Abstract
Dietary saturated fat (SFA) intake has been associated with elevated blood lipid levels and increased risk for the development of chronic diseases. However, some animal studies have demonstrated that dietary SFA may not raise blood lipid levels when the diet is sufficient in omega-3 polyunsaturated fatty acids (n-3PUFA). Therefore, in a randomised cross-over design, we investigated the postprandial effects of feeding meals rich in either SFA (butter) or vegetable oil rich in omega-6 polyunsaturated fatty acids (n-6PUFA), in conjunction with n-3PUFA, on blood lipid profiles [total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) and triacylglycerol (TAG)] and n-3PUFA incorporation into plasma lipids over a 6-h period. The incremental area under the curve for plasma cholesterol, LDL-C, HDL-C, TAG and n-3PUFA levels over 6 h was similar in the n-6PUFA compared to SFA group. The postprandial lipemic response to saturated fat is comparable to that of n-6PUFA when consumed with n-3PUFA; however, sex-differences in response to dietary fat type are worthy of further attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1940s, research on dietary saturated fats (SFA) has suggested adverse effects on health [1–3]. However, the data is heterogeneous and both epidemiological and interventional studies have produced contradictory results. Most studies have observed that SFA were positively associated with blood lipids and chronic disease risk [4–6], although some have found no association [7–13] or even a negative association [14, 15]. On the contrary, polyunsaturated fatty acids (PUFA) have been associated with protective effects on health and suggested as an alternative for saturated fat consumption, although not all PUFA have the same protective effects. Omega-3 polyunsaturated fatty acids (n-3PUFA) are known for their potential in the management of hyperlipidaemia [16, 17] and for their anti-arrhythmic [18, 19], anti-aggregatory [20] and anti-inflammatory potential, therefore contributing to the prevention of chronic diseases such as coronary heart disease [21]. However, omega-6 polyunsaturated fatty acids (n-6PUFA) are precursors of pro-inflammatory eicosanoids and although their cholesterol-lowering benefits have been reported [2, 22, 23], an increase in their intake has also been associated with an increase in the death rate from cardiovascular disease, coronary heart disease and all-cause mortality [24]. Additionally, the competition between n-3 and n-6PUFA has been broadly discussed [25–33] and it is well known that the n-6PUFA metabolism is favoured over n-3PUFA metabolism. The impact on blood lipid levels of a diet combining n-6PUFA and n-3PUFA has not being analysed in human trials.
Studies using animal models have indicated that saturated fats raise blood lipid levels only when the diet is deficient in n-3PUFA [34, 35]. Furthermore, chronic and acute interventional studies with healthy subjects demonstrated lower blood triacylglycerol (TAG) [36, 37] after consuming a combination of SFA and long chain n-3PUFA (LCn-3PUFA) when compared with an SFA diet or meal.
Postprandial studies comparing saturated fat-rich meals and n-6PUFA-rich meals are contradictory and do not consider n-3PUFA on the meal or in the subject’s daily diet. Some authors have observed no difference in postprandial plasma TAG in men who were healthy, overweight or with metabolic syndrome [38, 39] consuming high SFA (dairy-based) or n-6PUFA (vegetable oil) meals. While one study observed postprandially lower plasma TAG [40] and another observed higher plasma TAG [41] after consuming a high SFA meal (butter and palm oil + coconut butter, respectively), both compared to a high n-6PUFA meal. Furthermore, plasma cholesterol was not assessed in any postprandial study comparing SFA and n-6PUFA.
To date, the postprandial effects of a combination of LCn-3PUFA with different background fat types on blood lipids have not been studied in humans. Different fat types are usually considered in isolation, although consumed in combination in usual diets. Furthermore, dietary and postprandial effects of high n-6PUFA or SFA intake supplemented with LCn-3PUFA have also not been directly compared in human studies, although analysed individually in separate studies [36, 37, 42]. We have previously hypothesised that saturated fat consumption would not raise blood lipid levels if the diet was sufficient in n-3PUFA [43]. Therefore, in this study, we examined blood lipid levels [total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and TAG] and LCn-3PUFA levels in plasma when the background fat of the meal was high either in saturated fat or in n-6PUFA.
