Abstract
Evidence is accumulating that high serum concentrations of triacylglycerols (TAG) are, like LDL cholesterol, causally related to cardiovascular disease. A recent meta-analysis has indicated that plant stanol ester (PSE) intake not only lowered LDL cholesterol, but also serum TAG concentrations, especially in subjects with high baseline TAG concentrations. We therefore evaluated the effects of PSE supplementation on lipid metabolism in a population with elevated fasting TAG concentrations. In a randomized, placebo-controlled, parallel study, 28 subjects with elevated TAG concentrations (>1.7 mmol/L) were studied. After a 1-week run-in period during which a control margarine was used, subjects consumed for 3 weeks either control or PSE-enriched margarine (2.5 g/day of plant stanols). Serum plant stanol concentrations increased in all subjects receiving the PSE-enriched margarines, demonstrating good compliance. PSE supplementation significantly decreased serum total (6.7%, P = 0.015) and LDL cholesterol (9.5%, P = 0.041). A significant interaction between baseline TAG concentrations and PSE intake was found; PSE intake lowered TAG concentrations, particularly in subjects with high baseline TAG concentrations (>2.3 mmol/L; P = 0.009). Additionally, a significant interaction between baseline total number of LDL particles (LDL-P) and PSE intake was found (P = 0.020). PSE consumption lowered LDL-P, primarily in subjects with elevated baseline values; this was mainly due to a non-significant decrease in the number of atherogenic small LDL-P. Circulating levels of hs-CRP, glucose, and insulin were not changed after PSE intake. Taken together, PSE supplementation not only lowered LDL cholesterol, but also serum TAG concentrations, especially in subjects with overt hypertriglyceridemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The link between high serum low-density lipoprotein (LDL) cholesterol concentrations and cardiovascular disease (CVD) has been clearly established [1]. However, evidence is accumulating that high serum concentrations of triacylglycerols (TAG)—also known as hypertriglyceridemia—and low serum concentrations of high-density lipoprotein (HDL) cholesterol are also causally related to CVD [1]. TAG are major lipids in chylomicrons and very-low-density lipoprotein (VLDL) particles. These particles are closely related to the metabolism of other lipoproteins, including HDL. High TAG and low HDL often occur together, frequently with normal concentrations of LDL cholesterol, increased concentrations of small dense (sd) LDL and apoB, and insulin resistance [1]. This lipid abnormality is a fundamental characteristic of patients with the metabolic syndrome, a condition strongly associated with the risk to develop type II diabetes (DM2) and CVD [1].
Interestingly, a recent meta-analysis has indicated that consumption of plant stanol esters (PSE) not only lowers serum LDL cholesterol, but also serum TAG concentrations, in particular in subjects with high baseline TAG concentrations [2]. This meta-analysis was based on five trials carried out in our department and included almost 400 subjects. The reason that these effects have not been observed in individual studies may have been due to a lack of statistical power, as effects were only marginal in subjects with normal serum TAG concentrations. We therefore decided to design for the first time a study to specifically evaluate the effects of PSE on the serum lipoprotein profile in a population with elevated fasting serum TAG concentrations.
Subjects and methods
Subjects
Subjects, aged between 18 and 70 years, were recruited among an already existing cohort of patients diagnosed with familial combined hyperlipidemia (FCHL) at the Academic Hospital Maastricht (AZM). Patients were characterized by a specific phenotype, i.e. serum total cholesterol concentrations >6.5 mmol/L and/or elevated serum TAG concentrations (>2.3 mmol/L) at different visits before taking medication. Also subjects, who had serum TAG concentrations between 1.7 and 4.0 mmol/L, as indicated in earlier studies at our department, were approached. A fasting serum TAG concentration >1.7 mmol/L was chosen as lower boundary as this concentrations is considered to be elevated [3], while subjects with concentrations >4.0 mmol/L frequently need to be treated with medication [4].
