Abstract
We have recently witnessed substantial progress with immunotherapy for selected diseases. Checkpoint inhibitors and chimeric antigen receptor T (CAR-T) cells are among the most promising agents. Whereas much of the early success with CAR-T cells has been demonstrated with hematological malignancies, important barriers remain for the application of CAR-T cell therapies for the management of metastatic solid tumors. The challenges are particularly apparent when considering primary and metastatic tumors in the liver. At baseline, the intrahepatic space is immunosuppressive and this feature is exploited by malignant cells. Fortunately, our evolving understanding of liver immune cell biology is allowing the development of novel immunotherapeutic strategies for the treatment of liver tumors. Furthermore, the unique anatomic features of the liver make possible highly selective immunotherapeutic delivery approaches that may maximize antitumor efficacy while limiting off-target damage to healthy tissues. This review summarizes the immunobiology of the intrahepatic space and how this knowledge enables identification of hurdles and potential solutions to the barriers facing immunotherapy for liver tumors.
Similar content being viewed by others
Introduction
The liver is a unique immunological organ, with an abundance of immune cells demonstrating a strong tendency toward promotion of tolerance or immune suppression. The propensity toward immune tolerance in the liver is advantageous for maintenance of normal biological function, as the liver is constantly bathed in foreign antigens and bacterial byproducts found in the portal blood. Unfortunately, the capacity of intrahepatic immune cells to suppress immunity and inflammation create fertile ground for primary and metastatic liver tumors to develop and progress. Suppressor cells in the liver are impediments to the development of effective antitumor immunotherapy strategies. A deeper understanding of liver immune cell biology will be essential for the development of novel immunotherapeutic approaches for liver tumors.
Liver immunology
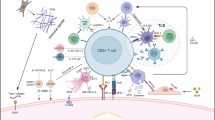
A perfectly balanced immune system protects our bodies from external pathogens and endogenous threats such as malignant cells, while avoiding damage to healthy tissue. Two main arms of the immune system work in concert toward this ideal—innate and adaptive immunity (Figure 1). The innate immune system provides a broad primary response that is not dependent on the exquisite antigen specificity of antibodies or T-cell receptors. The innate response is mediated by natural killer (NK) cells, macrophages and dendritic cells (DCs). Innate immune cells have the capacity to process and present antigen to cells capable of more specific and long-lasting adaptive responses. T and B cells mediate highly specific responses to particular antigens, in addition to rapid recall or memory responses. Although immune cells offer important protective functions, regulatory mechanisms to control immune system activation are essential for prevention of damage to normal tissues. This is particularly true in the liver.
Innate and adaptive immunity. (a) Innate immunity includes the body’s initial defences against infection. It includes certain complement proteins, epithelial barriers, natural killer (NK) cells, neutrophils (PMN), phagocytes such as macrophages (MAC) and antigen-presenting cells such as dendritic cells (DCs). Innate immune cells may directly kill tumor or infected cells and then present antigen to adaptive immune cells. (b), Adaptive immunity includes B-cell-mediated humoral (dissolved) and T-cell-mediated cellular components. The innate and adaptive immune systems communicate by direct cellular contact or cytokine secretion. We obtained permission from Elsevier to use this figure, which is appearing in an upcoming new edition of Jarnagin: Blumgart’s Surgery of the Liver, Biliary Tract, and Pancreas, 6th edn., Chapter 10: Liver immunology.
The liver contains an abundance of innate and adaptive immune cells, which are continuously exposed to ingested foreign antigens and bacterial products derived from gut flora. The liver is an immunologically rich and active organ, with a large number of DCs, Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), T cells, NK cells and B cells. Vigorous responses by liver immune cells to the steady stream of portal blood antigens would lead to a precarious situation with respect to liver damage and systemic inflammation.1 As such, intrahepatic immune cells are skewed toward tolerance, with overtly suppressive functions or quiescence at baseline.