Materials and Methods
Participants
The study population consisted of 26 healthy adults (18 women and 8 men) aged between 18 and 65 years. Participants were excluded if they were using lipid-lowering drugs (e.g. statins); had consumed fish oil supplements regularly within the past month; had regular consumption of two or more fish meals a week over the past month; had any history of congestive heart failure, stroke, myocardial infarction, coronary artery bypass graft, or atherosclerotic CVD; had history of diabetes; had history of gastrointestinal or liver disease; were smokers; or were pregnant or breast feeding. The number of study subjects was calculated to obtain 80 % power and produce a significant increase in plasma total cholesterol of about 5 % with standard deviation of 0.5 mmol/L [44]; and 22 participants were necessary to produce the desired effect.
Study Design
The study was a randomised controlled, cross-over, acute postprandial study. Following an overnight fast, participants visited the university on two occasions and consumed one of two meals, consisting of 150 g mashed potato mixed with either 38 g butter (SFA meal) or 32 g sunflower oil (n-6PUFA meal). Two hundred millilitres of water and 3 × 1 g fish oil capsules [100:500 mg eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) (EPAX 1050TG, Norway)] were also consumed as part of each of the meals. A similar dose of LCn-3PUFA (1.5 g) has been shown to be effective in a previous postprandial study [45]. The SFA meal contained 19 g carbohydrates, 3.8 g protein and 31.8 g fat (20.6 g saturated, 8.3 g monounsaturated, 0.7 g n-6PUFA and 2.1 g n-3PUFA) and the n-6PUFA meal contained 18.8 g carbohydrates, 3.5 g protein and 32.2 g fat (3.4 g saturated, 8 g monounsaturated, 19.1 g n-6PUFA and 1.9 g n-3PUFA). Test meals were consumed within 15 min. Blood was collected after an overnight fast and 1, 3, 4 and 6 h post meal consumption. Following a minimum of 1-week washout period, the same procedure was repeated, after consumption of the alternate meal. A 24-h food recall was used to measure the participants’ nutrient intake prior to the study meal. Blood lipid profile (total cholesterol, LDL-C, HDL-C and TAG) was measured at each time point. All volunteers gave written informed consent before participation. The University of Newcastle Human Research Ethics Committee approved the study (protocol H-2012-0117), and the study was registered with the Australia New Zealand Trial registry as ACTRN12612000654853.
Plasma Lipid Profile
Blood was collected in lithium heparin vacutainers, plasma was immediately separated from erythrocytes by centrifugation (3,000g × 10 min at 4 °C) and analysed for lipid profile (total cholesterol, LDL-C and HDL-C and TAG) by the Hunter New England Area Pathology Service.
Plasma Fatty Acid Composition
Blood was collected in EDTA vacutainers and plasma was immediately separated from the erythrocytes by centrifugation (1,000g × 15 min at 4 °C) and stored at −80 °C until analysis. Incorporation of fatty acids into plasma was determined using gas chromatography following transesterification. Fatty acids were methylated according to the method reported by Lepage and Roy [46] and C:19 was used as an internal standard; the methyl ester products were then separated, identified and quantified using a 30 m × 0.25 mm (DB-225) fused carbon–silica column, coated with cyanopropylphenyl (J & W Scientific, Folsom, CA) and Hewlett Packard 6890A series gas chromatograph with Chemstations Version A.04.02 for gas chromatographic analysis [47]. Fatty acid peaks were identified by comparison with standard mixtures of fatty acid methyl esters of known composition and concentration.
Statistical Analysis
Postprandial lipemia and plasma LCn-3PUFA were measured for each time point and the incremental area under the curve (iAUC) determined using the trapezoidal rule. All data is presented as mean and standard deviation. Measurements obtained for area were compared using paired t test and Wilcoxon signed-rank test for parametric and non-parametric data, respectively. The two-way analysis of variance with repeated measurements was applied to analyse meal effect over time (interaction meal × time) and the one-way analysis of variance with repeated measurements was applied to analyse the existence of difference between baseline and subsequent time points within meal. Change from baseline to each time point within meal was assessed using paired t test and Wilcoxon signed-rank test for parametric and non-parametric data, respectively. For all tests a P value lower than 0.05 was considered statistically significant. Stata IC 11.2 (StataCorp LP) was used to perform the statistical analysis.
