrifuged at 1300 g for 15 min at 4 °C within 60 min after sampling. Blood samples taken in serum tubes (Becton Dickinson) were allowed to clot for 30 min at 21 °C, followed by centrifugation at 1300 g for 15 min at 21 °C to obtain serum. Serum and plasma aliquots were directly snap-frozen in liquid nitrogen and stored at −80 °C until analysis. All samples from one subject were analyzed within the same analytical run.
Lipids and (apo)lipoproteins
In all fasting serum samples, serum total cholesterol (CHOD-PAP method; Roche Diagnostics Systems, Hoffmann-La Roche Ltd, Basel, Switzerland), high-density lipoproteins (HDL)-cholesterol (HDL-C) (CHOD/PAP method; Roche Diagnostics Systems, Hoffmann-La Roche) after precipitation of apoB-containing lipoproteins by adding phosphotungstic acid and magnesium ions (precipitation method; Monotest cholesterol, Boehringer, Mannheim, Germany) and TAG with correction for free glycerol (GPO Trinder; Sigma Diagnostics, St Louis, MO, USA) were analyzed enzymatically. LDL-C was calculated using the Friedewald equation.9 ApoB-100 and apoA-1 were measured using an immunoturbidimetric reaction (UNI-KIT apoB and UNI-KIT apoA-1, Roche, Basel, Switzerland). In addition, serum total cholesterol and TAG concentrations were also analyzed in the postprandial samples taken at all indicated time points.
Lipoprotein profiles
Serum lipoprotein profiles were determined using NMR spectroscopy (Liposcience, Raleigh, NC, USA) in EDTA plasma at 5 time points during the postprandial tests (T0, T60, T120, T180 and T240), as well as in fasting samples obtained at the end of each 3-week intervention periods, that is, days 28 and 85. Concentrations (nmol/l for VLDL and LDL particles and μmol/l for HDL particles) of the following subclasses were analyzed: large VLDL (>60 nm), medium VLDL (35–60 nm), small VLDL (27–35 nm), intermediate-density lipoprotein (IDL) (23–27 nm), large LDL (21.2–23 nm), small LDL (18–21.2 nm), medium small LDL (19.8–21.2 nm), very small LDL (18–19.8 nm), large HDL (8.8–13 nm), medium HDL (8.2–8.8 nm) and small HDL (7.3-8.2 nm). As NMR distinguishes lipoprotein subclasses on the basis of particle size alone, the largest VLDL fraction also includes the chylomicrons, which are especially present in the postprandial samples.10
Serum plant sterols and stanols
Concentrations of plant sterols, stanols and cholesterol precursors were determined at the start and at end of the 3-week intervention periods, that is, in serum samples from days 8 and 28, and from days 64 and 85, as described.11
Glucose, insulin, free fatty acid and high-sensitive C-reactive protein concentrations
Plasma glucose (Roche Diagnostic Systems, Hoffmann-La Roche) and free fatty acid (Wako Biochemicals) concentrations were measured in NaF plasma at all time points during the postprandial test days, as well as in fasting samples obtained on days 25, 28, 81 and 85. Serum insulin concentrations were determined at the same time points with a human insulin-specific radioimmunoassay (RIA) kit (Linco Research, St Charles, MO, USA). High-sensitive C-reactive protein was analyzed with a highly sensitive immunoturbidimetric assay (Kamiya Biomedical Company, Seattle, WA, USA).
Statistical analysis
A paired t-test was used to compare differences in fasting concentrations of the variables at the end of the intervention and control periods. For each subject, results of the two measurements taken at week 3 (days 25 and 28) and at week 12 (days 81 and 85) were averaged before statistical analysis. High-sensitive C-reactive protein concentrations were not normally distributed and analyzed with the nonparametric Mann–Whitney U-test. Changes over time of variables measured during the postprandial test were analyzed by linear-mixed models with diet and time as fixed factors and with diet × time as an interaction term. If this term was not significant, it was omitted from the model. Post hoc tests with Bonferroni's correction were carried out if factor time was significant to compare each concentration with baseline concentrations. At the postprandial test days, the iAUC was calculated using the trapezoidal rule12 for serum total cholesterol, TAG, glucose, insulin and all lipoprotein subfractions.