Participants were further selected for the study according to the following inclusion criteria: no history of CVD such as congestive heart failure or recent (<6 months) event (acute myocardial infarction, CVA), type I and II diabetes mellitus, epilepsy, asthma, COPD (chronic obstructive pulmonary disease), inflammatory bowel diseases, cancer, or rheumatoid arthritis; no use of diuretics; no abuse of drugs and/or alcohol; willing to abstain from alcohol 3 days before blood sampling; no pregnant or breast-feeding women; and no use of an investigational product 30 days before the study. The Medical Ethical Committee of the University of Maastricht had approved the study. Participants were given a detailed description of the experimental protocol and purpose of the study before they gave their written informed consent. Twenty-nine volunteers were selected for the study. One subject withdrew in the third week of the study because of difficulty in performing venipuncture. All other 28 volunteers, 16 men and 12 women, completed the study. Baseline characteristics did not differ between the treatment groups, except for the number of smokers (Table 1).
Experimental design
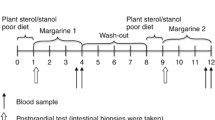
The study had a randomized, double-blind, placebo-controlled, parallel design. Two weeks before the start of the actual study and during the 4 weeks of the study, subjects had to stop [in consultation with their general practitioner (GP)] the intake of their regular cholesterol-lowering medication (statins or cholesterol-absorption inhibitors) when appropriate. No other classes of cholesterol-lowering drugs were used. Besides serum LDL and HDL cholesterol concentrations, this medication can also lower serum TAG concentrations. During the first 2 weeks of the study (before the run-in period), subjects were given a control margarine containing 60% absorbable fats. After these 2 weeks, the volunteers returned for blood sampling to the university. Further participation (and thus continuation of the medication-free period) was allowed only if serum total cholesterol concentrations were <8.0 mmol/L and TAG concentrations <4.0 mmol/L. Otherwise, subjects were send back to their GP and were advised to (re)start lipid-lowering medication according to the Dutch Cholesterol Consensus [4]. When serum cholesterol concentrations were <8.0 mmol/L and TAG concentrations between 1.7 and 4.0 mmol/L, subjects continued to consume daily 20 g of the control margarine for one more week. At the end of this run-in period, subjects were randomly divided over two groups, stratified for gender. The following 3 weeks (experimental period), one group continued to consume the control margarine, while the other group consumed daily 20 g of the experimental margarine, which had a similar fat content and fatty acid composition as the control margarine, but provided 2.5 g plant stanols. Plant stanols were made by saturation of plant sterols from tall oil, with a distribution of sitostanol (77.7%), campestanol (16.7%), sitosterol (2.2%), campesterol (1.8%), brassicasterol (0.1%), stigmasterol (0.1%), and other phytosterols (1.4%) (Raisio Group, Finland). The free plant stanols were esterified with fatty acids derived from sunflower oil before incorporation into the margarine. The margarine was packed in tubs of 140 g, equivalent to margarine for 7 days. Subjects had to divide the content of one tub into seven equal pieces of 20 g of margarine. One piece of 20 g had to be used on bread/crackers each day. All products were coded with a color label to blind the subjects and the investigators. All margarine leftovers had to be returned to the department and were weighed for the calculation of average daily margarine intake.
Participants recorded in diaries any signs of illness, medication used, menstrual phase, alcohol consumption, any deviations of the study protocol, and any of the following experienced complaints: headache, stomach complaints, nausea, bloated feeling, flatulence, diarrhea, constipation, itching, eruptions/rashes, fatigue and dizziness. Other possible side effects were monitored at the end of the run-in (day 21) and experimental period (day 42) by assessing parameters reflecting liver function.
Subjects also recorded their food intake for the previous 3 weeks at days 21 and 42 by completing food-frequency questionnaires (FFQ) to estimate their energy and nutrient intakes (Table 2). FFQ were checked by a registered dietician in the presence of the subjects. Body weight without shoes or heavy clothes was recorded at each visit.