Results
Twenty-six healthy subjects (18 womens and 8 mens) participated in the study. Their general characteristics, as an average over their their visits, are presented in Table 1. All participants consumed both meals and did not show any sign of intolerance to the meals or supplements.
At baseline men and women did not differ significantly in age, body mass index (BMI) and blood levels of TAG, LDL-C and LCn-3PUFA (Table 1). However, women presented with a higher percentage body fat, total cholesterol and HDL-C than men (Table 1). Nutrient intake of subjects the day before each dietary intervention is presented in Table 2 and did not differ between test meals. Postprandial change in total SFA was significantly higher (P < 0.001) after the SFA meal, while change in total n-6PUFA was significantly higher (P < 0.001) after the n-6PUFA meal (data not shown).
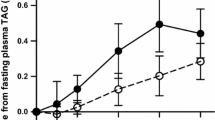
Postprandial metabolic measures following the two meals consumed for all subjects, men and women are presented in Table 3. When comparing the two test meals for all subjects, no significant difference was observed in iAUC for plasma TAG, total cholesterol, LDL-C, HDL-C and LCn-3PUFA. However, iAUC for total cholesterol in men was higher after the SFA meal compared with the n-6PUFA meal, with the difference reaching borderline statistical significance (P = 0.05) (Table 3).
There were time-dependent changes in TAG for all subjects and for women after the consumption of the saturated fat meal (P < 0.001 for both) and n-6PUFA meal (P = 0.004 and P = 0.002, respectively) (Table 3). There was a significant increase in TAG from baseline to 3 h (P < 0.001 for all subjects and women); at 6 h, TAG levels were not significantly different than baseline, indicating that TAG levels returned to baseline values after 6 h of meal consumption, particularly for women. For men, however, TAG levels increased significantly at 3 h after consuming the saturated fat meal (P = 0.017) and the n-6PUFA meal (P = 0.042). TAG were still significantly higher than the baseline levels 6 h after the saturated fat meal (P = 0.036), although decreasing from 4 to 6 h, and tended to remain higher after the n-6PUFA meal (P = 0.069) and still increased between 4 and 6 h (Table 3).
Plasma levels of total cholesterol did not present a time-dependent effect after consumption of the n-6PUFA meal for all subjects, men or women. However, after consumption of the saturated fat meal, the levels of total cholesterol were significantly higher than the baseline values at 4 and 6 h for all subjects (P = 0.004 and P = 0.032, respectively) and at 3, 4 and 6 h for men (P = 0.041, P = 0.014 and P = 0.017, respectively). Although total cholesterol levels started decreasing 4 h after the saturated fat meal consumption for all subjects, cholesterol levels were still increasing for men at 6 h (Table 3).
Levels of LDL-C were time dependant for all subjects and women after n-6PUFA meal consumption (P = 0.042 and P = 0.030, respectively) and for all subjects and men after the saturated fat meal consumption (P = 0.007 and P = 0.015, respectively). Levels of LDL-C were significantly higher than the baseline after 6 h of saturated fat meal consumption for all subjects and men (P = 0.019 and P = 0.017, respectively). After consumption of the n-6PUFA meal, there was a significant decrease in LDL-C at 1 and 3 h for all subjects (P = 0.016 and P = 0.009, respectively) and women (P = 0.026 and P = 0.020, respectively), followed by an increase at 4 and 6 h, returning to the levels observed at baseline (Table 3).
There was no time-dependent variation of HDL-C after the consumption of the saturated fat meal or n-6PUFA meal, for all subjects, women or men (Table 3).
There was a time-dependent increase in LCn-3PUFA when participants consumed both diets. Plasma LCn-3PUFA was higher than baseline at all times from 1 h for all subjects summed and for women after consumption of both meals; and from 3 h for men after consumption of both meals (Table 3).