The tolerogenic properties of liver immune cells protect us from overexuberant responses to portal venous antigens but limit the ability of our immune systems to fight intrahepatic neoplasia. The propensity of primary and metastatic tumors to thrive in the liver is in part reflective of the tolerogenic nature of the intrahepatic milieu.2, 3 Intrahepatic tolerance to specific antigens is initiated upon uptake, processing and presentation of soluble antigens by antigen-presenting cells (APCs). LSECs capture and process antigen but are unable to activate T cells on their own.4 DCs are also thought to promote tolerance in the liver, as studies have shown that these cells are less reactive than their counterparts elsewhere in the body.5 Thorn et al.6 have also shown that B cells can be suppressed in the liver that may also promote immune suppression, and liver T cells demonstrate tolerogenic properties.5
Tumor-infiltrating lymphocytes (TILs)
Studies of TILs have provided important insight into the nature and limitations of the endogenous response to intrahepatic neoplasms. TILs may be used for therapeutic or prognostic purposes.7 They are believed to represent an immune response to tumor antigens, although their presence does not imply effective antitumor immunity. Liver tumors, both primary and metastatic, have been evaluated extensively with regards to the presence of TILs in resected specimens and their prognostic value. Several types of TILs have been detected in liver malignancies, including T cells, NK cells, macrophages and B cells. CD8+ and CD4+ T cells are the most extensively studied liver TIL subsets as they are critical mediators of antitumor cellular immune responses. Regulatory T cells (Tregs), identified as FOXP3+, are often present in liver tumors and represent mediators of intrahepatic immunosuppression.8, 9, 10
TILs have been evaluated in an attempt to predict clinical outcomes for primary liver tumors, including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma. Li et al.11 showed that elevated tumor-associated macrophage and memory T-cell infiltration were a prognostic factor for disease-free survival and overall survival in resectable HCC. Mathai et al.10 reported that higher ratio of FOXP3+/CD8+ cells was associated with poorer differentiated tumors, recurrence and decreased overall survival. Interleukin 17 positive (IL17+) T cells and FOXP3+ cells are also believed to promote the progression and affect the prognosis of HCC by decreasing the level of CD8+ T cells within the tumor.12, 13 Takagi et al.14 evaluated CD4+ and CD8+ levels in resected intrahepatic cholangiocarcinoma and found that they correlated with DC density and outcome.
The liver is also a common site of metastatic disease, particularly from intra-abdominal cancers. Colorectal cancer liver metastasis (LM) patients who present with resectable disease have a 10-year survival of 17%, and fewer than 20% are surgical candidates at presentation.15 Studies evaluating TILs in primary colorectal cancer have shown the type, location and density of TILs to be associated with outcomes.16 TILs are also present in colorectal cancer LM specimens, with CD8+ T-cell density being predictive of long-term survival.17 Major histocompatibility complex (MHC) class I expression and its association with increased CD8+ T-cell density was correlated with increased overall survival and time to recurrence.18 Increased FOXP3 to CD8+ or CD4+ T-cell ratios were predictors of poor outcome in resected colorectal cancer LM patients, indicating the importance of suppressor–effector cell interactions.8 Tregs have also been correlated with outcome following resection of neuroendocrine tumor LM.19 Study of TILs from primary hepatic tumors and hepatic metastases have provided significant prognostic information regarding outcomes. Although the prognostic utility of TILs has been well demonstrated, a robust TIL response is generally insufficient to protect most patients from disease progression and death. As noted earlier, the suppressive tendencies of liver immune cells may have a large role in rendering TIL responses ineffective in addition to thwarting immununotherapeutic efforts.