Blood sampling
Five fasting blood samples were drawn during the study. All venipunctures were generally carried out by the same person, at the same location, and at the same time of the day (between 07:45 and 11:00 a.m.) on days 14, 21, 32, 37, and 42. Blood samples were taken from a forearm vein using vacutainers under minimal stasis with the subject in a supine position. On the morning of sampling, subjects were not allowed to smoke, to eat or to drink (except water). Volunteers had been fasting since 10 p.m. on the day preceding blood sampling. In addition, subjects were not allowed to drink alcohol 3 days before sampling.
Blood was collected in a 10-mL serum tube (Becton–Dickinson Vacutainer Systems, Breda, The Netherlands) for analysis of lipids (total cholesterol, HDL cholesterol, TAG), apolipoproteins (apoA-I, apoB-100), plant sterols and stanols, insulin, hs-CRP, and indices of liver function. Serum tubes were kept at room temperature after blood sampling. At least 1 h after venipuncture, serum was obtained by centrifuging at 2,000×g for 30 min at 4 °C. Serum was aliquoted and thereafter stored at −80 °C. At the same time, 4 mL of blood was also collected in a NaF tube (Becton–Dickinson Vacutainer Systems, Breda, The Netherlands) for analysis of glucose. Plasma was obtained immediately by centrifugation at 2,000×g for 30 min at 4 °C. Plasma was then divided into aliquots, snap-frozen, and stored at −80 °C. At days 21 and 42, blood was also collected in a 10-mL EDTA tube (Becton–Dickinson Vacutainer Systems, Breda, The Netherlands) for analysis of the plasma lipoprotein profile (lipoprotein particles). EDTA plasma was obtained directly by centrifugation of the EDTA tube at 2,000×g for 30 min at 4 °C. Plasma was then divided into aliquots, snap-frozen, and subsequently stored at −80 °C until analysis.
Side effects
Serum samples from days 21 and 42 were analyzed to determine parameters of liver function (alanine transaminase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, and total bilirubin). Measurements were carried out on a Beckman Coulter Synchron LX20 PRO Clinical System (Beckman Coulter Inc., Fullerton, CA, USA). All samples from one subject were analyzed in the same analytical run.
Inflammation
Serum samples from days 21 and 42 were analyzed to determine hs-CRP with a highly sensitive immunoturbidimetric assay (Kamiya Biomedical Company, Seattle, WA, USA). All samples from one subject were analyzed in the same analytical run.
Lipid metabolism
Concentrations of total cholesterol (ABX Diagnostics, Montpellier, France), HDL cholesterol (precipitation method; Roche Diagnostics Corporation, Indianapolis, IN, USA), and TAG corrected for free glycerol (Sigma–Aldrich Chemie, Steinheim, Germany) were analyzed enzymatically in all serum samples. Apolipoprotein concentrations (apoA-I, apoB-100) were analyzed in serum samples from days 14, 21, 37, and 42 using an immunoturbidimetric method (ABX Diagnostics, Montpellier, France). Serum LDL cholesterol concentrations were calculated by the formula of Friedewald et al. [5]. The plasma lipoprotein profile (lipoprotein particles) was analyzed with NMR by Liposcience (NMR LipoProfile test, Liposcience Inc., Raleigh, NC, USA). All samples from one subject were analyzed in the same analytical run.
Glucose metabolism
Plasma samples from days 14, 21, 37, and 42 were analyzed enzymatically to determine glucose concentrations by using the hexokinase method (Roche Diagnostic Systems, Hoffmann-La Roche Ltd., Basel, Switzerland). Insulin was measured using an ELISA method (DRG Instruments GmbH, Germany). The HOMA index, a measure of insulin sensitivity, was calculated [6]. All samples from one subject were analyzed in the same analytical run.
Serum plant sterols and stanols
Plant sterols and stanols (campestanol, sitostanol, campesterol, sitosterol, lathosterol) were determined in serum samples from days 21 and 42 as described [7]. All samples from one subject were analyzed in the same analytical run.