Discussion
The present study was designed to evaluate if meals containing either butter as a source of saturated fat or vegetable oil rich in n-6PUFA as the background fat, in association with LCn-3PUFA, would differentially impact postprandial blood lipid profiles and incorporation of LCn-3PUFA into plasma lipids. Equivalent ingested amounts of saturated fat (butter) or n-6PUFA (sunflower oil) in combination with LCn-3PUFA supplementation induced similar postprandial responses for plasma cholesterol pools (total cholesterol, HDL-C and LDL-C), suggesting comparable responses in lipoprotein metabolism. Saturated fats did not affect postprandial blood lipid profiles differently than n-6PUFA, when consumed in combination with LCn-3PUFA, indicating that LCn-3PUFA may have counteracted the well-described hyperlipidaemic effects of saturated fats [1, 48–50].
Epidemiological studies [3, 5] have indicated a positive association between dietary saturated fat and serum cholesterol, although Samuelson and co-workers [14] revealed a negative correlation between saturated fats and serum cholesterol. Saturated fats have also been shown to increase LDL-C level by reducing LDL receptor (LDL-r) expression [48, 51], consequently reducing the number of receptors and LDL-C uptake by the liver. Other mechanisms have also been proposed, a meal high in SFA has been shown to increase lecithin:cholesterol acyltransferase activity and to reduce the influx of free cholesterol from plasma to cell [52]. Chronic and acute clinical trials with healthy subjects comparing a diet high in saturated fat, with and without LCn-3PUFA supplementation, demonstrated a decrease in blood TAG and very low density lipoprotein cholesterol (VLDL-C) and an increase in LDL-C in the long term [37] and tendency for lower TAG postprandially [36] when LCn-3PUFA was supplemented concurrently (2.4 g EPA + DHA daily and 0.07 g EPA + DHA [9:77] per kg of body weight, respectively). Men with metabolic syndrome, on the other hand, presented similar TAG change when a high saturated fat meal (palm oil) was compared to the same meal supplemented with LCn-3PUFA (4 g fish oil providing 48 % EPA and 25 % DHA) [42]. Notably, both the meals provided similar amounts of n-6PUFA, causing an ambiguity regarding the effectiveness of the LCn-3PUFA supplementation. Notably, most of the studies reported in the literature were long-term studies and to date none of the postprandial studies assessed blood cholesterol levels, especially when LCn-3PUFA are co-supplemented.
Despite the well-documented ability of n-6PUFA to reduce blood lipid levels [5, 41, 49, 51, 53], the n-6PUFA meal did not affect blood lipid levels differently than the SFA meal. In contrast, Mekki et al. [40] observed a lower area under the TAG curve after the consumption of a butter-based meal compared to a sunflower oil-based meal in healthy young men, while Jackson et al. [41] observed a higher area under the TAG curve after a saturated fat meal (palm oil + cocoa butter) compared to an n-6PUFA meal (safflower oil). Other authors compared SFA-based (dairy fat) and n-6PUFA-based meals observing no difference in plasma TAG area under the curve between test meals for men who were overweight [39], healthy or with metabolic syndrome [38]. However, none of these authors fed LCn-3PUFA to their subjects or controlled for LCn-3PUFA present in erythrocytes or in their usual diets. In addition, n-6PUFA were inversely correlated with total cholesterol in epidemiological studies [5] and were shown to reduce LDL-C in animals [51] by upregulating LDL receptor synthesis and consequently increasing LDL-C uptake by the liver. The results observed in this study are suggestive of the competition [26, 28, 29] between n-3 and n-6PUFA, as n-3PUFA has been shown to induce an increase in LDL-C owing to their capacity to downregulate LDL-r [54], thus modulating the hypocholesterolaemic effect of n-6PUFA. It is noteworthy that, when both the meals were co-supplemented with LCn-3PUFA, similar postprandial lipoprotein responses were induced, despite the fact that the saturated fat meal provided an additional 95 mg of cholesterol and that saturated fat consumption was positively correlated with cholesterol levels in previous studies [5]. Other acute postprandial studies have not compared cholesterol change between test meals. Furthermore, maximum postprandial changes were observed with the two fat sources (30 g fat/meal) after 3–4 h.