Biology of liver immunosuppressive cells
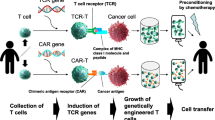
As noted earlier, the liver has unique population of immune cells that promote immune tolerance as evidenced by liver allografts requiring less immunosuppressive therapy as compared with other solid organ transplants.20 Although the tolerogenic nature of liver immune cells facilitate allograft acceptance, it is an impediment to cancer immunotherapy. Cancer immunotherapies include chimeric antigen receptor T (CAR-T) cell, antibodies that block immunoinhibitory or checkpoint molecules, and vaccines that attempt to induce a natural immune response against cancer. CAR-T cells are genetically engineered to express receptors that recognize an antigen on tumor cells.21 However, hepatic immunosuppressive cells limit CAR-T cell performance within the intrahepatic space.22 Detailed studies of intrahepatic immune cells have shed light on the mechanisms promoting liver tolerance and opportunities for enhancing immunotherapy for liver tumors.
Lymphocytes
The lymphocyte pool in the liver is comprised of conventional T cells, γδ T cells, NK and NK T cells (NKT) and B cells.5, 6, 23, 24, 25 Conventional liver CD4+ and CD8+ T cells have unique functional properties, while NKT and γδ T cells are particularly abundant. Most human liver CD4+ and CD8+ T cells are in an activated state and express CD25 and CD69.26 In mice, liver CD4+ T cells secrete both interferon γ (IFN γ) and IL4, indicative of T helper type 1 (Th1) and Th2 cell types.27 Among CD4+ T cells, Th1 programming is suppressed in the liver, resulting in a functional bias toward Th2 functionality.28 Liver Th2 cells produce high levels of IL4 and IL10, which thwart antitumor immune responses.
Intrahepatic CD4+ T cells are also reprogrammed into Tregs under neoplastic and inflammatory conditions.8, 29 Tregs with high expression levels of CD25 and Foxp3 are responsible for peripheral tolerance and protect the liver from immune-mediated damage.30 In HCC specimens, Tregs downregulate costimulatory molecules and decrease the secretion of IL12 and tumor necrosis factor α (TNFα) and promote tolerance.31 The liver also contains Th17 cells which secrete inflammatory cytokines that promotes hepatic inflammation and fibrosis. Liver Th17 cells may expand in response to programmed death-1 (PD-1, CD279) signaling and contribute to immunosuppression.12, 32 Intrahepatic T-cell subsets cooperate to create a tolerogenic milieu, which limits antitumor immunity.
NK cells may promote hepatic tolerance by producing suppressive factors such as transforming factor-beta (TGFβ) and IL10, which inhibit DC function. This in turn induces the expansion of immunosuppressive Treg cells.33, 34 NKT cells have properties of both T cells and NK cells and share several of their surface markers. Activated NKT cells secrete IFNγ and IL4 similar to Th1 and Th2 cells, respectively.35 NKT cells are known to clear hepatic infections and have a role in the developing inflammatory diseases. NKT cells are known to perform tumor surveillance and mediate tumor rejection by secreting IFNγ.36 Also, NKT cells can suppress T-cell proliferation and thereby cause immunosuppression in the liver.5 As such, liver NK and NKT function is highly contextual.
Liver sinusoid endothelial cells
LSECs line the hepatic sinusoids and are thus well positioned to take up and process the bulk of portal venous antigens.37 LSECs are efficient APC and process antigens at levels similar to DCs.4, 38 LSECs are also capable of recruiting hepatic leukocytes via CD54 (intercellular adhesion molecule-1), CD106 (vascular cell adhesion molecule-1), vascular adhesion protein-1, CD44 and hyaluronan.39, 40 LSECs express low levels of MHCII and co-stimulatory molecules41 and can induce CD4 T-cell reprogramming into suppressive IL10- and IL4-producing cells.42 The tolerogenic effect of LSECs are also mediated by IL10 secretion and PD-1/programmed death-ligand 1 (PD-L1) signaling.43
Dendritic cells
DCs are a rare population in the liver, have poor immunostimulatory capability and contribute to intrahepatic tolerance.44 Hepatic DCs are less immunostimulatory compared with splenic DCs.45 There are four distinct DC subtypes (CD8α+CD11b−, CD8α−CD11b+, CD8α+CD11blow/−, CD8αlow/−CD11blow/−) with specific functions. CD8α+CD11b− and CD8α−CD11b+, which account for only 20% of total DC population in the liver, activate T cells causing an immunostimulatory effect. However, the other more predominant subtypes of hepatic DCs, CD8α+CD11blow/− and CD8αlow/−CD11blow/−, are poor T-cell stimulators. Thus, these two populations may contribute to tolerance. Direct physical interaction between Tregs and DCs inhibit DC maturation even in the presence of granulocyte macrophages colony-stimulating factor (GM-CSF), TNFα or IFNγ.46 Human hepatic DCs are also tolerogenic in comparison to autologous blood DCs.2