Statistics
The sample size was calculated at 14 subjects per group to provide 80% statistical power to detect a true difference in fasting serum TAG concentrations equal or greater than 0.50 mmol/L between both groups.
Before the statistical analyses were carried out, serum lipid and lipoprotein concentrations from days 14 and 21 and days 37 and 42 were averaged for each subject. Plasma glucose and insulin concentrations from days 14 and 21 and days 37 and 42 were averaged as well. Normality was tested by the Shapiro–Wilk test. Hs-CRP concentrations and indices of liver function were not normally distributed and analyzed accordingly by the non-parametric Mann–Whitney test. Differences in baseline characteristics between the control and intervention groups, and differences in changes in dietary intakes were analyzed by the independent Student’s t test. Metabolic parameters were analyzed by ANCOVA. To answer the question whether baseline subject characteristics influenced response-to-treatment, the baseline value of that variable was included (as a covariate) into the model together with an interaction term (baseline value × treatment). The interaction term was only included in the final statistical model if statistically significant. Values are presented as means ± SD. Pearson correlation coefficients were determined for the relationship between the PSE-induced changes in TAG and the changes in the different lipoprotein particles. Differences were considered statistically significant at a P < 0.05. Statistical analyses were performed using SPSS 11.0 (version 11.0.3) for Macintosh OS X (version 10.3.9).
Inspection of the diaries revealed that five subjects had consumed alcohol during the 3-day period before blood sampling. For this reason, their corresponding TAG values for that particular day were omitted from the analyses. In addition, one subject had to be excluded from the statistical analyses, because of alcohol consumption on days 14, 37, and 42. Another subject was excluded, because he confirmed that he had continued his use of lipid-lowering medication during a part of the study, as indicated by a questionnaire filled out at the end of the study. Therefore in the final statistical analyses, the data of 26 subjects were used.
Results
Side effects
Liver function was assessed to monitor side effects. Throughout the study, all variables remained within the normal range for all subjects and no treatment effects were present (data not shown).
Dietary intake and body weight
As calculated from the returned tubs, average daily consumption of margarine on the control and PSE diets was 19.3 ± 1.9 and 19.3 ± 1.8 g, respectively. Average daily plant stanol intake was therefore 2.41 ± 0.23 g. Changes in daily energy intake and nutrient composition of the diets did not differ among the treatment groups (Table 2). Changes in body weight were similar for the control and PSE groups (P = 0.579), and were −0.10 ± 0.92 and 0.11 ± 0.74 kg, respectively.
Inflammation
Concentrations of hs-CRP, a marker for inflammation, also did not change during the study. Five subjects had, unrelated to the dietary treatments, on one or more occasion hs-CRP concentrations >9 mg/L [8]. When these subjects were excluded from the analysis, conclusions did not change (data not shown).
Lipid metabolism
Compared to the control diet, consumption of PSE significantly decreased serum total and LDL cholesterol concentrations by 6.7% (P = 0.015) and 9.5% (P = 0.041), respectively. In addition, serum apoB-100 concentrations were significantly lowered by 7.1% (P = 0.007). Changes in serum HDL cholesterol and apoA-1 concentrations did not differ among the diet groups (Table 3). A significant interaction between baseline TAG value and PSE intake was found (P = 0.009). PSE consumption lowered TAG concentrations by 11% in subjects with high baseline TAG concentrations (>2.3 mmol/L). For subjects with lower baseline serum TAG concentrations (<2.3 mmol/L), PSE consumption did not affect serum TAG concentrations.
Further, a significant interaction between the baseline number of the total LDL particles (LDL-P) and PSE intake was found (P = 0.020; Table 4). Supplementation of PSE lowered the total number of LDL-P, primarily in subjects with elevated baseline values. This decrease was reflected by a reduction of similar magnitude in the number of sdLDL particles, but this decline did not reach statistical significance (P = 0.150).