LCn-3PUFA content increased gradually over 6 h following consumption of the two test meals supplemented with the same amount of n-3PUFA. However the overall change in plasma LCn-3PUFA did not differ between the two meals consumed. These results are in contrast to a chronic feeding study [34] showing a greater increase in plasma LCn-3PUFA when animals were fed a high saturated fat diet compared with those fed a high n-6PUFA diet, supplemented with the same level of n-3PUFA. We have also previously demonstrated that the amount of n-3PUFA required to cause any beneficial effects was lower in rats consuming diets rich in SFA than those on n-6PUFA [35]. Nevertheless, none of the previous studies have reported postprandial changes in LCn-3PUFA on dietary interventions containing different background fats, particularly in human subjects. Differences in LCn-3PUFA driven by combination with other fats (e.g. SFA or n-6PUFA) would be more likely to be observed in the long term, when competition between fat types would be sustained over weeks or months, rather than just a few hours. Competition in the metabolism of n-3PUFA and n-6PUFA has been demonstrated, comprising fatty acid elongation and desaturation and eicosanoids formation [18, 26, 28–30]; changes being also observed in cell membrane composition, fluidity and permeability [55].
Our study population was a mix of men and pre- and postmenopausal women (8, 10 and 8 subjects, respectively). Data presented suggested that men may in general have a slower fat metabolism when compared to women. Plasma TAG levels were increasing even after 6 h of consuming fatty meals in men, while TAG levels peaked at 3 h and then dropped back to baseline values at 6 h in women. Consistent with our findings, Couillard et al. [56] reported different postprandial peaks and clearance of plasma TAG in men and women, and suggested an impaired TAG clearance in men compared to women. Furthermore, Koutsari et al. [57] reported higher area under the plasma TAG curve for men than women. Additionally, there was no difference between the two test meals in the rate of postprandial cholesterol clearance in men or women, and slower cholesterol clearance was evident in men despite adequate LCn-3PUFA supplementation. Although not statistically significant, men presented a tendency for higher cholesterol response after consumption of the SFA meal than after the n-6PUFA meal, suggesting a more pronounced change in postprandial plasma cholesterol than in women. The expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR) has previously been shown to differ in men and women; however, whether this would influence their response to dietary fat treatments remains to be seen [58]. Additionally, oestrogen was shown to promote LDL-r gene expression, increasing the number of LDL-r in the presence of higher concentration of estrogen and cholesterol clearance in women [59]. Nevertheless, these mechanisms may not account for the differences in postprandial lipid metabolism following consumption of meals differing in fat composition. The present study was not designed to specifically assess sex differences; therefore the observed differences between men and women need to be confirmed with a study design adequately powered for both sexes. However, the physiological differences between men and women suggest that in future studies men and women, as well as different menopausal statuses, should be considered during study planning and designing, to account for gender and hormonal differences.
Butter fat was used as the source of saturated fat in this study, although it differs in fatty acid composition from other sources [60] and may present different metabolic pathways and effect on plasma lipids [61]. Butter is rich in stearic acid, which has been shown to cause no increase in cholesterol levels [62], and in short and medium chain fatty acids, which are more easily absorbed than long chain SFA [63]. Short and medium chain SFA bind to albumin for transport to the liver, bypassing assembling into triglycerides and packaging into chylomicrons, unlike long chain fatty acids [63]. Therefore, further studies are necessary to understand if the results observed in this study would also be applicable to other saturated fats such as those present in chocolate, red meats and vegetable oils including palm and coconut oil. The authors also acknowledge that the study design was limited by not having two additional treatment groups, namely butter alone and sunflower oil alone, so that the real effect of the fish oils could be better established.
Postprandial responses in terms of plasma lipid profiles and plasma LCn-3PUFA concentrations were similar when subjects consumed either SFA or n-6PUFA in association with n-3PUFA, providing support for our hypothesis [43] that saturated fats do not raise blood lipid levels, compared to n-6PUFA, when co-administered with adequate LCn-3PUFA. Long-term intervention trials are warranted to substantiate the importance of LCn-3PUFA status in determining the effects of saturated fat-rich diets versus n-6PUFA-rich diets.