Kupffer cells
KCs are found within the liver sinusoids and constitute 80–90% of tissue macrophages in the body.47 Depletion of KCs causes loss of oral tolerance and KC are less immunogenic than macrophages in other organs.2, 5 At baseline, they are more skewed toward tolerance. KCs can secrete anti-inflammatory cytokines or immunosuppressive factors (IL10, nitric oxide, TGFβ) in addition to pro-inflammatory cytokines (TNFα, IL6).48, 49, 50, 51 They can inhibit T-cell proliferation and the secretion of IL10 can induce the activation of Tregs thereby causing tolerance.52 KCs can also express both PD-1 and PD-L1, which are known immunomodulatory molecules. PD-L1–PD-1 interactions between KCs, effector T cells and LSECs can modulate disease activity.53
Myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous cell population of myeloid origin that have been reported in association with in liver tumors and in several inflammatory conditions, including sepsis, hepatitis and viral infections. T-cell suppression caused by MDSCs is mediated in part by L-arginine depletion by arginase 1 or by reactive oxygen species.54 Liver MDSCs expand in response to GM-CSF secreted by tumor cells and GM-CSF enhances their capacity to suppress immune responses22, 55 (Figure 2) through exploitation of STAT3, indoleamine 2,3-dioxygenase (IDO) and PD-L1.22, 56
Tumor-derived granulocyte macrophages colony-stimulating factor (GM-CSF) drives indoleamine 2,3-dioxygenase (IDO) and programmed death-ligand 1 (PD-L1) expression in liver myeloid-derived suppressor cells (L-MDSCs). In all, 2.5 × 105 L-MDSCs were cultured with or without 5 × 104 MC38 tumor cells. Anti-GM-CSF and anti-GM-CSF receptor antibodies were added at the start or after 1 day of culture. After 48 h, L-MDSCs were purified for IDO and PD-L1 expression measurement (a and b). Isotype controls were used to define background staining and to set the threshold for positive IDO and PD-L1 expression. IDO/PD-L1 co-expression was visualized in L-MDSCs/MC38 cultures with or without anti-GM-CSF and anti-GM-CSF receptor (c). Bars show the expression averages of L-MDSCs isolated from four tumor-bearing livers and are representative of three experiments. Error bars are based on s.e.m. values. P-values were calculated using a two-tailed t-test. Previously published in Thorn et al.89
Hepatic stellate cells (HSCs)
HSCs are located in the luminal sinusoidal space of Disse. HSCs are derived from bone marrow precursors in mice and store vitamin A.57 Once activated, HSCs metabolize vitamin A and produce extracellular matrix that induces hepatic fibrosis and cirrhosis.58, 59 HSCs express MHCI/MHCII and CD86 but are poor APCs. HSCs are capable of producing retinoic acid and TGFβ that induce Tregs.60 On contact with activated T cells, HSCs express PD-L1, which attenuates T-cell response by increasing apoptosis.61
Immunoinhibitory signaling
Immune checkpoint molecules prevent the development of unregulated immune response and autoimmune tissue damage. Some of the well-known immnuoinhibitory receptors include cytotoxic T-lymphocyte antigen-4 (CTLA-4), PD-1, T-cell immunoglobulin domain and mucin domain-3 (TIM3) and lymphocyte-activation gene (LAG3).62, 63, 64 CTLA-4 counteracts CD28 costimulatory receptor activity on T cells. CTLA-4 and CD28 have common ligands in CD80 and CD86. However, the affinity of CTLA-4 for CD80 and CD86 is much higher than CD28 and thus prevents engagement of CD28. CTLA-4 blockade is known to enhance CD4 helper T cells and limit Treg suppressive activity.65, 66, 67, 68
PD-1 causes immune tolerance in the tumor microenvironment.69, 70, 71, 72 Unlike CTLA-4, PD-1 limits T-cell activity in the peripheral tissue during inflammation and autoimmunity. PD-1 gets overexpressed during T-cell activation and is associated with proliferation of Tregs in the presence of its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC).70 Recently, it was reported that there was a molecular interaction between PD-L1 expressed on APCs and CD80 expressed on T cells thereby eliciting inhibitory signals.73, 74 PD-1 is also expressed on other cell types such as B cells and NK cells, which limit their lytic activities.75, 76 However, PD-1 predominantly regulates effector T-cell proliferation and cytokine production, while CTLA-4 regulates early T-cell activation.