Pearson's correlation coefficients were determined between changes in the different parameters after the 3-week interventions and postprandial changes in serum lipid, TAG, free fatty acid, glucose and insulin concentrations at baseline. A P-value ⩽0.05 was considered statistically significant. All data are presented as means±standard deviation and all analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics and dietary intake data
Twenty subjects started the study. Owing to flu the evening before the first test day and tonsillitis 1 week before the second test day for which antibiotics had to be used, two subjects dropped out. Both events occurred during the week in which a plant sterol-poor diet was consumed, that is, before plant stanol ester consumption started. Therefore, these events were considered not to be related to the active ingredients. Baseline characteristics of the 18 subjects who completed the study are shown in Table 1. Energy and nutrient intakes did not differ between the two periods (supplementary table 1). There were also no significant changes in body weight during the study.
Serum lipid, lipoproteins, glucose, insulin, free fatty acids and inflammation
Three-weeks consumption of the margarine enriched with plant stanol esters reduced serum total cholesterol and LDL-C concentrations by 0.38±0.42 mmol/l (7.1%; P<0.01) and by 0.30±0.41 mmol/l (9.5%; P<0.01), respectively. Serum apoB100 concentrations were reduced by 0.07±0.09 g/l (8.6%; P<0.01). Serum HDL-C, TAG and apoA-1 concentrations did not change (Table 2). As compared with the control period, the number of total VLDL-CM, small VLDL and large LDL particles decreased by 17.2±27.8 (26.6%; P=0.02), 9.4±15.5 mmol/l (27.6%; P=0.02) and 56.4±109.3 mmol/l (12.3%; P=0.04), respectively, during the plant stanol ester period (Table 3). Glucose, insulin, free fatty acid and high-sensitive C-reactive protein concentrations did not differ between the placebo and intervention periods (Table 2).
Serum plant sterols, plant stanols, lathosterol and desmosterol
As expected, consumption of plant stanol esters decreased cholesterol-standardized concentrations of serum sitosterol by 44.6±27.6 102 × μmol/mmol cholesterol (P<0.01) and of campesterol by 71.3±44.7 102 × μmol/mmol cholesterol (P<0.01). Serum cholesterol-standardized concentrations of sitostanol concentrations increased by 16.9±6.5 102 × μmol/mmol cholesterol (P<0.01) and of campestanol by 7.3±3.3 102 × μmol/mmol cholesterol (P<0.01). For markers of endogenous cholesterol synthesis, no significant effect was found for lathosterol, whereas for desmosterol, concentrations significantly increased by 4.2±8.6 102 × μmol/mmol cholesterol (P=0.05; Table 2).
Predictive value of the postprandial test for chronic lipid-lowering efficacy
The major aim of the study was to evaluate whether metabolic characteristics of the postprandial response could be used to predict the response after 3-week plant stanol ester consumption. In this study, the serum total and LDL-C-lowering effect ranged from −14 to +9% for total cholesterol and from −25 to +15% for LDL-C, respectively.
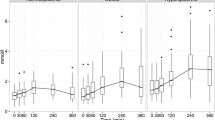
Interestingly, the iAUC of the postprandial glucose concentration obtained during the control period and the change in fasting total cholesterol after 3 weeks of plant stanol ester consumption were strongly correlated (r=0.66, P<0.01) (Figure 2). Moreover, comparable positive correlations were found between the iAUC of the postprandial glucose concentration and the changes in fasting concentrations of LDL-C (r=0.76, P<0.01), apoB100 (r=0.68, P<0.01), total VLDL (r=0.50, P=0.04), small VLDL (r=0.47, P=0.05) and IDL (r=0.48, P=0.05). The iAUC for insulin was not predictive for any of these parameters. In contrast to the iAUC for glucose, the baseline lathosterol/campesterol ratio, which has been suggested to predict the decrease in LDL-C after plant stanol ester consumption13 did not correlate with the observed LDL-C response after 3 weeks of plant stanol ester consumption (r=0.25, P=0.31). In agreement, this ratio also did not correlate with the iAUC for glucose (r=0.05, P=0.84).
Relationship between the postprandial iAUC of glucose with the changes in the concentrations of total cholesterol (a), LDL-C (b), apoB100 (c), total VLDL (d), small VLDL (e) and IDL (f). Each correlation plot consists of 18 data points corresponding to the number of subjects who consumed in random order a shake enriched with or without 4 g of plant stanol esters.
Effects of plant stanol esters on postprandial lipemia and glycemia
Fasting concentrations of serum total cholesterol, TAG, glucose and insulin before the 4-h postprandial period did not differ significantly. After the meals, serum total cholesterol concentrations decreased and those of TAG increased over time (P=0.02 and 0.01, respectively; Figure 3). These changes were comparable after the plant stanol ester and control meals. In addition, the iAUC of serum total cholesterol and TAG were comparable after consumption of both meals (P=0.55 and 0.27, respectively). Glucose and insulin concentrations increased after both meals. The factor time was significant for both conditions, but changes did not differ between the two meals. The iAUCs of glucose and insulin were also comparable after consumption of both meals (P=0.07 and 0.13, respectively).