Abbreviations
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- EPA:
-
Eicosapentaenoic acid
- HDL-C:
-
High density lipoprotein cholesterol
- iAUC:
-
Incremental area under the curve
- LCn-3PUFA:
-
Long chain omega-3 polyunsaturated fatty acids
- LDL-C:
-
Low density lipoprotein cholesterol
- PUFA:
-
Polyunsaturated fatty acids
- n-3PUFA:
-
Omega-3 polyunsaturated fatty acids
- n-6PUFA:
-
Omega-3 polyunsaturated fatty acids
- SFA:
-
Saturated fat
References
Hegsted DM, Mcgandy RB, Myers L, Stare FJ (1965) Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr 17:281–295
Keys A, Anderson JT, Grande F (1957) Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 273:959–966
Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MT, Kromhout D, Nedeujkovic S, Punsar S, Seccareccia F, Toshima H (1986) The diet and 15-year death rate in the Seven Countries Study. Am J Epidemiol 124:903–915
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn R, Buzina R, Dontas AS, Fidanza F, Giaipaoli S, Jansen A, Karvonen M, Katan M, Nissinen A, Nedeljkovic S, Pekkanen J, Pekkarinen M, Punsar S, Rasanen L, Simic B, Toshima H (1995) Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med 24:308–315
Shekelle RB, Shryock AM, Paul O, Lepper M, Stamler J, Liu S, Raynor WJ (1981) Diet, serum cholesterol, and death from coronary heart disease. N Engl J Med 304:65–70
Yamagishi K, Nettleton JA, Folsom AR (2008) Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 156:965–974
Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, Newman AB, Visser M, Kritchevsky SB (2011) Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis 21:430–437
Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC (1999) Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr 70:1001–1008
Hu FB, Stampfer MJ, Manson JE, Rim E, Colditz GA, Rosner BA, Hennekens CH, Willett WC (1997) Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 337:1491–1499
Mirmiran P, Ramezankhani A, Azizi F (2009) Combined effects of saturated fat and cholesterol intakes on serum lipids: Tehran Lipid and Glucose Study. Nutrition 25:526–531
Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC (2005) Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses’ Health Study. Am J Epidemiol 161:672–679
Posner BM, Cobb JL, Belanger AJ, Cupples A, D’Agostino RB, Stokes J (1991) Dietary lipid predictors of coronary heart disease in men. Arch Intern Med 115:1181–1187
Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC (2001) Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 73:1019–1026
Samuelson G, Bratteby L, Mohsen R, Vessby B (2001) Dietary fat intake in healthy adolescents: inverse relationships between the estimated intake of saturated fatty acids and serum cholesterol. Br J Nutr 85:333–341. doi:10.1079/BJN2000279
Tholstrup T, Marckmann P, Jespersen J, Vessby B, Jart A, Brittmarie S (1994) Effect on blood lipids, coagulation, and fibrinolysis of a fat high in myristic acid and a fat high in palmitic acid. Am J Clin Nutr 60:919–925
Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ (2002) Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 76:1007–1015
Eslick GD, Howe PRC, Smith C, Priest R, Bensoussan A (2009) Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol 136:4–16
Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 77:937–946. doi:10.1016/j.bcp.2008.10.020
Christensen JH, Christensen MS, Dyerberg J, Schmidt EB (1999) Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr 70:331–337
Mantzioris E, Cleland LG, Gibson RA, Neumann MA, Demasi M, James MJ (2000) Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr 72:42–48
Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC (2005) Effect of different antilipidemic agents and diets on mortality. Arch Intern Med 165:725–730
Ahrens EH, Insull W, Blomstrand R, Hirsch J, Tsaltas T, Peterson M (1957) The influence of dietary fats on serum lipid levels in man. Lancet 1:944–953
Shepherd J, Packard CJ, Grundy SM, Yeshurun D, Gotto J, Antonio M, Taunton D (1980) Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J Lipid Res 21:91–99
Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR (2013) Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 346. doi:10.1136/bmj.e8707
Cho HP, Nakamura MT, Clarke SD (1999) Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem 1:471–477
Friesen RW, Innis SM (2010) Linoleic acid is associated with lower long-chain n-6 and n-3 fatty acids in red blood cell lipids of Canadian pregnant women. Am J Clin Nutr 91:23–31
Goyens PLL, Spilker ME, Zock PL, Katan MB, Mensink RP (2006) Conversion of alfa-linolenic acid in humans is influenced by the absolute amounts of alfa-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 84:44–53
Liou YA, Innis SM (2009) Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-γ-linolenic and eicosapentaenoic acid in plasma of adult men. Prostaglandins Leukot Essent Fatty Acids 80:201–206. doi:10.1016/j.plefa.2009.02.003
Novak EM, Dyer RA, Innis SM (2008) High dietary ω-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res 1237:136–145
Novak EM, King DJ, Innis SM (2012) Low linoleic acid may facilitate Δ6 desaturase activity and docosahexaenoic acid accretion in human fetal development. Prostaglandins Leukot Essent Fatty Acids 86:93–98
Rahm JJ, Holman DT (1964) Effect of linoleic acid upon the metabolism of linolenic acid. J Nutr 84:15–19
Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TAB, Allen NE, Key TJ (2005) Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr 82:327–334
Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 47:147–155. doi:10.1016/j.plipres.2007.12.004
MacDonald-Wicks LK, Garg ML (2004) Incorporation of n-3 fatty acids into plasma and liver lipids of rats: importance of background dietary fat. Lipids 39:545–551
Garg ML, Thomson ABR, Clandinin MT (1990) Interactions of saturated, n-6 and n-3 polyunsaturated fatty acids to modulate arachidonic acid metabolism. J Lipid Res 31:271–277
Newens KJ, Thompson AK, Jackson KG, Wright J, Williams CM (2011) DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. Am J Clin Nutr 94:742–748. doi:10.3945/ajcn.110.009233
Rivellese AA, Maffettone A, Vessby B, Uusitupa M, Hermansen K, Berglund L, Louheranta A, Meyer BJ, Riccardi G (2003) Effects of dietary saturated, monounsaturated and n-3 fatty acids on fasting lipoproteins, LDL size and post-prandial lipid metabolism in healthy subjects. Atherosclerosis 167:149–158. doi:10.1016/s0021-9150(02)00424-0
Bonham MP, Linderborg KM, Dordevic A, Larsen AE, Nguo K, Weir JM, Gran P, Luotonen MK, Meikle PJ, Cameron-Smith D, Kallio HPT, Sinclair AJ (2013) Lipidomic profiling of chylomicron triacylglycerols in response to high fat meals. Lipids 48:39–50
Masson CJ, Mensink RP (2011) Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J Nutr 141:816–821. doi:10.3945/jn.110.136432
Mekki N, Charbonnier M, Borel P, Leonardi J, Juhel C, Portugal H, Lairon D (2002) Butter differs from olive oil and sunflower oil in its effects on postprandial lipemia and triacylglycerol-rich lipoproteins after single mixed meals in healthy young men. J Nutr 132:3642–3649
Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM (2005) Acute effects of meal fatty acids on postprandial NEFA, glucose and apo E response: implications for insulin sensitivity and lipoprotein regulation? Br J Nutr 93:693–700. doi:10.1079/BJN20051410
Tulk HMF, Robinson LE (2009) Modifying the n-6/n-3 polyunsaturated fatty acid ratio of a high-saturated fat challenge does not acutely attenuate postprandial changes in inflammatory markers in men with metabolic syndrome. Metab Clin Exp 58:1709–1716
Dias CB, Garg R, Wood LG, Garg ML (2014) Saturated fat consumption may not be the main cause of increased blood lipid levels. Med Hypotheses 82:187–195. doi:10.1016/j.mehy.2013.11.036
Karvonen HM, Tapola NS, Uusitupa MI, Sarkkinen ES (2002) The effect of vegetable oil-based cheese on serum total and lipoprotein lipids. Eur J Clin Nutr 56:1094–1101
Kagan ML, West AL, Zante C, Calder PC (2013) Acute appearance of fatty acids in human plasma—a comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis 12:102
Lepage G, Roy GC (1986) Direct trans-esterification of all classes of lipid in an onestep reaction. J Lipid Res 27:114–120
Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML (2002) Increased plasma fatty acid concentrations after respiratory exacerbations are associated with elevated oxidative stress in cystic fibrosis patients. Am J Clin Nutr 75:668–675
Mustad VA, Etherton TD, Cooper AD, Mastro AM, Pearson TA, Jonnalagadda SS, Kris-Etherton PM (1997) Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J Lipid Res 38:459–468
Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlström H, Risérus U (2012) Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 95:1003–1012. doi:10.3945/ajcn.111.030114
Bergeron N, Havel RJ (1995) Influence of diets rich in saturated and omega-6 polyunsaturated fatty acids on the postprandial responses of apolipoproteins B-48, B-100, E, and lipids in triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 15:2111–2121. doi:10.1161/01.atv.15.12.2111
Mustad VA, Ellsworth JL, Cooper AD, Kris-Etherton PM, Etherto TD (1996) Dietary linoleic acid increases and palmitic acid decreases hepatic LDL receptor protein and mRNA abundance in young pigs. J Lipid Res 37:2310–2323
Castro GR, Fielding CJ (1985) Effects of postprandial lipemia on plasma cholesterol metabolism. J Clin Investig 75:874–882. doi:10.1172/JCI111786
Fernandez ML, West KL (2005) Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 135:2075–2078
Wilkinson J, Higgins JA, Fitzsimmons C, Bowyer DE (1998) Dietary fish oils modify the assembly of VLDL and expression of the LDL receptor in rabbit liver. Arterioscler Thromb Vasc Biol 18:1490–1497. doi:10.1161/01.ATV.18.9.1490
Stubbs CD, Smith AD (1984) The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta Rev Biomembr 779:89–137. doi:10.1016/0304-4157(84)90005-4
Couillard C, Bergeron N, Prud’homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després J-P (1999) Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol 19:2448–2455. doi:10.1161/01.atv.19.10.2448
Koutsari C, Zagana A, Tzoras I, Sidossis LS, Matalas AL (2004) Gender influence on plasma triacylglycerol response to meals with different monounsaturated and saturated fatty acid content. Eur J Clin Nutr 58:295–502
De Marinis E, Martini C, Trentalance A, Pallottini V (2008) Sex differences in hepatic regulation of cholesterol homeostasis. J Endocrinol 198:635–643. doi:10.1677/joe-08-0242
Distefano E, Marino M, Gillette JA, Hanstein B, Pallottini V, Brüning J, Krone W, Trentalance A (2002) Role of tyrosine kinase signaling in estrogen-induced LDL receptor gene expression in HepG2 cells. Biochim Biophys Acta Mol Cell Biol Lipids 1580:145–149. doi:10.1016/S1388-1981(01)00197-4
Jensen RG (2002) The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci 85:295–350. doi:10.3168/jds.S0022-0302(02)74079-4
Legrand P, Beauchamp E, Catheline D, Pédrono F, Rioux V (2010) Short chain saturated fatty acids decrease circulating cholesterol and increase tissue PUFA content in the rat. Lipids 45:975–986. doi:10.1007/s11745-010-3481-5
Parodi PW (2009) Has the association between saturated fatty acids, serum cholesterol and coronary heart disease been over emphasized? Int Dairy J 19:345–361
Ramı́rez M, Amate L, Gil A (2001) Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev 65(Supplement 2):S95–S101. doi:10.1016/S0378-3782(01)00211-0
Acknowledgments
Authors are grateful to EPAX Norway AS (Norway) for providing, free of charge, the fish oil concentrate capsules; Ms Melissa Fry for assistance with the fatty acid analysis; the Centre for Physical Activity and Nutrition for part funding of the project and the Hunter Medical Research Institute (HMRI) Register of Volunteers. CBD was supported by a scholarship from Coordenação Nacional de Desenvolvimento Científico e Tecnológico—CNPq.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dias, C.B., Phang, M., Wood, L.G. et al. Postprandial Lipid Responses do not Differ Following Consumption of Butter or Vegetable Oil when Consumed with Omega-3 Polyunsaturated Fatty Acids. Lipids 50, 339–347 (2015). https://doi.org/10.1007/s11745-015-4003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4003-2