Other immune checkpoint markers of interest are LAG3 and TIM3, which are implicated in inhibiting lymphocyte activity and in anergy. These receptors are upregulated during T-cell activation. LAG3 signaling enhances immunosuppressive activity of Treg cells and inhibits the effector activity of CD8 T cells.77, 78 LAG3–MHCII interaction enhances T-cell proliferation and effector T-cell function in vivo and in vitro. PD-1 and LAG3 are co-expressed on anergic or exhausted T cells.79, 80 TIM3 co-expresses with PD-1 on tumor-specific CD8 T cells and their dual blockade enhance T-cell proliferation and cytokine secretion.81, 82, 83
Overcoming immune suppression in the liver for antitumor immunotherapy
Few attempts have been made to specifically target intrahepatic neoplasms with immunotherapy and augment therapy through modulation of liver immune cells. Effective immunotherapy for liver tumors will require an effective tumor killing strategy, efficient intrahepatic delivery and agents capable of reversing suppressive function of liver immune cells. Intrahepatic tolerance can be reversed by depleting suppressor cells, activating hepatic immune cells or blocking the immune checkpoints. CAR-T cells are among the more promising immunotherapy technologies to emerge in recent years, given that highly specific immune responses can be manufactured as opposed to induced.
CAR-T cells have demonstrated success in hematological malignancies and have shown activity in solid tumors. CAR-T cells are autologous T cells that are bioengineered to express an immune receptor that is activated upon tumor antigen binding. We have reported the biological activity and safety of anti-carcinoembryonic antigen (anti-CEA) CAR-T cell hepatic artery infusions (HAIs) in patients with heavily pretreated large tumor burdens.84, 85 CAR-T cell therapies for LM will likely be enhanced by strategies to inhibit the function of intrahepatic-suppressor cells, among which MDSC are particularly problematic. Liver MDSCs may be susceptible to inhibition of PD-1/PD-L1 interactions or IDO.22 MDSCs express IDO and PD-L1, which mediate T-cell suppression.56 Combinatorial immunotherapeutic approaches will enhance the antitumor activity of CAR-T cells.
Enhancing the efficacy and safety of liver immunotherapy through targeted delivery
In addition to addressing intrahepatic immunosuppression, regional delivery approaches have shown promise as a strategy to enhance biological activity and reduce off-target toxicity when targeting liver tumors with cellular immunotherapeutics.84, 85 The rationale for regional, intrahepatic infusion of CAR-T cell stems from the experiences with regional chemotherapy and immune cell treatments. Delivery of chemotherapy directly into the liver for metastasis treatment permits maximal exposure of the tumors to the agent, while minimizing the effects on healthy tissues elsewhere. This principle has been well demonstrated in patients receiving HAI of chemotherapy for LM. Response rates are consistently higher with HAI and systemic effects are minimized.86 The regional infusion of lymphocytes into the liver has been demonstrated to be feasible and safe, with up to 80% of radiolabeled lymphocytes infused via the hepatic artery persist in the liver for up to 120 h.87
We recently completed a phase I trial to test CAR-T cell HAI to determine whether direct regional delivery of CAR-T cell to LM is safe and associated with signals of clinical efficacy.84, 85 Six patients completed the protocol, and three received anti-CEA CAR-T cell HAIs alone in dose-escalation manner (108, 109 and 1010 cells). We treated three additional patients with the maximum planned CAR-T cell HAI dose (1010 cells × 3) along with IL2 infusional support. Four patients had >10 LMs, and patients were heavily pretreated with conventional systemic therapy.