Serum sterols and stanols
Cholesterol-standardized serum sterol and stanol concentrations are presented in Table 5. A significant interaction between baseline concentrations of cholesterol-standardized sitostanol and response-to-treatment was found (P = 0.011). Consumption of PSE increased cholesterol-standardized sitostanol concentrations, in particular in subjects with elevated baseline values. Additionally, eating the PSE-enriched margarine significantly increased cholesterol-standardized serum campestanol concentrations (P < 0.001). Plant stanol concentrations increased in every subject receiving the PSE-enriched margarines, which demonstrates good compliance with the study protocol.
Compared to the control diet, cholesterol-standardized serum sitosterol (P = 0.008) and campesterol (P = 0.020) concentrations significantly decreased after PSE consumption, indicating decreased intestinal cholesterol absorption [9]. A significant interaction between baseline cholesterol-standardized lathosterol concentrations and plant stanol intake was found (P = 0.043). Daily intake of PSE increased this marker for endogenous cholesterol synthesis, especially in subjects with elevated baseline values.
Glucose metabolism
Changes in plasma glucose and insulin concentrations, and HOMA index did not differ among the diet groups (data not shown).
Correlations
Significant correlations were found between PSE-induced changes in concentrations of TAG and large VLDL particles (r = 0.713, P < 0.001), large LDL-P (r = −0.575, P = 0.002), and sdLDL particles (r = 0.438, P = 0.025). Further, PSE-induced changes in TAG significantly correlated with medium HDL particle concentrations (r = 0.394, P = 0.047). Regarding the remaining lipoprotein particles, no significant correlations were found with the PSE-induced changes in TAG (data not shown).
Discussion
This study was specifically designed to examine the effects of PSE on serum TAG concentrations in subjects with elevated serum concentrations of TAG. In line with our recent meta-analysis [2], a significant interaction between baseline TAG concentrations and PSE intake was found. Also, we have recently shown that plant stanols lowered serum TAG concentrations in subjects with the metabolic syndrome [10]. Based on the meta-analysis [2], the expected decrease in serum TAG concentrations was on average 0.09 mmol/L for “borderline high” baseline TAG concentrations (1.7–2.2 mmol/L; [3]) and a daily plant stanol intake of 2.5 g. At a similar intake, the expected decline in serum TAG concentrations at “high” baseline TAG values (2.3–4.0 mmol/L; [3]), was on average 0.19 mmol/L. Compared to these predicted effects, our results are slightly different since a reduction in serum TAG concentrations was hardly present at baseline TAG values <2.3 mmol/L. In contrast, at “high” baseline TAG concentration, the average decrease was larger than anticipated, i.e. 0.35 mmol/L. It is possible that the estimates from the meta-analysis for subjects selected on disturbances in TAG metabolism are less precise, because most of the subjects in the meta-analysis had serum TAG concentrations <2.0 mmol/L.
As TAG in the fasted state are mainly transported by VLDL particles, it can be suggested that VLDL metabolism is changed after consumption of PSE. However, plasma concentrations of VLDL particles (large, medium, small) were not significantly altered after the PSE diet. TAG-rich lipoproteins (TRL), such as chylomicrons, VLDL (small, large), and TRL remnants play a significant role in the pathogenesis of atherosclerosis, the main cause of CVD [1]. It has been proposed that the potential atherogenicity of the large VLDL particles resides in their susceptibility to oxidation, which promotes foam cell formation by a mechanism analogous to that of oxidized LDL [11]. In support, however, of an effect on VLDL metabolism, a significant correlation was found between the PSE-induced changes in TAG and large VLDL particle concentrations. Regarding medium and small VLDL particles, no significant correlations were found between the change in these particle numbers and the change in TAG concentrations. Possibly, the variation in response of the different VLDL particles was too large to reach statistically significant dietary effects.