Mean changes (±s.e.m.) in serum concentrations of total cholesterol (a), TAG (b), insulin (c) and plasma concentrations of glucose (d) and free fatty acids (e) following a shake enriched with (●) or without (○) plant stanol esters in a randomized crossover study with normolipidemic subjects (n=18). Data were analyzed using linear-mixed models. After consumption of the shake, there was a decrease in the concentration of total cholesterol and an increase in the concentration of TAG, insulin, glucose and free fatty acids, which was significant for the time factor (P⩽0.05). Values between the shakes did not differ (P>0.05). #After Bonferroni's correction significantly different from baseline (P⩽0.05).
As shown in supplementary Table 2, there were no significant changes in lipoprotein profiles over the 4-h postprandial period after intakes of the plant stanol ester and control meals. We only observed some diet-independent effects such as increases over time in the total numbers of VLDL-CM, large VLDL-CM and medium VLDL particles. No effect was found on concentrations of small VLDL particles. Interestingly, the concentrations of large and medium HDL particles were immediately increased after consuming the shake and those of small HDL particles decreased. Surprisingly, postprandial concentrations of large LDL particles, which is recognized as a postprandial appearing lipoprotein subclass,14 tended to decrease (P=0.06). The total number of LDL, as well as those of the various small LDL particles increased during the 4-h follow-up period.
As expected, there was a strong positive correlation between the iAUC of the postprandial TAG concentration and the iAUC of the postprandial concentration of large VLDL-CM particles (r=0.76, P<0.01 and r=0.88, P<0.01 for the control and the plant stanol ester periods, respectively). The iAUC of the postprandial TAG concentrations also correlated positively with the iAUC of the postprandial concentration of medium VLDL particles in the control group (r=0.57, P=0.01). For the other lipoprotein particles, no significant correlations were found.
Discussion
The mechanism underlying the beneficial effects of plant stanol esters on fasting serum LDL-C and possibly TAG concentrations15, 16, 17 is still actively evaluated and debated. Although the effects on LDL-cholesterol concentrations originate from reduced intestinal cholesterol absorption, the explanation for the reduction in serum TAGs has not been unraveled yet. To further understand these mechanisms and variations in responsiveness, acute dietary challenge studies may be helpful.8,18 After the intake of an acute single dose of plant stanol esters, however, no major postprandial changes were observed in parameters related to lipid and glucose metabolism. Yet, we found a clear association between the iAUC of the postprandial glucose concentration at baseline and changes in the concentrations of total cholesterol, LDL-C, apoB100, total VLDL, small LDL and IDL after 3 weeks of plant stanol ester consumption. This suggests that subjects with a more pronounced postprandial glucose response are less sensitive for the chronic LDL-C-lowering effect of plant stanol ester consumption.
As expected,16 19 3 weeks of consumption of plant stanol esters lowered serum LDL-C concentrations in normolipidemic subjects. In addition, we found reductions in the number of total VLDL particles, and in the concentrations of small VLDL and of large LDL particles. No decrease in small dense LDL particles was observed, which could be explained by the fact that these are metabolic products of the large TAG-rich VLDL particles,20 which were unchanged. Earlier studies have reported a significant reduction in the total number of LDL particles16 or in large and medium VLDL particles21 after consumption of plant stanol esters. However, these studies were conducted in subjects with familial hypercholesterolemia and in subjects diagnosed with the metabolic syndrome, and it cannot be excluded that effects in these populations are different. In contrast to our results, others have found in normolipidemic subjects a reduction in the number of large VLDL and IDL particles after plant stanol ester consumption,17 for which we have no explanation. Thus, on the basis of these limited number of studies, it is not possible to draw a conclusion on the effects of plant stanol esters on lipoprotein subclasses.