No patient suffered a grade 3 or 4 adverse events related to the CAR-T cell HAIs and there were no deaths related to the study intervention. Importantly, regional infusion seemed to avoid severe cytokine release syndrome and significant off-target effects from direct destruction of normal CEA+ tissue by CAR-T cells. Febrile adverse events were observed in four patients. A single patient experienced a marked increase in the peripheral eosinophil count. Given the reported association between IL2 infusion and cardiac thrombosis with other features of Loeffler’s syndrome,88 we obtained an echocardiogram and electrocardiogram, which were normal
Normal liver parenchyma and biliary structures were well preserved following CAR-T cell HAIs. Biopsies from normal liver did not demonstrate increased levels of inflammation or fibrosis following CAR-T cell HAI whether or not systemic IL2 was administered. Although all patients experienced transient elevations of alkaline phosphatase, total bilirubin and aspartate aminotransferase levels, the majority of values did not deviate significantly from baseline levels. Portal pressures and liver synthetic function were not adversely affected by the CAR-T cell HAIs, as reflected by no patient becoming thrombocytopenic or coagulopathic.
One patient remains alive with stable disease at 44 months following CAR-T cell HAI and five patients died of progressive disease. Among the patients in the cohort who received systemic IL2 support, CEA levels decreased 37% (range 19–48%) from baseline. Biopsies demonstrated an increase in LM necrosis or fibrosis in four of the six patients. Elevated serum IFNγ levels correlated with serum CEA responses. As CAR-T cell HAIs were well tolerated and associated with evidence of tumor cell killing in our subjects, further clinical testing of this approach alone and in combinatorial manner is underway. HAI of CAR-T cells for the treatment of liver tumor is a promising approach for enhancing clinical activity while avoiding some of the more problematic systemic effects of CAR-T cell infusions, including severe cytokine release syndrome.
Summary
The liver contains an abundance of diverse immune cells that work in concert to create a highly tolerogenic milieu. Though hepatic tolerance is beneficial in liver transplantation and maintenance of normal physiological homeostasis, intrahepatic immunosuppression limits the effectiveness of endogenous and therapeutic antitumor immunity. Hepatic immunosuppression is mediated by a variety of cell types, cytokines and immunoinhibitory molecules. Reversal of intrahepatic immunosuppression will be an important element in combinatorial immunotherapy approaches designed to target intrahepatic tumors.
References
Knolle PA, Gerken G . Local control of the immune response in the liver. Immunol Rev 2000; 174: 21–34.
Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol 2009; 182: 1901–1911.
Jewell AP . Is the liver an important site for the development of immune tolerance to tumours? Med Hypotheses 2005; 64: 751–754.
Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP . Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol 2004; 173: 230–235.
Katz SC, Pillarisetty VG, Bleier JI, Kingham TP, Chaudhry UI, Shah AB et al. Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology 2005; 42: 293–300.
Thorn M, Point GR, Burga RA, Nguyen CT, Joseph Espat N, Katz SC . Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1+CD11b+ myeloid cells. J Leukoc Biol 2014; 96: 883–894.
Rosenberg SA, Spiess P, Lafreniere R . A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233: 1318–1321.
Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 2013; 20: 946–955.
Khan H, Pillarisetty VG, Katz SC . The prognostic value of liver tumor T cell infiltrates. J Surg Res 2014; 191: 189–195.
Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM . Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol 2012; 36: 980–986.