Daily consumption of 2.4 g plant stanols also significantly decreased serum LDL cholesterol concentrations by 9.5%. This change is consistent with the estimated mean change in LDL cholesterol concentrations of −8.9% for daily intakes of 2.0 to 2.4 g plant sterols or stanols [12]. Serum apoB-100 concentrations were lowered by 7.1%, which is in the same range as the reductions for total and LDL cholesterol. As expected, no effects on serum HDL cholesterol and apoA-1 concentrations were found. Plant sterols and stanols lower serum LDL cholesterol by interfering with the absorption of cholesterol in the intestine [13]. As a consequence, endogenous cholesterol synthesis and LDL receptor-mediated cholesterol uptake will increase, which will lead to decreased serum LDL cholesterol [14]. In support of this working mechanism and in agreement with previous studies [15, 16], consumption of PSE significantly lowered concentrations of markers reflecting cholesterol absorption and increased concentrations of a marker reflecting endogenous cholesterol synthesis. In our study, the serum LDL cholesterol-lowering effect was mainly related to a decrease (−11.2%) in the atherogenic sdLDL particles, but this effect was not statistically significant. A significant positive correlation however was found between the plant stanol-induced changes in TAG and sdLDL particle concentrations. sdLDL particles are particularly considered to be atherogenic, since these particles are retained preferentially by the artery wall and are readily oxidized [1, 17]. Generation of small, dense LDL occurs by intravascular lipoprotein remodeling as a result of metabolic disturbances, which are frequently present in patients with DM2 and the metabolic syndrome [17]. The common underlying predisposing factor may be the development of a fatty liver resulting in hypertriglyceridemia, due to in particular large VLDL particles. Large VLDL particles are a precursor for the synthesis of sdLDL by LPL mediated processes [18].
Hypertriglyceridemia is also associated with an overproduction of other cardiovascular risk factors, such as glucose, hs-CRP, plasminogen activator inhibitor-1 (PAI-1), fibrinogen, and coagulation factors [1]. We therefore looked into the effects of the PSE diet on glucose metabolism in our hypertriglyceridemic study population. However, consumption of PSE did not favorably alter glucose and insulin concentrations, and consequently the HOMA index (insulin resistance measurement) was also unchanged. These effects are in line with earlier findings in healthy subjects, where glucose concentrations were not changed after supplementation of a reduced-calorie orange juice beverage enriched with plant sterols (2 g/day) [19]. In addition, daily consumption of plant sterol ester (1.9 g plant sterols) and PSE (2.0 g plant stanols)-enriched spreads for 10 weeks did not change plasma glucose and serum insulin concentrations in hypercholesterolemic subjects [20].
There are some limitations of this study. First, we did not measure effects on postprandial TAG concentrations. As PSE consumption interferes with intestinal cholesterol absorption, it can be suggested that effects on postprandial lipid metabolism are even more pronounced. Studies on the postprandial effects of phytosterols are however scarce. In healthy subjects, 2-week PSE consumption (3 g/day plant stanols) only tended to diminish postprandial TAG concentrations [21]. In addition, the use of PSE (3 g/day plant stanols)-enriched margarine did not decrease postprandial triglyceridemia in statin patients [22]. Another limitation is the generalization of our findings to different etiologies of hypertriglyceridemia. Like hypercholesterolemia, hypertriglyceridemia has a broad genetic base and cannot be extrapolated depending on the mechanism.
To summarize, consumption of PSE significantly lowered serum LDL cholesterol in subjects with elevated TAG concentrations, primarily by a decrease in the atherogenic sdLDL particles. The PSE-induced TAG-lowering in these subjects was related to their baseline TAG concentrations, and was particularly evident in subjects with high serum TAG concentrations. Taken together, these findings show that functional foods enriched with PSE are not only of benefit to lower increased serum LDL cholesterol concentrations, but also those of TAG in subjects with overt hypertriglyceridemia.