In general, plant stanol ester intervention studies show a large inter-individual variation in the cholesterol-lowering efficacy.13 To explain this inter-individual variation, various studies have focussed on genetic background22 or individual characteristics such as being a cholesterol absorber or not.23 For this, the plasma ratio of lathosterol to campesterol is frequently used.24 However, in our study this ratio did not correlate with the changes in the fasting lipid and lipoprotein profile. We did find, however, a strong positive correlation between the postprandial iAUC of glucose with changes in total cholesterol, LDL-C, apoB100, total VLDL, small VLDL and IDL. It should be noticed that the matrix (shake vs margarine) and the dose of plant stanol esters (4 vs 3 g) were different in the acute and semi-long-term intervention. However, it is not likely that this will affect the predictive value, as it was shown previously that the food matrix was not a determinant of the LDL-C-lowering efficacy of plant stanols.25 Our findings regarding a potential link with glucose concentrations are in agreement with the observations of Watts et al.,26 who suggested that subjects who are insulin resistant have a reduced susceptibility to interventions that lower intestinal cholesterol absorption. If true, the hypocholesterolemic effect of plant sterols/stanols in a metabolic syndrome population should be lower because of the lower intestinal absorption observed in these subjects.27,28 In contrast, on the basis of the available data from a number of controlled intervention studies, we have earlier concluded that the response toward plant sterol and stanol esters seemed to be larger in subjects with the metabolic syndrome.13 The question still remains as to how we can explain the inconsistency between the results from those studies and the current study together with the study from Watts et al.26 It should, however, be realized that no side-by-side comparisons regarding LDL-C-lowering responsiveness in healthy and, for example, diabetic subjects have been made. A logical explanation for our results could be that it relates not only to glucose but also to the regulatory effects of insulin. However, the iAUC for insulin, as well as the homeostatic model assessment index were not predictive, indicating that it is not insulin sensitivity as such that predicts responsiveness. Clearly, the data presented here suggest that the effects on cholesterol metabolism owing to the inhibition of intestinal cholesterol absorption could be mediated via changes in determinants of glucose metabolism. Future studies should therefore focus on transcription factors linking lipid, glucose and insulin metabolism such as sterol regulatory element-binding protein-2, carbohydrate-responsive element-binding protein and sterol regulatory element-binding protein-1c.
Unfortunately, we could not observe any plant stanol ester-induced effect on the postprandial lipoprotein profile, which is in agreement with the 4-h postprandial study of Gylling et al.29 It could be possible that the acute 4-h postprandial follow-up period in our study was not long enough to observe any significant changes in the postprandial lipid and lipoprotein profile induced by the plant stanol ester treatment. In addition, results could be different when subjects were studied after chronic intake of plant stanol esters.
In summary, the results of our study demonstrate that a single dose of plant stanol esters does not have an acute effect on postprandial glucose, lipid and lipoprotein metabolism. However, glucose responses during a postprandial test at baseline seemed predictive for individual responses in lipid and lipoprotein metabolism after 3 weeks of plant stanol ester consumption. This may suggest that the plant stanol ester-induced effects on cholesterol metabolism are associated with subjects' characteristics of glucose metabolism.
References
Baumgartner S, Mensink RP, Plat J . Plant sterols and stanols in the treatment of dyslipidemia: new insights into targets and mechanisms related to cardiovascular risk. Curr Pharm Des 2011; 17: 922–932.
De Smet E, Mensink RP, Plat J . Effects of plant sterols and stanols on intestinal cholesterol metabolism: suggested mechanisms from past to present. Mol Nutr Food Res 2012; 56: 1058–1072.
Brufau G, Kuipers F, Lin Y, Trautwein EA, Groen AK . A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One 2011; 6: e21576.
Rideout TC, Chan YM, Harding SV, Jones PJ . Low and moderate-fat plant sterol fortified soymilk in modulation of plasma lipids and cholesterol kinetics in subjects with normal to high cholesterol concentrations: report on two randomized crossover studies. Lipids Health Dis 2009; 8: 45.
Ottestad I, Ose L, Wennersberg MH, Granlund L, Kirkhus B, Retterstol K . Phytosterol capsules and serum cholesterol in hypercholesterolemia: a randomized controlled trial. Atherosclerosis 2013; 228: 421–425.
Bozzetto L, Annuzzi G, Corte GD, Patti L, Cipriano P, Mangione A et al. Ezetimibe beneficially influences fasting and postprandial triglyceride-rich lipoproteins in type 2 diabetes. Atherosclerosis 2011; 217: 142–148.
Relas H, Gylling H, Miettinen TA . Effect of stanol ester on postabsorptive squalene and retinyl palmitate. Metabolism 2000; 49: 473–478.
van Dijk SJ, Mensink M, Esser D, Feskens EJ, Muller M, Afman LA . Responses to high-fat challenges varying in fat type in subjects with different metabolic risk phenotypes: a randomized trial. PLoS One 2012; 7: e41388.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem 2004; 50: 1189–1200.