Li YW, Qiu SJ, Fan J, Gao Q, Zhou J, Xiao YS et al. Tumor-infiltrating macrophages can predict favorable prognosis in hepatocellular carcinoma after resection. J Cancer Res Clin Oncol 2009; 135: 439–449.
Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z et al. Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol 2014; 29: 851–859.
Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, Gao YT et al. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 2012; 86: 329–337.
Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol 2004; 35: 881–886.
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007; 25: 4575–4580.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–1964.
Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol 2009; 16: 2524–2530.
Turcotte S, Katz SC, Shia J, Jarnagin WR, Kingham TP, Allen PJ et al. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol Res 2014; 2: 530–537.
Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gonen M, Espat NJ et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxford) 2010; 12: 674–683.
Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969; 223: 472–476.
Emtage PC, Lo AS, Gomes EM, Liu DL, Gonzalo-Daganzo RM, Junghans RP . Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res 2008; 14: 8112–8122.
Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 2015; 64: 817–829.
Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol 1998; 28: 84–90.
Crispe IN, Mehal WZ . Strange brew: T cells in the liver. Immunol Today 1996; 17: 522–525.
Crispe IN . Hepatic T cells and liver tolerance. Nat Rev Immunol 2003; 3: 51–62.
Pruvot FR, Navarro F, Janin A, Labalette M, Masy E, Lecomte-Houcke M et al. Characterization, quantification, and localization of passenger T lymphocytes and NK cells in human liver before transplantation. Transpl Int 1995; 8: 273–279.
Klugewitz K, Topp SA, Dahmen U, Kaiser T, Sommer S, Kury E et al. Differentiation-dependent and subset-specific recruitment of T-helper cells into murine liver. Hepatology 2002; 35: 568–578.
Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN . Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol 2002; 169: 2407–2413.
Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol 2011; 187: 1150–1156.
Stross L, Gunther J, Gasteiger G, Asen T, Graf S, Aichler M et al. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology 2012; 56: 873–883.
Chen X, Du Y, Huang Z . CD4+CD25+ Treg derived from hepatocellular carcinoma mice inhibits tumor immunity. Immunol Lett 2012; 148: 83–89.
Licata LA, Nguyen CT, Burga RA, Falanga V, Espat NJ, Ayala A et al. Biliary obstruction results in PD-1-dependent liver T cell dysfunction and acute inflammation mediated by Th17 cells and neutrophils. J Leukoc Biol 2013; 94: 813–823.
Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol 2004; 173: 6072–6081.
Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology 2007; 120: 73–82.
Tyznik AJ, Verma S, Wang Q, Kronenberg M, Benedict CA . Distinct requirements for activation of NKT and NK cells during viral infection. J Immunol 2014; 192: 3676–3685.
Sun R, Gao B . Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma). Gastroenterology 2004; 127: 1525–1539.
Horst AK, Neumann K, Diehl L, Tiegs G . Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 2016; 13: 277–292.
Schurich A, Berg M, Stabenow D, Bottcher J, Kern M, Schild HJ et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol 2010; 184: 4107–4114.
Knolle PA, Limmer A . Control of immune responses by savenger liver endothelial cells. Swiss Med Wkly 2003; 133: 501–506.
Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 1997; 99: 2782–2790.
Knolle PA, Uhrig A, Hegenbarth S, Loser E, Schmitt E, Gerken G et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol 1998; 114: 427–433.
Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S et al. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J Immunol 1999; 162: 1401–1407.
Carambia A, Frenzel C, Bruns OT, Schwinge D, Reimer R, Hohenberg H et al. Inhibition of inflammatory CD4 T cell activity by murine liver sinusoidal endothelial cells. J Hepatol 2013; 58: 112–118.
Lau AH, Thomson AW . Dendritic cells and immune regulation in the liver. Gut 2003; 52: 307–314.
Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP . Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol 2004; 172: 1009–1017.
Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S . Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA 2008; 105: 10113–10118.
Bilzer M, Roggel F, Gerbes AL . Role of Kupffer cells in host defense and liver disease. Liver Int 2006; 26: 1175–1186.