Abbreviations
- TAG:
-
Triacylglycerols
- PSE:
-
Plant stanol ester
- LDL-P:
-
LDL particles
- LDL:
-
Low-density lipoprotein
- CVD:
-
Cardiovascular disease
- HDL:
-
High-density lipoprotein
- VLDL:
-
Very-low-density lipoprotein
- sd:
-
Small dense
- DM2:
-
Type II diabetes
- FCHL:
-
Familial combined hyperlipidemia
- AZM:
-
Academic Hospital Maastricht
- GP:
-
General practitioner
- hs-CRP:
-
High-sensitivity C-reactive protein
- FFQ:
-
Food-frequency questionnaires
- TRL:
-
TAG-rich lipoproteins
- PAI-1:
-
Plasminogen activator inhibitor-1
- n.a.:
-
Not applicable
References
Malloy MJ, Kane JP (2001) A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med 47:111–136
Naumann E, Plat J, Kester AD, Mensink RP (2008) The baseline serum lipoprotein profile is related to plant stanol induced changes in serum lipoprotein cholesterol and triacylglycerol concentrations. J Am Coll Nutr 27:117–126
Haymore BR, Parks JR, Oliver TG, Glister BC (2005) Hypertriglyceridemia. Hospital Physician, 17–24
Syllabus behandeling en preventie van coronaire hartziekten door verlaging van de plasmaconcentratie. Utrecht: Centraal Begeleidingsorgaan voor de Intercollegiale Toetsing, 1998
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Plat J, Mensink RP (2001) Effects of diets enriched with two different plant stanol ester mixtures on plasma ubiquinol-10 and fat-soluble antioxidant concentrations. Metabolism 50:520–529
Shine B, de Beer FC, Pepys MB (1981) Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta 117:13–23
Nissinen MJ, Gylling H, Miettinen TA (2008) Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br J Nutr 99:370–378
Plat J, Brufau G, Dallinga-Thie GM, Dasselaar M, Mensink RP (2009) A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J Nutr 139:1143–1149
Havel RJ (2000) Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol 11:615–620
Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R (2003) Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 78:965–978
Plat J, Nichols JA, Mensink RP (2005) Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J Lipid Res 46:2468–2476
Plat J, Mensink RP (2002) Effects of plant stanol esters on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase mRNA expression in mononuclear blood cells of healthy men and women. Faseb J 16:258–260
Vuorio AF, Gylling H, Turtola H, Kontula K, Ketonen P, Miettinen TA (2000) Stanol ester margarine alone and with simvastatin lowers serum cholesterol in families with familial hypercholesterolemia caused by the FH-North Karelia mutation. Arterioscler Thromb Vasc Biol 20:500–506
Theuwissen E, Mensink RP (2007) Simultaneous intake of {beta}-glucan and plant stanol esters affects lipid metabolism in slightly hypercholesterolemic subjects. J Nutr 137:583–588
Packard CJ (2003) Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans 31:1066–1069
Cohn JS, Marcoux C, Davignon J (1999) Detection, quantification, and characterization of potentially atherogenic triglyceride-rich remnant lipoproteins. Arterioscler Thromb Vasc Biol 19:2474–2486
Devaraj S, Autret BC, Jialal I (2006) Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am J Clin Nutr 84:756–761
Hallikainen M, Lyyra-Laitinen T, Laitinen T et al (2006) Endothelial function in hypercholesterolemic subjects: effects of plant stanol and sterol esters. Atherosclerosis 188:425–432
Relas H, Gylling H, Miettinen TA (2000) Effect of stanol ester on postabsorptive squalene and retinyl palmitate. Metabolism 49:473–478
Castro Cabezas M, de Vries JH, Van Oostrom AJ, Iestra J, van Staveren WA (2006) Effects of a stanol-enriched diet on plasma cholesterol and triglycerides in patients treated with statins. J Am Diet Assoc 106:1564–1569
Acknowledgements
We thank the study participants for their cooperation and enthusiasm. We also thank the technical and dietary staff from our department for their support. The study was supported financially by the Raisio Group Finland.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Theuwissen, E., Plat, J., van der Kallen, C.J. et al. Plant Stanol Supplementation Decreases Serum Triacylglycerols in Subjects with Overt Hypertriglyceridemia. Lipids 44, 1131–1140 (2009). https://doi.org/10.1007/s11745-009-3367-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3367-6