Plat J, Mensink RP . Effects of diets enriched with two different plant stanol ester mixtures on plasma ubiquinol-10 and fat-soluble antioxidant concentrations. Metabolism 2001; 50: 520–529.
Matthews JN, Altman DG, Campbell MJ, Royston P . Analysis of serial measurements in medical research. BMJ 1990; 300: 230–235.
Plat J, Mackay D, Baumgartner S, Clifton PM, Gylling H, Jones PJ . Progress and prospective of plant sterol and plant stanol research: report of the Maastricht meeting. Atherosclerosis 2012; 225: 521–533.
Dayspring T . Images, lipoproteins, drugs, and atherothrombotic events. Menopause 2009; 16: 632–634.
Rideout TC, Harding SV, Jones PJ . Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol Nutr Food Res 2010; 54: S7–13.
Theuwissen E, Plat J, van der Kallen CJ, van Greevenbroek MM, Mensink RP . Plant stanol supplementation decreases serum triacylglycerols in subjects with overt hypertriglyceridemia. Lipids 2009; 44: 1131–1140.
Plat J, Mensink RP . Plant stanol esters lower serum triacylglycerol concentrations via a reduced hepatic VLDL-1 production. Lipids 2009; 44: 1149153.
Strassburg K, Esser D, Vreeken RJ, Hankemeier T, Muller M, van Duynhoven J et al. Postprandial fatty acid specific changes in circulating oxylipins in lean and obese men after high-fat challenge tests. Mol Nutr Food Res 2014; 58: 591–600.
Katan MB, Grundy SM, Jones P, Lau M, Miettinen T, Paoletti R . Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003; 78: 965–978.
Packard CJ, Shepherd J . Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol 1997; 17: 3542–3556.
Plat J, Brufau G, Dallinga-Thie GM, Dasselaar M, Mensink RP . A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J Nutr 2009; 139: 1143–1149.
Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res 2002; 43: 486–494.
Rideout TC, Harding SV, Mackay DS . Metabolic and genetic factors modulating subject specific LDL-C responses to plant sterol therapy. Can J Physiol Pharmacol 2012; 90: 509–514.
Thuluva SC, Igel M, Giesa U, Lutjohann D, Sudhop T, von Bergmann K . Ratio of lathosterol to campesterol in serum predicts the cholesterol-lowering effect of sitostanol-supplemented margarine. Int J Clin Pharmacol Ther 2005; 43: 305–310.
Demonty I, Ras RT, van der Knaap HC, Duchateau GS, Meijer L, Zock PL et al. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J Nutr 2009; 139: 271–284.
Watts GF, Chan DC, Ooi EM, Nestel PJ, Beilin LJ, Barrett PH . Fish oils, phytosterols and weight loss in the regulation of lipoprotein transport in the metabolic syndrome: lessons from stable isotope tracer studies. Clin Exp Pharmacol Physiol 2006; 33: 877–882.
Paramsothy P, Knopp RH, Kahn SE, Retzlaff BM, Fish B, Ma L et al. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am J Clin Nutr 2011; 94: 1182–1188.
Hernandez-Mijares A, Banuls C, Jover A, Sola E, Bellod L, Martinez-Triguero ML et al. Low intestinal cholesterol absorption is associated with a reduced efficacy of phytosterol esters as hypolipemic agents in patients with metabolic syndrome. Clin Nutr 2011; 30: 604–609.
Gylling H, Hallikainen M, Simonen P, Miettinen HE, Nissinen MJ, Miettinen TA . Serum and lipoprotein sitostanol and non-cholesterol sterols after an acute dose of plant stanol ester on its long-term consumption. Eur J Nutr 2012; 51: 615–622.
Acknowledgements
We thank all study participants for their cooperation and enthusiasm. We also thank the technical and dietary staff from our department for their support. We thank Anja Kerksiek for the analysis of the plant sterols, stanols and cholesterol precursors. ED, RPM and JP designed the research; ED conducted the research; ED, RPM and JP analyzed the data; ED, RPM Dl and JP wrote the paper; and JP had primary responsibility for final content. All authors read and approved the final manuscript. The study was financially supported by Raisio, Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
De Smet, E., Mensink, R., Lütjohann, D. et al. Acute effects of plant stanol esters on postprandial metabolism and its relation with changes in serum lipids after chronic intake. Eur J Clin Nutr 69, 127–133 (2015). https://doi.org/10.1038/ejcn.2014.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.200
- Springer Nature Limited