Bingisser RM, Tilbrook PA, Holt PG, Kees UR . Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 1998; 160: 5729–5734.
Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL . In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol 1991; 138: 395–402.
Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G . Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol 1995; 22: 226–229.
You Q, Cheng L, Kedl RM, Ju C . Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 2008; 48: 978–990.
Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015; 62: 279–291.
Xie Z, Chen Y, Zhao S, Yang Z, Yao X, Guo S et al. Intrahepatic PD-1/PD-L1 up-regulation closely correlates with inflammation and virus replication in patients with chronic HBV infection. Immunol Invest 2009; 38: 624–638.
Youn JI, Gabrilovich DI . The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40: 2969–2975.
Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther 2016; 23: 188–198.
Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther 2016; 23: 188–198.
Weiskirchen R, Tacke F . Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr 2014; 3: 344–363.
Trautwein C, Friedman SL, Schuppan D, Pinzani M . Hepatic fibrosis: concept to treatment. J Hepatol 2015; 62 (1 Suppl): S15–S24.
Lee YA, Wallace MC, Friedman SL . Pathobiology of liver fibrosis: a translational success story. Gut 2015; 64: 830–841.
Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol 2013; 190: 2009–2016.
Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004; 40: 1312–1321.
Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res 2014; 74: 1045–1055.
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res 2014; 74: 1924–1932.
Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011; 208: 395–407.
Egen JG, Allison JP . Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 2002; 16: 23–35.
Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R . Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1994; 1: 793–801.
Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA 2002; 99: 11790–11795.
Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM et al. Reversal of the TCR stop signal by CTLA-4. Science 2006; 313: 1972–1975.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027–1034.
Ishida Y, Agata Y, Shibahara K, Honjo T . Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–3895.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203: 883–895.
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001; 291: 319–322.
Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010; 116: 1291–1298.
Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol 2011; 187: 1097–1105.
Fanoni D, Tavecchio S, Recalcati S, Balice Y, Venegoni L, Fiorani R et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett 2011; 134: 157–160.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71: 5393–5399.
Goldberg MV, Drake CG . LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol 2011; 344: 269–278.
Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G et al. Role of LAG-3 in regulatory T cells. Immunity 2004; 21: 503–513.
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10: 29–37.
Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 2009; 182: 6659–6669.
Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One 2012; 7: e30852.
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207: 2175–2186.
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC . Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207: 2187–2194.
Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res 2015; 21: 3149–3159.
Saied A, Licata L, Burga RA, Thorn M, McCormack E, Stainken BF et al. Neutrophil:lymphocyte ratios and serum cytokine changes after hepatic artery chimeric antigen receptor-modified T-cell infusions for liver metastases. Cancer Gene Ther 2014; 21: 457–462.
Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009; 27: 3465–3471.
Keilholz U, Scheibenbogen C, Brado M, Georgi P, Maclachlan D, Brado B et al. Regional adoptive immunotherapy with interleukin-2 and lymphokine-activated killer (LAK) cells for liver metastases. Eur J Cancer 1994; 30A: 103–105.
Junghans RP, Manning W, Safar M, Quist W . Biventricular cardiac thrombosis during interleukin-2 infusion. N Engl J Med 2001; 344: 859–860.
Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther 2016; 23: 188–198.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Guha, P., Reha, J. & Katz, S. Immunosuppression in liver tumors: opening the portal to effective immunotherapy. Cancer Gene Ther 24, 114–120 (2017). https://doi.org/10.1038/cgt.2016.54
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2016.54
- Springer Nature America, Inc.
This article is cited by
-
Novel treatment paradigms for metastatic uveal melanoma
Cancer Gene Therapy (2022)
-
Regional infusion of a class C TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition
Cancer Gene Therapy (2022)
-
Monocytic and granulocytic myeloid-derived suppressor cell plasticity and differentiation are organ-specific
Oncogene (2021)
-
Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity
Nature Protocols (2021)
-
Comparison of immune profiles between hepatocellular carcinoma subtypes
Biophysics Reports (2020)